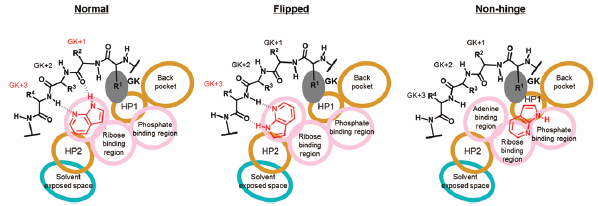
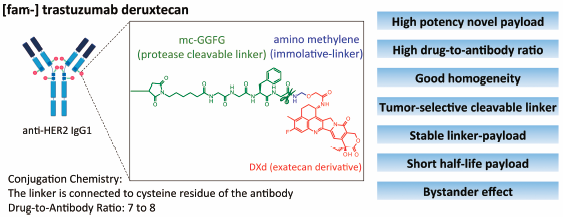
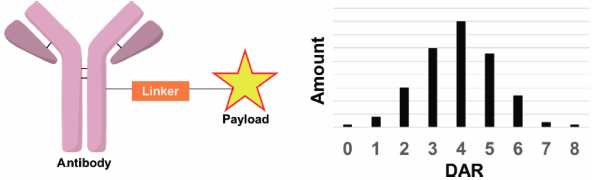
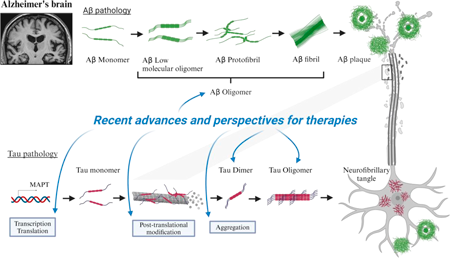
Chemical and Pharmaceutical Bulletin
日本薬学会は,1880年に創立された,我が国では歴史ある学会の一つです.現在,約15,000人の会員を擁しており,毎月3誌の学術誌を刊行しております.英文による学術誌の一つとして「Chemical and Pharmaceutical
Bulletin」(Chem. Pharm. Bull.)は, 1953年にPharmaceutical Bulletinとして創刊され,その後Chem. Pharm. Bull. と名称を変え,薬学と健康科学に関する化学分野をカバーしています.二つ目として,「Biological and
Pharmaceutical Bulletin」(Biol. Pharm. Bull.) があり,これは1978年に創刊されたJournal Pharmacobio-Dynamicsを起源としており,更に1953年に創刊され,2012年に内容を引き継いだJournal of Health Scienceの後継誌として,薬学と健康科学に関する生物学分野を領域としています.英文と和文両方にて構成される学術誌として,「YAKUGAKU ZASSHI」(薬学雑誌)があり,本学術誌は学会創立の翌年(1881年)に創刊され,最も長い歴史を有しています.薬学雑誌では,和文による原著論文・総説等のほか,臨床薬学領域研究については英文による投稿も受け付けています. 日本薬学会におけるこれら学術誌のスコープは,基礎研究から臨床研究に至る幅広い分野に渡りますが,いずれも薬学・健康科学をベースとしています。3誌に投稿された論文の平均審査期間は,現在,投稿された方へ最初の判定を通知するまでに約1か月ですが,更なる時間短縮を目指しています.3誌ともにJ-STAGEにて無料公開しており,研究成果を世に広める一助となることを期待しております.皆様の研究成果をChem. Pharm. Bull.やBiol. Pharm. Bull.,薬学雑誌へ積極的にご投稿下さいますよう,よろしくお願い申し上げます.
学術誌編集委員長
中川 秀彦
名古屋市立大学大学院薬学研究科
もっと読む
学術誌編集委員長
中川 秀彦
名古屋市立大学大学院薬学研究科
公益社団法人 日本薬学会
が発行
収録数 28,507本
(更新日 2026/02/04)
(更新日 2026/02/04)
Online ISSN : 1347-5223
Print ISSN : 0009-2363
ISSN-L : 0009-2363
Print ISSN : 0009-2363
ISSN-L : 0009-2363
1.3
2024 Journal Impact Factor (JIF)
2024 Journal Impact Factor (JIF)
ジャーナル
査読
オープンアクセス
HTML
Scopus Pubmed J-STAGE Data
Scopus Pubmed J-STAGE Data