2013 Volume 61 Issue 5 Pages 559-566
2013 Volume 61 Issue 5 Pages 559-566
A method has been developed for the measurement of transport activities in membrane vesicles obtained from Sf9 cells for 3β-hydroxy-Δ5-bile acids by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Calibration curves for the bile acids were linear over the range of 10 to 2000 pmol/mL, and the detection limit was less than 1 pmol/mL for 3β-hydroxy-Δ5-bile acids using selected reaction monitoring analysis. The analytical method was applied to measurements of transport activities in membrane vesicles obtained from human multidrug resistance-associated protein 2-, 3-, and human bile salt export pump-expressing Sf9 cells for conjugated 3β-hydroxy-Δ5-bile acids. The present study demonstrated that human multidrug resistance-associated protein 3 vesicles accepted conjugated 3β-hydroxy-Δ5-bile acids along with common bile acids such as glycocholic acid and taurolithocholic acid 3-sulfate.
Bile acids are excreted into bile by canalicular ATP (ATP)-binding cassette (ABC) transporters, such as multidrug resistance-associated protein 2 (MRP2, ABCC2) and bile salt export pump (BSEP, ABCB11).1,2) Canalicular secretion of bile acids constitutes the major driving force for the generation of bile flow. Biliary excretion of monovalent bile acids, such as taurine- and glycine-conjugated bile acids, is mediated by BSEP, and divalent bile acids conjugated with sulfuric acid or glucuronic acid are excreted into bile by MRP2.1,2) It is thought that any dysfunction of these transporters induces cholestasis.1,2) Mutations in MRP2 lead to Dubin–Johnson syndrome, and mutations in BSEP cause progressive familial intrahepatic cholestasis type 2 (PFIC-2).1,2) In contrast to MRP2 and BSEP, multidrug resistance-associated protein 3 (MRP3, ABCC3), localized at the basolateral membrane in hepatocytes, excretes organic anions and bile acids from hepatocytes to the sinusoid.3,4) Human MRP3 (hMRP3) has been shown to transport not only monovalent bile acids but also divalent bile acids.4–6) By hMRP3, monovalent bile acids such as glycocholic acid (G-CA) are transported, at high rates, even if at low affinity.4,5) Divalent bile acids, such as taurolithocholic acid-3-sulfate (T-LCA-3S), are transported by hMRP3 with high affinity.6) The transport properties of bile acids mediated by human BSEP (hBSEP), human MRP2 (hMRP2), and hMRP3 have been investigated by examining the ATP-dependent transport of common bile acids, such as glycocholic acid (G-CA), taurocholic acid (T-CA) and taurolithocholic acid-3-sulfate (T-LCA-S), in isolated bile canalicular membrane vesicles or in membrane vesicles obtained from these ABC transporter-expressing cells.1–6) In those investigations, bile acids labeled with a radioactive isotope (RI) have been widely used as substrates.
It has been reported that 3β-hydroxy-Δ5-bile acids such as 3β,7α,12α-trihydroxy-5-cholenoic acid (Δ5-3β,7α,12α-ol) and 3β,7α-dihydroxy-5-cholenoic acid (Δ5-3β,7α-ol) as shown in Fig. 1, are the major bile acids excreted in the urine of patients with 3β-hydroxy-Δ5-C27-steriod dehydrogenase/isomerase (HSD3B7) deficiency.7–15) Deficiency of this enzyme is thought to cause chronic liver injury in childhood.7–11) The above ABC transporters may play key roles in excretion of these 3β-hydroxy-Δ5-bile acids from the liver. It has also been reported that in cholestasis, up-regulation of hepatic hMRP3 is an adaptive response, providing an alternative excretory pathway to help address the overload of bile acids.3,16,17) Therefore, hMRP3 may be involved in urinary excretion of these 3β-hydroxy-Δ5-bile acids. However, the transport properties of these transporters for these unusual bile acids have not been established because of the difficulty in obtaining these RI-labeled bile acids. The transport properties of 3β-hydroxy-Δ5-bile acids by these ABC transporters may be affected, because their structures and physical properties are significantly different from those of the common bile acids that are present in human biological fluids.

There have been several reports on the separation and quantification of 3β-hydroxy-Δ5-bile acids by gas chromatography-mass spectrometry (GC-MS).9,10,12) The GC-MS method may be applicable for measurements of transport activities of these unusual bile acids in membrane vesicles without using RI labels. However, under the alkaline or acidic conditions usually used for deconjugation in the GC-MS method, 3β-hydroxy-Δ5-bile acids are expected to be converted into their dehydrated products or complicated degradation products.
In our previous study, measurements of the transport activities of common bile acids in membrane vesicles obtained from hMRP3-expressing Sf9 cells were carried out by high-performance liquid chromatography-electrospray ionization coupled to tandem mass spectrometry (LC/ESI-MS/MS) using selected reaction monitoring (SRM).18) This LC/ESI-MS/MS method, which makes prior deconjugation unnecessary, appears to be suitable for measurements of 3β-hydroxy-Δ5-bile acid transport activities by membrane vesicles. The present paper deals with an application of the LC/ESI-MS/MS method to measurement of ATP-dependent transport activities of 3β-hydroxy-Δ5-bile acids in membrane vesicles obtained from hMRP2-, hMRP3-, and hBSEP-expressing Sf9 cells.
Glycine- and taurine-conjugates and 3-sulfates of 3β-hydroxy-Δ5-bile acids were all stock samples synthesized in our laboratory.19) Chemical structures of 3β-hydroxy-Δ5-bile acids and their abbreviations are shown in Fig. 1. G-CA, T-CA and T-LCA-S were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.) and used after chromatographic purification. [2,2,3,4,4-2H5]-Glycoursodeoxycholic acid (G-UDCA-d5) was chemically synthesized in our laboratory (isotopic purity >98.5%) and used as an internal standard (IS) for the determination of bile acids by LC/ESI-MS/MS. Oasis HLB 96-well plate cartridges were purchased from Waters Co. (Milford, MA, U.S.A.) and were washed successively with ethanol (0.5 mL) and water (1 mL) prior to use. ATP disodium salt (ATP) and adenosine monophosphate disodium salt (AMP) were purchased from Oriental Yeast (Tokyo, Japan). hBSEP-, hMRP2- and hMRP3-expressing Sf9 membrane vesicles were purchased from GenoMembrane, Inc. (Kanagawa, Japan). Acetonitrile (MeCN), ethanol, methanol and water were of HPLC grade and ammonium acetate was of analytical grade, and all of them were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan).
LC-MS/MS ConditionsLC/ESI-MS/MS analysis was performed using a 4000Q-Trap hybrid triple quadrupole linear ion-trap mass spectrometer (AB SCIEX, Foster City, CA, U.S.A.) equipped with an ESI probe. High-purity nitrogen was produced by a nitrogen generator, 12E-SDA (System Instruments, Tokyo, Japan). The ion source was operated in the negative ion-mode using the following settings: ion spray voltage, −4000 V; ion source heater temperature, 550°C; source gas 1, 55 psi; source gas 2, 85 psi; and curtain gas setting, 30 psi. Analytes were monitored by SRM. Mass transitions and MS parameters are shown in Table 1. The chromatographic system consisted of an Agilent 1200 series HPLC system (Agilent Technologies, Palo Alto, CA, U.S.A.). Gradient chromatographic separation of bile acids was performed on an Ascentis Express C18 column (50 mm×2.1 mm i.d., 2.7-µm particles, Supelco, Bellefont, PA, U.S.A.) at ambient temperature. Mobile phase A was 15% MeCN–10 mm ammonium acetate (adjusted to pH 7.0 with aqueous ammonia solution), and mobile phase B was 90% MeCN–10 mm ammonium acetate (adjusted to pH 7.0 with aqueous ammonia solution)). The mobile phase was delivered at a flow rate of 0.35 mL/min. The gradient program was as follows: mobile phase B was increased from 0 to 48% over a period of 8 min and then from 48 to 100% over a period of 3 min. The column was washed with 100% B for 1 min and re-equilibrated at 0% B for 5 min.
| Bile acid | [M−H]− (m/z) | SRM transition (m/z) | C.E.a) (V) | R.T.b) (min) | Linear regression equationc) | LODd) (pmol/mL) |
|---|---|---|---|---|---|---|
| G-Δ5-3β,7α-ol | 446.3 | 446.3→74.0 | 62 | 5.6 | A=3.8×10−3 C−0.042e) | 0.5 |
| T-Δ5-3β,7α-ol | 496.3 | 496.3→80.0 | 124 | 5.9 | A=3.1×10−3 C+0.673e) | 0.5 |
| Δ5-3β,7α-ol-S | 469.2 | 469.2→97.0 | 130 | 5.4 | A=3.1×10−3 C+0.046e) | 1.0 |
| G-Δ5-3β,7α-ol-S | 526.2 | 526.2→446.3 | 42 | 4.6 | A=1.5×10−3 C−0.244e) | 1.0 |
| T-Δ5-3β,7α-ol-S | 576.2 | 287.6→478.3 | 32 | 4.8 | A=7.4×10−3 C+0.185e) | 0.5 |
| G-Δ5-3β,7α,12α-ol | 462.3 | 462.3→74.0 | 70 | 4.4 | A=2.8×10−3 C−0.068e) | 0.5 |
| T-Δ5-3β,7α,12α-ol | 512.3 | 512.3→80.0 | 124 | 4.7 | A=1.2×10−3 C+0.324e) | 0.3 |
| Δ5-3β,7α,12α-ol-S | 485.2 | 485.2→97.0 | 84 | 4.0 | A=3.1×10−3 C−0.022e) | 1.0 |
| G-Δ5-3β,7α,12α-ol-S | 542.2 | 542.2→462.3 | 46 | 3.7 | A=3.1×10−3 C−0.006e) | 1.0 |
| T-Δ5-3β,7α,12α-ol-S | 592.2 | 295.6→494.3 | 34 | 3.9 | A=8.6×10−3 C−0.107e) | 0.5 |
| G-UDCA-d5 (I.S.) | 453.3 | 453.3→74.0 | 70 | 5.8 |
a) Collision energy. b) Retention time. c) Calibration curves were constructed by plotting peak area ratios of each concentration of bile acid (10, 20, 40, 100, 200, 400, 1000, 2000 pmol/mL) to that of the IS (400 pmol/mL). d) Limit of detection. e) A, peak area ratio; C, concentration (pmol/mL).
Human MRP2-, MRP3-, and BSEP-expressing Sf9 membrane vesicles were suspended in a transport buffer. For transport studies with hBSEP-membrane vesicles, 10 mm Hepes-Tris buffer (pH 7.4, containing 100 mm KNO3, 10 mm Mg(NO3)2, 50 mm sucrose) was used as the transport buffer, and 50 mm 3-(N-morpholino)propanesulfonic acid (MOPS)-Tris buffer (pH 7.0, containing 70 mm KCl and 7.5 mm MgCl2) was used as the transport buffer for transport studies with hMRP2 and hMRP3 membrane vesicles. Membrane vesicles obtained from Sf9 cells transfected with the virus vector alone (mock vesicles) were used as controls. The membrane transport study was performed using a rapid filtration technique.4,5,20) Briefly, the incubation mixture contained appropriate amounts of bile acid, 4 mm ATP or AMP and 25 µg of membrane protein in 50 µL of transport buffer. The mixture was incubated in a test tube at 37°C. The transport reaction was stopped by the addition of 0.2 mL of ice-cold stop buffer. The stop buffer solutions used were as follows: 10 mm Hepes-Tris buffer (pH 7.4, containing 100 mm KNO3 and 50 mm sucrose) for hBSEP vesicles and 40 mm MOPS-Tris buffer (pH 7.0, containing 70 mm KCl) for hMRP2 and hMRP3 vesicles. The mixture was passed through a 1.0-µm glass fiber filter (Millipore Co., Billerica, MA, U.S.A.) and then washed five times with 0.2 mL of ice-cold stop buffer. The bile acid transported into vesicles retained on the filter was eluted with ethanol (1.4 mL) after the addition of IS (20 pmol) on the filter. Sample preparation of the filtrate for LC/ESI-MS/MS analysis using solid phase extraction (SPE) was performed by the method previously reported.18) SPE was performed using Oasis HLB 96-well plate cartridges, a vacuum manifold and a vacuum source. After evaporation of the filtrate, the residue was dissolved in 0.1 m phosphate buffer (pH 7.4, 0.2 mL), and the solution was loaded onto an Oasis HLB 96-well plate cartridge at a flow rate of approximately 4 mL/min. The cartridge was sequentially washed with water (0.4 mL). After the cartridge had been dried under vacuum for 2 min, the bile acids were eluted with ethanol (0.4 mL). The eluate was evaporated to dryness under reduced pressure. The residue was dissolved with 50 µL of mobile phase A, and an aliquot (5 µL) was injected into the LC/ESI-MS/MS system.
ATP-dependent transport activity was calculated by subtracting the transport activity in the presence of AMP from that in the presence of ATP. To estimate the kinetic parameters for the uptake of bile acids by membrane vesicles, the initial uptake rate was fitted to the following equation by means of nonlinear least-square regression analysis using KaleidaGraph (Synergy Software, Reading, PA, U.S.A.):
 |
where v, s, Km and Vmax are uptake rate (pmol/min/mg), concentration of bile acid (µm), Michaelis–Menten constant (µm) and maximum uptake rate (pmol/min/mg), respectively. The results are presented as means±standard error (S.E.).
Method ValidationFor preparing standard stock solutions, glycine- and taurine-conjugated bile acids and sulfated bile acids were dissolved in methanol at a concentration of 200 µm. Samples were diluted to concentrations of 10, 20, 50, 100, 200, 500, 1000, and 2000 pmol/mL using methanol. An IS stock solution containing 400 pmol/mL of stable isotope-labeled bile acids was also prepared in methanol. A 50-µL aliquot of each standard solution was mixed with 50 µL IS solution and evaporated under nitrogen gas at room temperature. The residue was dissolved in 50 µL of mobile phase A, and 5 µL of this solution was injected into the LC/ESI-MS/MS system. Calibration curves were constructed by plotting the peak area-ratio of each bile acid to those of IS versus the weights of the bile acid. To determine the recovery rates of SPE using Oasis HLB 96-well plate cartridges, blank extracts of an incubation mixture with mock vesicles were prepared. The recovery rates of SPE were tested by adding known concentrations of bile acids (10, 100, 1000 pmol/mL) to blank extracts.
It has been reported that 3β-hydroxy-Δ5-bile acids exist mainly as glycine- and taurine-conjugated forms and 3-sulfated forms in biological fluids.7,10,15) In the present study, various conjugated bile acids (Fig. 1) were used as substrates of membrane vesicles for the following experiments.
LC/ESI-MS/MS Analysis of 3β-Hydroxy-Δ5-bile AcidsFirst, we investigated the fragmentation of deprotonated bile acids in the negative ion mode using 50% MeCN–10 mm ammonium acetate (adjusted to pH 7.0 by adding an aqueous ammonia solution) as the mobile phase. The glycine- and taurine-conjugated 3β-hydroxy-Δ5-bile acids (G-Δ5-3β,7α-ol, G-Δ5-3β,7α,12α-ol and T-Δ5-3β,7α-ol, T-Δ5-3β,7α,12α-ol), and the nonamidated 3β-hydroxy-Δ5-bile acid 3-sulfates (Δ5-3β,7α-ol-S and Δ5-3β,7α,12α-ol-S) gave a deprotonated ion [M−H]− as a base peak. The glycine-conjugates and the nonamidated sulfates gave a sole product ion at m/z 74 and m/z 97, respectively. For taurine-conjugates, product ions were observed at m/z 80, m/z 107 and m/z 124 and the most abundant product ion was at m/z 80. On the other hand, bile acids conjugated with amino acid and sulfuric acid gave characteristic fragment ions. Taurine and sulfuric acid-conjugated bile acids, T-Δ5-3β,7α,12α-ol-S and T-Δ5-3β,7α-ol-S, showed doubly charged ions [M−2H]2− at m/z 295.6 and m/z 287.6 as an intense peak, with a low abundance of deprotonated ions [M−H]− at m/z 592.2 and at m/z 576.2, respectively. Furthermore, the doubly charged ions [M−2H]2− of these bile acids gave product ions [M−H−H2SO4]− (at m/z 494.3 for T-Δ5-3β,7α,12α-ol-S and at m/z 478.3 for T-Δ5-3β,7α-ol-S) and [HSO4]− at m/z 97, whereas product ions [M−H−H2SO4]− showed the most intense peak. Glycine and sulfuric acid-conjugated bile acids (G-Δ5-3β,7α,12α-ol-S and G-Δ5-3β,7α-ol-S) gave the deprotonated ion [M−H]− at m/z 542.2 and m/z 526.2, respectively. These bile acids gave product ions [M−HSO3]− (m/z 462.3 for G-Δ5-3β,7α,12α-ol-S and m/z 446.3 for G-Δ5-3β,7α-ol-S) and [HSO4]− at m/z 97. These results are consistent with previously reported findings.18,21–25) Therefore, we selected these precursor ions and product ions as monitoring ions for SRM analysis and optimized the collision energy to obtain the highest signal-to-noise ratio (Table 1). Typical SRM chromatograms for authentic samples of conjugated 3β-hydroxy-Δ5-bile acids are shown in Fig. 2, indicating simultaneous separation and determination of all bile acids within 6 min. Calibration curves for all bile acids were linear over the range of 10–2000 pmol/mL, with linear correlation coefficients of greater than 0.999 for all bile acids. The deviations of calibration standards were less than 10% (n=5) for all points in the calibration range. The detection limit was less than 1.0 pmol/mL (S/N=5) for all bile acids using blank extracts of an incubation mixture with mock vesicles (25 µg of membrane protein) and was sufficient for application to the measurement of ATP-dependent transport activities of bile acids in membrane vesicles obtained from Sf9 cells. The LC/ESI-MS/MS parameters of the reference bile acids are summarized in Table 1. Prior to LC/ESI-MS/MS analysis, the bile acid transported into membrane vesicles that was retained on the glass fiber filter was eluted with ethanol. The bile acids were completely recovered from the first 1.4 mL of ethanol. After evaporation of the eluents, the residue was passed through an Oasis HLB 96-well plate cartridge to remove any impurities. The recovery rates of SPE using Oasis HLB 96-well plate cartridges described in Experimental were tested by adding known concentrations of conjugated 3β-hydroxy-Δ5-bile acids to blank extracts. The relative recoveries of each bile acid were obtained as 96.6 to 104.3% of the added amounts of their standard samples, and the coefficients of variation were less than 4.9%, by this clean-up procedure (Table 2). Figure 3 shows typical SRM chromatograms of incubation mixtures of hMRP3-expressing membrane vesicles with G-Δ5-3β,7α,12α-ol-S and G-Δ5-3β,7α-ol-S as substrates. All bile acids were clearly detected without endogenous contaminants.

| Bile acid | Relative recovery (%, mean±S.D.,a) n=5) | ||
|---|---|---|---|
| Concentration added 10 pmol/mL | Concentration added 100 pmol/mL | Concentration added 1000 pmol/mL | |
| G-Δ5-3β,7α-ol | 100.7±2.0 (2.0) | 100.9±3.1 (3.0) | 99.2±3.0 (3.1) |
| T-Δ5-3β,7α-ol | 100.4±3.2 (3.2) | 99.5±4.9 (4.9) | 98.4±4.3 (4.4) |
| Δ5-3β,7α-ol-S | 100.7±1.2 (1.2) | 101.9±1.0 (1.0) | 100.4±2.2 (2.4) |
| G-Δ5-3β,7α-ol-S | 104.3±3.4 (3.3) | 101.9±0.9 (0.9) | 100.0±2.5 (2.5) |
| T-Δ5-3β,7α-ol-S | 96.6±2.7 (2.8) | 99.7±1.3 (1.3) | 99.6±2.1 (2.1) |
| G-Δ5-3β,7α,12α-ol | 100.9±4.6 (4.6) | 99.8±1.8 (1.8) | 100.3±3.3 (3.3) |
| T-Δ5-3β,7α,12α-ol | 99.7±2.9 (2.9) | 102.7±3.2 (3.2) | 101.9±2.8 (2.8) |
| Δ5-3β,7α,12α-ol-S | 100.8±2.4 (2.4) | 101.6±1.8 (1.8) | 101.7±1.2 (1.2) |
| G-Δ5-3β,7α,12α-ol-S | 102.6±1.6 (1.5) | 101.9±0.9 (0.9) | 102.0±3.6 (3.5) |
| T-Δ5-3β,7α,12α-ol-S | 101.0±1.7 (1.7) | 103.9±1.8 (1.8) | 101.2±4.7 (4.6) |
a) Standard deviation. Values in parentheses represent the coefficient value.

The LC/ESI-MS/MS method was applied to measurements of the ATP-dependent transport activities of 3β-hydroxy-Δ5-bile acids by membrane vesicles obtained from hMRP2-, hMRP3-, and hBSEP-expressing Sf9 cells. It has been reported that G-CA and T-CA are typical substrates of hBSEP and that hMRP2 shows a high affinity for T-LCA-S.1,2) In contrast to hBSEP and hMRP2, hMRP3 has been shown to transport not only monovalent bile acids but also divalent bile acids.4–6) In the present study, glycine-, and taurine-conjugated 3β-hydroxy-Δ5-bile acids were used as substrates for hBSEP vesicles. 3-Sulfated 3β-hydroxy-Δ5-bile acids and their glycine- and taurine-conjugates were used as substrates for hMRP2 vesicles. For hMRP3 vesicles, both monovalent (glycine- and taurine-conjugates) and divalent 3β-hydroxy-Δ5-bile acids (3-sulfates, and 3-sulfated glycine and taurine conjugates) were used as substrates.
Measurements of the ATP-dependent transport activities of conjugated 3β-hydroxy-Δ5-bile acids by hBSEP vesicles and hMRP2 vesicles were carried out first. The ATP-dependent transport activities of T-CA and T-LCA-S by these membrane vesicles were also measured as positive control experiments using a previously reported method.18) The results are shown in Fig. 4. No significant ATP-dependent uptake of the tested 3β-hydroxy-Δ5-bile acids by hBSEP and hMRP2 vesicles was observed compared to the ATP-dependent uptake by mock vesicles. Moreover, time dependence of the ATP-dependent uptake of these bile acids was not observed (data not shown).

Human BSEP- or hMRP2-expressing membrane vesicles or mock vesicles (each 25 µg of protein) were incubated in a transport buffer containing bile acids (5 µm) for 1 min at 37°C in the presence of 4 mm ATP or AMP. ATP-dependent transport activity was calculated by subtracting the transport activity in the presence of AMP from that in the presence of ATP. Each value represents the mean±S.D. of 3 determinations.
The ATP-dependent transport activities of the various conjugated 3β-hydroxy-Δ5-bile acids by hMRP3 vesicles were also determined by the LC/ESI-MS/MS method. The ATP-dependent transport activities of G-CA and T-LCA-S by hMRP3 vesicles were determined as positive control experiments. The initial ATP-dependent uptake levels of the studied bile acids by hMRP3-expressing membrane vesicles and mock vesicles are shown in Fig. 5. We found that the initial ATP-dependent transport uptake levels of G-Δ5-3β,7α-ol (47.2±5.2 pmol/min/mg protein), T-Δ5-3β,7α,12α-ol (25.0±2.4 pmol/min/mg protein) and G-Δ5-3β,7α,12α-ol (36.5±1.7 pmol/min/mg protein) were comparable to that of G-CA (33.0±9.2 pmol/min/mg protein) and that the uptake of T-Δ5-3β,7α-ol (84.9±10.2 pmol/min/mg protein) was approximately 2-fold higher than that of G-CA. For trihydroxy 3β-hydroxy-Δ5-bile acid sulfates, Δ5-3β,7α,12α-ol-S, G-Δ5-3β,7α,12α-ol-S and T-Δ5-3β,7α,12α-ol-S, the ATP-dependent uptake activities were 91.9±8.3 pmol/min/mg protein, 97.6±6.0 pmol/min/mg protein and 66.1±4.4 pmol/min/mg protein, respectively. Furthermore, the ATP-dependent uptake levels of dihydroxy 3β-hydroxy-Δ5-bile acid sulfates, Δ5-3β,7α-ol-S (244.2±14.9 pmol/min/mg protein), G-Δ5-3β,7α-ol-S (278.4±14.0 pmol/min/mg protein) and T-Δ5-3β,7α-ol-S (203.5±7.1 pmol/min/mg protein), were higher that of T-LCA-S (142.7±18.4 pmol/min/mg protein). Although ATP-dependent uptake levels for trihydroxy 3β-hydroxy-Δ5-bile acid sulfates were lower than that for T-LCA-S, the hMRP3 vesicles showed ATP-dependent uptake for all sulfated 3β-hydroxy-Δ5-bile acids. Figure 6 shows the ATP-dependent uptake of Δ5-3β,7α-ol-S (A) and T-Δ5-3β,7α-ol (B), obtained by the subtraction of the uptake with AMP from that with ATP, by hMRP3 and mock vesicles for 5 min. The ATP-dependent uptake of bile acids by hMRP3 vesicles increased linearly up to 1 min. Time dependence of the ATP-dependent uptake of other conjugated 3β-hydroxy-Δ5-bile acids was also observed (data not shown).

Human MRP3-expressing membrane vesicles or mock vesicles (each 25 µg of protein) were incubated in a transport buffer containing bile acids (5 µm) for 1 min at 37°C in the presence of 4 mm ATP or AMP. ATP-dependent transport activity was calculated by subtracting the transport activity in the presence of AMP from that in the presence of ATP. Each value represents the mean±S.D. of 3 determinations.

Membrane vesicles (25 µg of protein) from hMRP3 and mock-expressing Sf9 cells were incubated in a transport buffer containing 5 µm of bile acid at 37°C in the presence of 4 mm ATP or AMP. Results are shown as ATP-dependent transport, calculated by subtracting the transport in the presence of AMP from that in the presence of ATP. Each point and vertical bar represents the mean±S.D. of 3 determinations.
Since ATP-dependent uptake of conjugated 3β-hydroxy-Δ5-bile acids by hMRP3 vesicles was observed, we performed a kinetic analysis study to further characterize the hMRP3-mediated transportation of the conjugated 3β-hydroxy-Δ5-bile acids. The concentration dependence of ATP-dependent transport activities of the conjugated 3β-hydroxy-Δ5-bile acids in hMRP3 vesicles was also determined by LC/ESI-MS/MS. The uptake was saturable for all tested bile acids (Fig. 7). The values of the kinetic parameters, Km and Vmax, for 3β-hydroxy-Δ5-bile acids were determined by nonlinear least regression analysis. The Km and Vmax (with S.E.) values are summarized in Table 3. To compare the transport rates for 3β-hydroxy-Δ5-bile acids by hMRP3 vesicles, the intrinsic clearance (Vmax/Km) values for each bile acid were also determined using the Km and Vmax values calculated by kinetic analysis (Table 3). The affinities and Vmax/Km values for 3-sulfated bile acids were higher than those for non-sulfated bile acids. The Km values of monovalent 3β-hydroxy-Δ5-bile acids except T-Δ5-3β,7α,12α-ol were comparable to the previously reported value of hMRP3-mediated transport of 14C-labeled G-CA (248±113 µm).5) The present study demonstrated that hMRP3 vesicles transported conjugated 3β-hydroxy-Δ5-bile acids except T-Δ5-3β,7α,12α-ol as efficiently as common bile acids, such as G-CA and T-LCA-S.
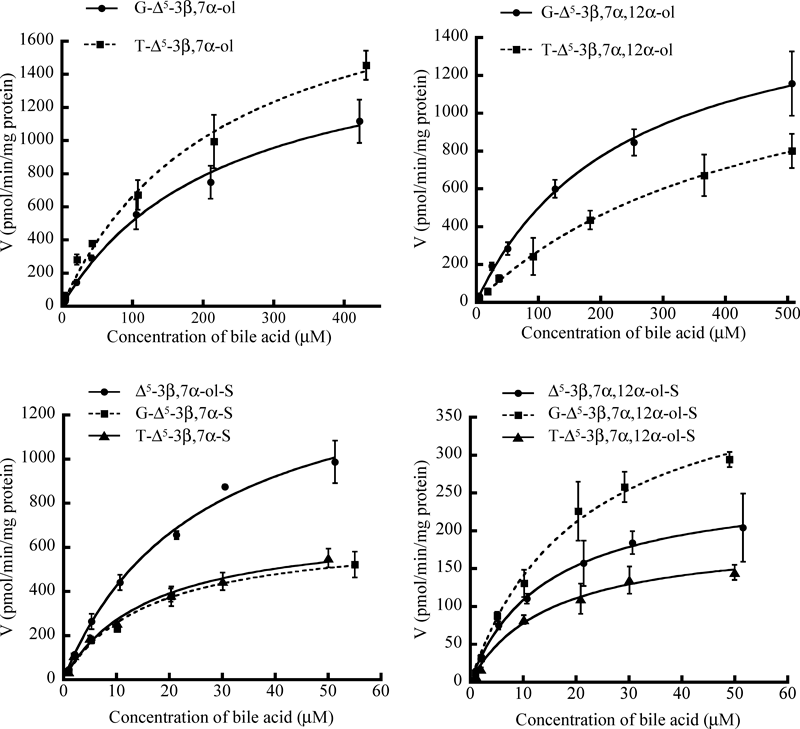
Membrane vesicles (25 µg of protein) from hMRP3-expressing Sf9 cells were incubated with various concentrations of bile acids with 4 mm ATP or AMP in a transport buffer for 1 min at 37°C. ATP-dependent transport activity was calculated by subtracting the transport activity in the presence of AMP from that in the presence of ATP. Each value represents the mean±S.D. of 4 determinations.
| Bile acid (substrate) | Km (µm) | Vmax (pmol/min/mg) | Vmax/Km (µL/min/mg) |
|---|---|---|---|
| G-Δ5-3β,7α-ol | 230±40 | 1685±144 | 7±1 |
| T-Δ5-3β,7α-ol | 229±51 | 2172±234 | 9±2 |
| Δ5-3β,7α-ol-S | 26±4 | 1512±102 | 58±10 |
| G-Δ5-3β,7α-ol-S | 16±3 | 669±50 | 42±8 |
| T-Δ5-3β,7α-ol-S | 16±3 | 706±47 | 44±9 |
| G-Δ5-3β,7α,12α-ol | 237±21 | 1681±70 | 7±1 |
| T-Δ5-3β,7α,12α-ol | 449±45 | 1491±170 | 3±1 |
| Δ5-3β,7α,12α-ol-S | 14±1 | 264±7 | 19±1 |
| G-Δ5-3β,7α,12α-ol-S | 21±3 | 430±29 | 20±3 |
| T-Δ5-3β,7α,12α-ol-S | 19±5 | 215±26 | 11±3 |
The Km and Vmax values for conjugated 3β-hydroxy-Δ5-bile acids were determined by nonlinear least-squares regression analysis using the data shown in Fig. 7. Each value represents the mean±S.E. of 4 determinations.
In this study, we used an LC/ESI-MS/MS method for measurements of transport activities of conjugated 3β-hydroxy-Δ5-bile acids in membrane vesicles obtained from hMRP2-, hMRP3-, and hBSEP-expressing Sf9 cells. This method is sufficiently sensitive for determination of conjugated 3β-hydroxy-Δ5-bile acids in membrane vesicles. The use of this LC/ESI-MS/MS method for measurements of bile acid transport activities may provide detailed information on the roles and functions of these ABC transporters.
Measurements of the ATP-dependent transport activities of conjugated 3β-hydroxy-Δ5-bile acids by membrane vesicles obtained from hMRP2-, hMRP3-, and hBSEP-expressing Sf9 cells were performed using the LC/ESI-MS/MS method. ATP-dependent uptake of conjugated 3β-hydroxy-Δ5-bile acids by hBSEP and hMRP2 vesicles was not observed (Fig. 4). These findings suggested that conjugated 3β-hydroxy-Δ5-bile acids were scarcely transported by hBSEP and hMRP2 vesicles. On the other hand, hMRP3 vesicles showed ATP-dependent uptake for all conjugated 3β-hydroxy-Δ5-bile acids (Fig. 5). A kinetic analysis study to further characterize the hMRP3-mediated transportation of the conjugated 3β-hydroxy-Δ5-bile acids was performed. The affinities and Vmax/Km values for 3-sulfated bile acids were higher than those for non-sulfated bile acids (Table 3). These results are consistent with results of previous studies indicating that divalent bile acids, such as T-LCA-S, are transported by hMRP3 with high affinity.4,5) The present study demonstrated that hMRP3 vesicles accepted conjugated 3β-hydroxy-Δ5-bile acids. hMRP3 may exhibit wider substrate specificity than that of hBSEP and hMRP2.
It has been reported that hMRP3 expression is highly induced in hepatocytes under cholestatic conditions.3,16,17) It is thought that hMRP3 may compensate for the impaired functions of hMRP2 and hBSEP and that hMRP3 functions as an efflux transporter from hepatocytes to circulating blood to switch the excretion route for organic anions, including bile acids, from bile to urine under pathologic conditions.3,16,17) Ichimiya et al. and Clayton investigated the bile acid profiles in urine of patients with HSD3B7 deficiency using FAB-MS, ESI-MS, and GC-MS methods and reported that sulfated 3β-hydroxy-Δ5-bile acids and their glycine conjugates were the major bile acids excreted in urine of the patients.10,15) The present study demonstrated that hMRP3 vesicles have high affinities for 3-sulfated 3β-hydroxy-Δ5-bile acids. hMRP3 may play a key role in the excretion of conjugated 3β-hydroxy-Δ5-bile acids.
It has been reported that hMRP4 is expressed at the basal membrane of the liver and is also involved in the efflux transport of bile acids, as well as hMRP3.3,26) It has also been shown that hMRP4 is highly up-regulated in the cholestatic human liver and that it plays a role in the removal of toxic organic anions from the liver under cholestatic conditions.3,26) hMRP4 may also be involved in the urinary excretion of 3β-hydroxy-Δ5-bile acids. Further studies on the transport properties of hMRP4 for 3β-hydroxy-Δ5-bile acids are in progress.