2014 年 62 巻 1 号 p. 72-76
2014 年 62 巻 1 号 p. 72-76
Laboratory tests of the decomposition of corticosteroids during activated sludge processing were investigated. Corticosteroid standards were added to activated sludge, and aliquots were regularly taken for analysis. The corticosteroids were extracted from the samples using a solid-phase extraction method and analyzed LC-MS. Ten types of corticosteroids were measured and roughly classified into three groups: 1) prednisolone, triamcinolone, betamethasone, prednisolone acetate, and hydrocortisone acetate, which decomposed within 4 h; 2) flunisolide, betamethasone valerate, and budesonide of which more than 50% remained after 4 h, but almost all of which decomposed within 24 h; and 3) triamcinolone acetonide, and fluocinolone acetonide of which more than 50% remained after 24 h. The decomposed ratio was correlated with each corticosteroid’s Log P, especially groups 2) and 3).
A number of studies aimed at detecting medical drugs in aquatic environment including sewage plant effluent, river water and tap water, have been reported in recent years.1–3) The drugs found in these water have been assumed to have originally entered the aquatic environment through human excretion, domestic wastewater (e.g., from bathing), discarded unused drugs, and from agricultural use. Although the concentrations that have been reported varied depending on the study period, the season, and the location, the concentrations have generally, with very few exceptions, been extremely low in the nanogram per liter (ppt) range. Hermaphroditic fish were frequently found in a river that received sewage plant effluent in England in the 1980s. The cause of which was found to be natural and/or artificial female hormones from human excretion that could not be decomposed in the sewage plant. Artificial female hormones have stronger effects than the natural hormones, so nanogram per liter concentrations of the artificial female hormones triggered the feminization of fish.4) These reports show that even nanogram per liter concentrations of these substances in the aquatic environment may have chronic adverse effects. Artificial female hormones are used medically to cause physiological activation, so even trace amounts might have unexpected effects including endocrine disruption. There have been a number of studies in Europe and the United States, in which analytical methods for these substances have been developed and the concentrations of the substances in the environment have been measured.5–18) To deal with the problem of these substances being present in the aquatic environment, environmental impact assessments are required under European Union directives (2001/83/EU) and United States federal regulations (21 CFR part 25), in EU and U.S.19,20) However, there are no regulations to control the environmental effects of medical drugs in Japan. The Japanese Ministry of Health, Labour and Welfare has started to develop laws for the environmental impact assessment of medical drugs to ensure the protection of the aquatic environment. About 30000 different medical drugs are thought to be available in Japan,21) but the environmental concentrations and dynamics of only a few these drugs have been studied.22–24)
We have previously found corticosteroids (CSs) at several nanograms per liter CSs in river water and samples taken from a sewage treatment plant.25,26) CSs are found in preparations such as analgesics, antiphlogistics, antipruritics, and astringents, which make up about 4.9% of the gross monetary value of medical drugs sold in Japan.21) Many of these products are used as ointments or creams, so it can be assumed that large proportions of the products are washed off without being metabolized or decomposed.
Sewage treatment plants typically use activated sludge treatments, using air and a biological floc that is composed of bacteria and protozoa. The treated wastewater is then disinfected with chlorine before being discharged to the aquatic environment (which could be a river, lake, swamp, or sea). In the biological treatment process the organic matter in the sewage water (both dissolved and dispersed particulates) is used as a nutrient for the cultured bacteria and protozoa, the organic matter being oxidized and decomposed to form water, bicarbonate, and other small molecules. This form of treatment is a major process in many facilities because a high degree of sewage purification can be achieved at relatively low cost. However, it has been reported that the process degrades different organic substrates with different efficiencies. For instance, fenoprofen and ibuprofen are relatively easily decomposed during activated sludge treatment, but carbamazepine and clofibric acid are not degraded during the treatment.27)
Following our previous research,22,23) here we present the results of an investigation into the degradation of CSs during activated sludge treatment to assess the different degrees of decomposition of different CSs during treatment. There has been one published study of the decomposition of CSs during wastewater treatment using a membrane bioreactor.28) In this study, however, we used activated sludge taken from a working sewage treatment plant.
The target compounds and their physiochemical properties (at >98% purity) are listed in Table 1, and their structures are shown in Fig. 1. Prednisolone (P), triamcinolone (T), betamethasone (B), prednisolone acetate (Pa), hydrocortisone acetate (Ha), betamethasone valerate (Bv), and triamcinolone acetonide (Tc) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Budesonide (Bu) was obtained from Sigma-Aldrich Japan (Tokyo, Japan). Flunisolide (F) and fluocinolone acetonide (Fc) were obtained from ICN Biomedicals (Irvine, CA, U.S.A.). P derivatives with the various substituent groups at different positions were chosen for this study to investigate whether the decomposition rate would be varied by activated sludge treatment.
| Compounds | MW | Log P (Octanol–Water) |
|---|---|---|
| P | 360.5 | 1.62 |
| T | 394.4 | 1.16 |
| B | 392.5 | 1.94 |
| Pa | 402.5 | 2.40 |
| Ha | 404.5 | 2.19 |
| F | 434.5 | 2.66 |
| Bv | 476.6 | 3.60 |
| Bu | 430.5 | 2.18 |
| Tc | 434.5 | 2.53 |
| Fc | 452.5 | 2.48 |

Super grade acetic acid, methanol, and sodium acetate, and HPLC and LC-MS grade acetonitrile were purchased from Wako Pure Chemical Industries, Ltd. Whatman glass microfiber filters (GF/C, 47 mm diameter; Sigma-Aldrich Japan), and Sep-Pak® C18, 100 mg (Waters, Massachusetts, U.S.A.) were used for sample treatments. Purified water (18 MΩ/cm) was produced using an SA-2000E system (EYELA, Tokyo, Japan) and a Milli-Q Direct system (Millipore, Billerica, MA, U.S.A.).
Standard SolutionsA stock standard solution (1000 µg/mL) of each target compound was prepared in methanol and stored in a dark bottle at 4°C until use. Working standard solutions at concentrations of 10–1000 µg/L were prepared by diluting the individual stock solutions with methanol just before use. Individual stock solutions were diluted ten-fold with methanol just before they were used in decomposition experiments.
Activated SludgeActivated sludge was obtained from the aeration tank of the Takase sewage treatment plant (Funabashi, Japan). The mixed liquor suspended solids content was 2.5 g/L and the pH was 6.8–7.5.
Decomposition ExperimentsSteroids were added to 500 mL of activated sludge from the aeration tank to reach a final concentration of 100 µg/L and the mixture was aerated the whole time. The decomposition experiments temperature was 19–21°C. A 20 mL aliquot of the mixture was taken immediately after the steroids were added and further 20 mL aliquots were collected periodically.
Pretreatment ConditionsEach sample was passed through a GF/C glass microfiber filter. The filtrate was analyzed as the liquid phase of the sample. Then the filter was washed with 5 mL of pure water. The filter was then sonicated with 5 mL of methanol for 5 min twice. The methanol (total of 10 mL) was analyzed as the sludge phase of the sample. A Sep-Pak® C18 cartridge was conditioned with 2 mL of pure water and 2 mL of methanol. The liquid phase and sludge samples were loaded onto the cartridge, respectively using a vacuum manifold (GL Science, Tokyo, Japan). The cartridge was washed with 5 mL of pure water and dried by allowing air to be sucked through the cartridge for 30 min. The CSs from the cartridge were eluted with 1 mL of methanol.
Instrumentation and Measurement ConditionsAnalyses were performed using an Agilent 1100 Series (Agilent Technologies, Santa Clara, CA, U.S.A.) under the conditions shown in Table 2. The [M+CH3COO]− ion was detected at high sensitivity in negative ion mode using 5 mM acetic acid(aq) as the mobile phase. The compounds were separated using a Develosil C30-UG-3 analytical column (2.0×150 mm, Nomura Chemical Co., Aichi, Japan) using a gradient mobile phase with acetonitrile (solvent A) and 5 mM acetic acid(aq) (Solvent B). Betamethasone and dexamethasone were poorly separated but the other steroids were adequately separated. A calibration curve (with a concentration range of 10–1000 µg/L) was prepared for each CS measurement cycle.
| LC conditions | MS conditions | |||
|---|---|---|---|---|
| Column | Develosil C30-UG-3 (2.0×150 mm) | Ionization | ESI negative | |
| Mobile phase | Solvent A: CH3CN | Nebulizer | N2 (40 psig) | |
| Solvent B: 5 mM CH3COOH | Drying gas | N2 (10 L/min, 350°C) | ||
| Gradient program | Time (min) | 0→10→15 | V-Cap | 3000 V |
| A (%) | 40→90→90 | Fragmentor | 125 V | |
| Flow rate of eluent | 0.2 mL/min | |||
| Column temp. | 40°C | |||
| Injection volume | 5 µL | |||
We investigated the decomposition of CSs in laboratory scale experiments using activated sludge taken from an aerobic treatment system in a sewage treatment plant. The CSs were analyzed by LC-MS (n=3). The detection limits (DLs) were calculated that the minimum injected mass gives an S/N=3. DLs ranged from 1.6 to 9.4 pg for each target compound injected. The relative standard deviation of determination for each compound was 6.0–16.1%. Samples were taken at different times from the treatment apparatus. The zero hour’s residual CS concentrations were expressed as 100%. The degradation profiles are shown in Fig. 2. The CSs could be divided into three groups from their degradation profiles. Group 1 included five CSs (P, T, B, Pa and Ha) which decomposed within 4 h of digestion (to below the limit of detection). Group 2 included three CSs (F, Bv, and Bu [1 and 2]) which 50% remained more than after 4 h of digestion. They were almost completely decomposed after 24 h. Group 3 included Tc and Fc. About 50% of them remained after 24 h of digestion. Two Bu peaks appeared in the chromatograms because Bu is a mixture of two epimeric forms. They showed different decomposition behavior during the digestion. All of the CSs in groups 2 and 3 have an esterified hydroxyl functional group on C17. Many medical drugs are made to become more active once inside the body. Since the CSs are usually used in external applications, the drug is designed to be stable to remain unchanged at the application site to achieve the desired effect. The stabilization might lead to cause the CSs difficult to degrade biologically, activated sludge.

Group 1: included five CSs (P, T, B, Pa and Ha), which decomposed within 4 h of digestion (to below the limit of detection). Group 2: included three CSs (F, Bv, and Bu [1 and 2]), of which more than 50% remained after 4 h of digestion, and which were almost completely decomposed after 24 h. Group 3: included Tc and Fc of which about 50% remained after 24 h of digestion.
The fluoride substitution sites in F and Tc are different (C6 for F and C9 for Tc), and F was almost fully degraded after 24 h of digestion whereas Tc was less than 50% degraded after 24 h. Replacing the hydrogen at C9 with a halogen, as in Tc, causes synthetic steroids to be comparatively hard to biodegrade because they become more structurally stable.29) This is likely to be the reason why Tc was harder to decompose than F in our experiments. This could also explain why the decomposition behaviors of Tc and Fc were similar (Fc also have a fluorine atom at C9). The lowest decomposition rate was found for Tc which has a cyclic configuration at C16 and C17, and a fluorine atom at C9.
The relationships between the physicochemical properties and decomposition rate of each CSs were investigated. The relationship between Log P and the residual ratio after 2 h of digestion are shown for each CS in Fig. 3. We found that the CSs could be roughly divided into two groups from this data. The CSs in one group were decomposed within 4 h of digestion (triangles), and the other CSs took more than 4 h of digestion to be decomposed (open circles). The open circle group showed the correlation between Log P and residual ratio (r=0.990). Higher lipophilicity appeared to be related to a higher decomposition rate. It has been noted that decomposition by activated sludge mainly occurs in the solid (sludge) phase,25) so substances that readily associate with the solid phase (i.e., the more lipophilic CSs) will be decomposed more readily. Figure 3 also implies that the decomposition processes were complex reaction system, because of the various decomposition patterns. Although the CSs near Log P of 2, the diversified residual ratio were observed. Since the activated sludge was sonicated with methanol, the microbes of cells in the activated sludge were broken and CSs in the activated sludge were thoroughly extracted. Although the decomposition process were not elucidated, these data imply CSs group with the triangle in Fig. 3 were rapidly absorbed and decomposed. On the other hand, the CSs belongs to the open circle group in Fig. 3 were assumed to be either absorbed slowly or hardly metabolized.
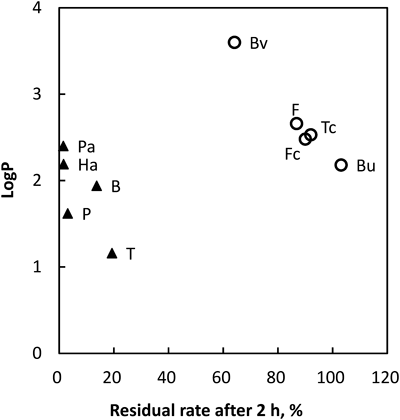
▲: Decomposed within 4 h. ○: Decomposed more than 4 h.
The decomposition rates using activated sludge can be predicted from the Log P values of medical drugs. The decomposition rates were found to depend on the positions of substituents in the molecules and to differ for different geometrical isomers. Although we only studied a limited kinds of CSs, our results show that some of the CSs were more stable than the others. Some of them were not able to be fully decomposed in 24 h of digestion by activated sludge. Since activated sludge is a complex microbial system, the organisms responsible for decomposing the CSs could not be identified in this study.
Medicinal substances are essential for treating illnesses preventing disease, and improving the quality of life for humans. It is difficult to regulate the release of medicinal drugs to the environment because of the variety of ways in which they are used and the side range of ways in which they may be released such as in human excreta, domestic wastewater (e.g., from bathing), and discarding unused drugs. However, it is essential to develop procedures to prevent or control the discharge of these substances into the environment to ensure that they do not adversely affect the aquatic environment.
We thank Funabashi City Takase treatment plant for providing samples.