2013 年 61 巻 6 号 p. 662-665
2013 年 61 巻 6 号 p. 662-665
A new flavonoid, 2,″4″-O-diacetylquercitrin (1), along with six known flavonoids (2–7) were isolated from the aerial parts of Melastoma sanguineum. The structure of the new flavonoid was established by extensive spectroscopic studies and chemical evidence. The inhibitory effects of isolated compounds (1–7) on advanced glycation end-products (AGEs) formation and rat lens aldose reductase (RLAR) in vitro were examined. Of the tested compounds, compound 1 was the strongest inhibitor of AGEs, with an IC50 of 11.46±0.44 µm. In the RLAR assay, all tested compounds exhibited greater inhibitory effects on RLAR than that of a positive control, 3,3-tetramethyleneglutaric acid (IC50=28.8±1.5 µm); compound 1 exhibited the strongest RLAR-inhibitory activity, with an IC50 of 0.077±0.003 µm.
Hyperglycemia, the primary clinical manifestation of diabetes, is important in the pathogenesis of diabetic complications.1,2) Various hyperglycemia-induced metabolic and hemodynamic derangements, including increased advanced glycation end-product (AGE) formation, increases in the polyol pathway, activation of protein kinase C isomers, and increases in the hexosamine pathway, contribute to the characteristic histopathological changes observed in diabetic complications.3,4) Evidence suggests that the accumulation of AGE, heterogeneous molecules that are derived from non-enzymatic glycation between amino acid residues and oxidative derivatives of glucose or pentose, is irreversible, causing structural and functional changes in proteins such as collagen, elastin, and albumin, leading to the development of numerous complications associated with diabetes, such as neuropathy, nephropathy, angiopathy, and retinopathy.5) Aldose reductase (AR; alditol/nicotinamide adenine dinucleotide phosphate+ (NADP+) oxidoreductase, E.C. 1.1.1.21) is the key enzyme in the polyol pathway that catalyzes the reduced nicotinamide adenine dinucleotide (NADH)-dependent reduction of glucose to sorbitol. During hyperglycemic events, elevated glucose levels enhance AR activity by increasing the glucose flux through the polyol pathway, inducing functional and morphological changes associated with diabetic complications, such as cataracts, neuropathy, and nephropathy.6,7) Therefore, developing pharmacological inhibitors that inhibit AGE formation or AR might provide a therapeutic approach for delaying and preventing diabetic complications.8,9)
Owing to their structural and chemical diversity, natural compounds provide numerous therapeutic agents that prevent can diseases and treat various ailments. Attention has focused on finding specific pharmacologically significant compounds from natural products. Melastoma sanguineum Sims is a genus of shrubs and small trees within the family Melastomataceae that is native to tropical and sub-tropical regions of Southeast Asia. However, no studies have examined the chemical constituents and biological activity of this plant.
In searching for novel treatments for diabetic complications from natural resources, we found that the 80% EtOH extract of the aerial parts of M. sanguineum inhibited both AGE formation (IC50 = 3.19 µg/mL) and rat lens AR (RLAR, IC50=3.27 µg/mL) considerably. Further phytochemical study of this plant resulted in the isolation of a new flavonoid, 2″,4″-O-diacetylquercitrin (1), along with six known flavonoids (2–7). This report describes the isolation and structural elucidation of these flavonoids, as well as the characterization of their inhibitory effects on AGE formation and RLAR.
The 80% EtOH extract of the aerial parts of M. sanguineum was suspended in H2O and partitioned successively with n-hexane, EtOAc, and n-BuOH. The EtOAc- and n-BuOH soluble fractions, both of which significantly inhibited AGE formation and RLAR, were subjected to a series of chromatographic techniques guided by inhibitory activity of AGE formation and RLAR, leading to the isolation of a new flavonoid (1) along with six known flavonoids (2–7) (Fig. 1).

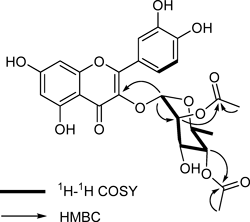
Compound 1 was obtained as a yellow amorphous powder with a negative specific rotation, [α]D25 −171.0° (c=0.1, MeOH). High resolution (HR)-electrospray ionization (ESI)-MS analysis of 1 yielded a molecular ion peak at m/z 531.1143 [M−H]−, in accordance with the molecular formula C25H24O13. A positive Mg/HCl test and the UV absorption maxima observed at 255 (band II) and 353 (band I) nm suggested the presence of a 3-O-substituted flavonol structure in 1.10) The IR spectrum exhibited absorption bands for a hydroxy group (3350 cm−1), α,β-unsaturated carbonyl group (1660 cm–1), and aromatic ring system (1610, 1502, 1450 cm−1). Acid hydrolysis of 1 yielded an aglycone and a monosaccharide unit. The 1H-NMR spectrum of 1 showed signals for a quercetin unit,11) a rhamnopyranosyl unit,12) and two acetyl groups (δH 2.07, 1.99, each 3H, s). The aglycone unit was characterized by two meta-coupled doublets at δH 6.37 (1H, d, J=2.0 Hz, H-8) and 6.20 (1H, d, J=2.0 Hz, H-6) and ABX aromatic system protons at δH 7.36 (1H, d, J=2.0 Hz, H-2′), 7.34 (1H, dd, J=8.0, 2.0 Hz, H-6′), and 6.92 (1H, d, J=8.0 Hz, H-5′). The signals at δH 5.40 (1H, d, J=1.5 Hz) and 0.94 (3H, d, J=6.0 Hz) were assigned to the anomeric proton and methyl group at C-6″, respectively, of a rhamnopyranosyl unit. The carbon signals at δC 103.3, 75.5, 73.6, 70.6, 69.8, and 17.8 of 1 further supported the presence of a rhamnopyranosyl unit, identified as l-rhamnose by comparison of its specific optical rotation, [α]D25 −7.2° (c=0.1, H2O), with that of authentic l-rhamnose, [α]D25 −8.8° (c=0.1, H2O). Moreover, the small coupling constant (J=1.5 Hz) of the anomeric proton indicated that the rhamnopyranosyl unit was linked in the α-configuration.12) The 13C-NMR spectrum, combined with the distortionless enhancement by polarization transfer (DEPT) data, showed that 1 contained 25 carbons, of which 15 carbon signals were assigned to a quercetin unit, six carbon signals to a rhamnopyranosyl unit, and four carbon signals (δC 172.9, 172.1, 21.2, 21.0) were assigned to two acetyl groups. These spectroscopic data were similar to those of quercitrin (6), except for the resonances of two acetyl groups, suggesting that 1 is a diacetylated form of 6. When the 13C-NMR spectrum of 1 was compared with that of 6, downfield shifts by about 1.6 and 2.3 ppm, respectively, were observed for C-2 and C-4 of the rhamnopyranosyl unit, suggesting that two acetyl groups are linked to these carbons, which was confirmed by the observed heteronuclear multiple bond connectivity (HMBC) cross-peaks of δH 4.22 (H-2″) and δC 172.1, and δH 5.02 (H-4″) and δC 172.9 (Fig. 2). Based on the above data, the structure of 1 was established as 2,″4″-O-diacetylquercitrin.
In addition, the six known compounds were identified as quercetin (2),11) astragalin (3),13) afzelin (4),14) isoquercitrin (5),15) quercitrin (6),14) and rutin (7)16) by comparing their physicochemical and spectral data to those in the literature. To our knowledge, this is the first report of the chemical constituents of M. sanguineum.
The inhibitory effects of the isolated compounds (1–7) on AGE formation and RLAR in vitro were examined using a described procedure,17) and the results are presented in Table 1. All of the tested compounds except compound 7 exhibited considerable inhibition of AGE formation, with IC50 values ranging from 11.46–89.91 µm, compared to that of aminoguanidine (AG, IC50=965.9±26.9 µm), the first AGE inhibitor for the treatment of diabetic nephropathy.18) Of the tested compounds, compound 1, with two acetyl groups in the rhamnopyranosyl unit, was the strongest inhibitor of AGEs, with an IC50 of 11.46±0.44 µm; its activity was approximately two times stronger than that of quercitrin (6, IC50=25.11±0.45 µm), which contains no acetyl moiety in the rhamnopyranosyl unit. In the RLAR assay, all tested compounds exhibited greater inhibitory effects on RLAR than that of a positive control, 3,3-tetramethyleneglutaric acid (TMG, IC50=28.8±1.5 µm); compound 1 exhibited the strongest RLAR-inhibitory activity, with an IC50 of 0.077±0.003 µm. This result is consistent with previous reports that compounds 2, 3, 5, and 6 were shown to exhibited potent AGE formation and AR inhibitory activity.17,19,20)
| Compounds | Inhibitory effect (IC50, µm)a) | |
|---|---|---|
| AGEs formationb) | RLAR | |
| 1 | 11.46±0.44 | 0.077±0.003 |
| 2 | 28.41±0.21 | 7.21±0.16 |
| 3 | 89.91±0.41 | 5.09±0.19 |
| 4 | 58.90±0.58 | 0.81±0.03 |
| 5 | 14.66±0.27 | 4.33±0.22 |
| 6 | 25.11±0.45 | 0.16±0.01 |
| 7 | NDc) | 5.39±0.86 |
| AGd) | 965.9±26.9 | — |
| TMGd) | — | 28.8±1.50 |
a) Results are expressed as means±S.D. (n=3) and IC50 indicates the concentration (µm) at which the inhibition percentage of the AGEs formation or RLAR was 50%, and the values were determined by regression analysis. b) After incubating for 14 d, the fluorescent reaction products were assayed on a spectrofluorometric detector. c) Not determined due to the low solubility of 7 in the reaction mixture. d) Aminoguanidine (AG) and 3,3-tetramethyleneglutaric acid (TMG) were used as positive controls.
Development and investigation of AGE and AR inhibitors, especially natural anti-AGE and AR agents with few side effects, might provide a potential therapeutic approach for delaying and preventing diabetes-related complications. Studies have been undertaken to discover natural compounds that inhibit AGE formation or AR and have found flavonoids to be potent AGE formation and AR inhibitors.21–23) Our results indicate that the flavonoid components isolated in this study contributed to the inhibitory effect of the aerial parts of M. sanguineum on AGE formation and RLAR. Furthermore, a new flavonol glycoside, 2″,4″-O-diacetylquercitrin (1), showed strong inhibitory activity against AGE formation and RLAR and represents a promising agent to prevent or treat diabetic complications and other related diseases.
Optical rotations were measured on a JASCO P-2000 digital polarimeter. IR spectra were recorded on a JASCO 100 IR spectrometer. All 1D (1H and 13C) and 2D (correlation spectroscopy (COSY), heteronuclear multiple quantum coherence (HMQC), and HMBC) NMR spectra were obtained using a Bruker Avance 500 NMR spectrometer with tetramethylsilane (TMS) as an internal standard. HR-ESI-MS were recorded on a Shimadzu LCMS-IT-TOF spectrometer. Column chromatography was performed using silica gel (70–230 mesh and 230–400 mesh, Merck), YMC-gel ODS-A (12 nm, S-75 µm, YMC), and Sephadex LH-20 (Amersham Pharmacia Biotech). Thin-layer chromatography (TLC) was performed on pre-coated silica gel 60 F254 (0.25 mm, Merck) and RP-18 F254s plates (0.25 mm, Merck). Spots were detected by UV light (254 nm) and spraying with 10% H2SO4 followed by heating.
Plant MaterialAerial parts of M. sanguineum were collected in Ha Nam province, Vietnam, in June 2007 and identified by Prof. J.-H. Kim of Gachon University, Republic of Korea. A voucher specimen (No. VN-072) was deposited in the Herbarium of the Diabetic Complications Research Team, Korea Institute of Oriental Medicine, Republic of Korea.
Extraction and IsolationThe air-dried aerial parts of M. sanguineum (3.8 kg) were extracted with 80% EtOH (3×20 L) at room temperature for seven days, filtered, and concentrated to yield an 80% EtOH extract (360 g). This extract (100 g) was suspended in H2O (4 L) and partitioned successively with n-hexane (3×4 L), EtOAc (3×4 L), and n-BuOH (3×4 L) to yield n-hexane-, EtOAc-, and n-BuOH-soluble fractions of 9.4, 12.0, and 36.2 g, respectively. The EtOAc-soluble fraction, which significantly inhibited AGE formation (IC50=7.43 µg/mL) and RLAR (IC50=2.39 µg/mL), was subjected to silica gel column chromatography (70–230 µm, 50×7.0 cm), and eluted with a gradient solvent system consisting of CHCl3–MeOH (100 : 1→0 : 1) to generate ten fractions (E1–E10). Fraction E9 (3.0 g) was applied to a silica gel column (70–230 µm, 60×5.0 cm), and eluted with a CHCl3–MeOH gradient solvent system (50 : 1→10 : 1), yielding five subfractions (E9.1–E9.5). Of these, Fraction E9.2 (1.0 g) was chromatographed further on a Sephadex LH-20 column (60×4.0 cm), and eluted using a MeOH–H2O gradient solvent system (30 : 70→80 : 20) to yield compounds 1 (20 mg), 2 (12 mg), and 3 (45 mg). Fraction E9.5 (0.3 g) was purified further over a Sephadex LH-20 column (60×2.5 cm), and eluted with MeOH–H2O (40 : 60) to yield compound 4 (50 mg). The n-BuOH-soluble fraction, which also significantly inhibited AGE formation (IC50=2.34 µg/mL) and RLAR (IC50=1.33 µg/mL), was applied to a silica gel column (70–230 µm, 50×9.0 cm), and eluted with a CHCl3–MeOH gradient solvent system (10 : 1→0 : 1) to generate 10 fractions (B1–B10). Of these, Fraction B2 (6.0 g) was chromatographed further in a Sephadex LH-20 column (60×5 cm) and eluted using a MeOH–H2O gradient solvent system (50 : 50→100 : 0) to generate seven subfractions (B2.1–B2.7). Then, Fractions B2.3 and B2.6 (150, 100 mg, respectively) were chromatographed separately in a Sephadex LH-20 column (60×2.5 cm). Eluting B2.3 with a MeOH–H2O gradient solvent system (30 : 70→50 : 50) yielded compounds 5 (8 mg) and 6 (13 mg) and eluting B2.6 with MeOH–H2O (30 : 70) yielded compound 7 (66 mg).
2″,4″-O-Diacetylquercitrin (1): Yellow amorphous powder. [α]D25 −171.0° (c=0.1, MeOH). UV (MeOH) λmax nm (log ε): 255 (4.10), 353 (4.35). IR (KBr) νmax cm−1: 3350, 1730, 1720, 1660, 1610, 1502, 1450, 1380, 1140. HR-ESI-MS m/z: 531.1143 [M−H]− (Calcd for C25H23O13−: 531.1144). 1H-NMR (500 MHz, MeOD): δH 7.36 (1H, d, J=2.0 Hz, H-2′), 7.34 (1H, dd, J=8.0, 2.0 Hz, H-6′), 6.92 (1H, d, J=8.0 Hz, H-5′), 6.37 (1H, d, J=2.0 Hz, H-8), 6.20 (1H, d, J=2.0 Hz, H-6), 5.40 (1H, d, J=1.5 Hz, H-1″), 5.02 (1H, t, J=9.6 Hz, H-4″), 4.38 (1H, dd, J=9.6, 6.0 Hz, H-5″), 4.22 (1H, dd, J=3.3, 1.5 Hz, H-2″), 3.90 (1H, dd, J=9.6, 3.3 Hz, H-3″), 2.07 and 1.99 (each 3H, s, CH3CO×2), 0.94 (3H, d, J=6.0 Hz, H-6″). 13C-NMR (125 MHz, MeOD): δC 179.6 (C-4), 172.9 and 172.1 (CH3CO×2), 166.0 (C-7), 163.3 (C-5), 159.4 (C-2), 158.7 (C-9), 150.0 (C-4′), 146.6 (C-3′), 136.3 (C-3), 123.1 (C-6′), 122.9 (C-1′), 116.9 (C-2′), 116.6 (C-5′), 106.0 (C-10), 103.3 (C-1″), 99.9 (C-6), 94.8 (C-8), 75.5 (C-4″), 73.6 (C-2″), 70.6 (C-3″), 69.8 (C-5″), 21.2 and 21.0 (CH3CO×2), 17.8 (C-6″).
Acid Hydrolysis of 1Compound 1 (4 mg) in 10% HCl–dioxane (1 : 1, 5 mL) was heated at 80°C for 3 h in a water bath. After cooling, the mixture was extracted with EtOAc (3×5 mL). The aqueous layer was neutralized with Na2CO3 and then concentrated to dryness and subjected to silica gel chromatography eluting with CHCl3–MeOH–H2O (7 : 3 : 0.1) to give l-rhamnose. l-Rhamnose was identified by comparison of specific optical rotation, [α]D25 −7.2° (c=0.1, H2O), and TLC analysis.
AGE Formation Inhibitory AssayAccording to a well-established method, the reaction mixture [bovine serum albumin (10 mg/mL, Sigma, St. Louis, MO, U.S.A.; 700 µL) in 50 mm phosphate buffer (pH 7.4) with 0.02% sodium azide) was added to 0.2 m fructose and glucose (100 µL). In screw cap tubes (1.5 mL), the reaction mixture was then mixed with 200 µL of serial diluted compounds or aminoguanidine (Sigma). After incubating at 37°C for 7 d, the fluorescent reaction products (200 µL) were transferred to 96-well plates and assayed on a spectrofluorometric detector (Bio-Tek, Synergy HT, U.S.A.; excitation wavelength, 350 nm; emission wavelength, 450 nm). AGEs assay was performed in triplicate. The concentration of each test sample giving 50% inhibition of the activities (IC50) was estimated from the least-squares regression line of the logarithmic concentration plotted against the remaining activity.
RLAR Inhibitory AssayAll animal procedures were approved by the local ethics committee for animal experiments (Approval date and number; February 17, 2011 and 11-018, respectively). Rat lenses were removed from the eyes of 8 weeks old Sprague-Dawley rats (Dae-Han Bio Link Co., Umsung, Korea) weighing 100–150 g and homogenized in 12 volumes of a 135 mm Na, K-phosphate buffer (pH 7.0) containing 0.5 mm phenylmethylsulfonyl fluoride and 10 mm 2-mercaptoethanol. The homogenate was centrifuged at 100000×g for 30 min, and the supernatant fluid was used as the RLAR. The incubation mixture contained 135 mm Na,K-phosphate buffer (pH 7.0), 100 mm Lithium sulfate, 0.03 mm NADPH, 1 mm dl-glyceraldehyde as a substrate, and 50 µL of enzyme fraction, with or without 25 µL of sample solution, in a total volume of 1.0 mL. The reaction was initiated by the addition of NADPH at 37°C and stopped by the addition of 0.3 mL of 0.5 m HCl. Then, 1 mL of 6 m NaOH containing 10 mm imidazole was added, and the solution was heated at 60°C for 10 min to convert NADP to a fluorescent product. Fluorescence was measured using a spectrofluorometric detector (Shimadzu RF-5301PC, Japan, Ex: 360, Em: 460 nm). The concentration of each test sample giving 50% inhibition of the activities (IC50) was estimated from the least-squares regression line of the logarithmic concentration plotted against the remaining activity. 3,3-Tetramethyleneglutaric acid (Aldrich) was used as a positive control.
This research was supported by a Grant [K11040, K12040] from the Korea Institute of Oriental Medicine (KIOM). The NMR and MS experiments were performed by the Korea Basic Science Institute (KBSI).