2014 年 62 巻 11 号 p. 1136-1140
2014 年 62 巻 11 号 p. 1136-1140
A bioassay-guided fractionation and chemical investigation of the MeOH extract from the twigs of Lindera glauca (SIEB. et ZUCC.) BLUME resulted in the isolation and identification of six lignans (1–6) including three new lignan derivatives, named linderuca A (1), B (2), and C (3). The structures of the new compounds (1–3) were determined on the basis of spectroscopic analyses, including two dimensional NMR and circular dichroism (CD) spectroscopy studies. The cytotoxic activities of the isolates (1–6) were evaluated by determining their inhibitory effects on human tumor cell lines. Compounds 1–5 showed antiproliferative activities against A549, SK-OV-3, SK-MEL-2, and HCT-15 cell lines with IC50 values of 7.79–29.42 µM. Based on the understanding that inflammation is a crucial cause of tumor progression, we also investigated the anti-inflammatory activities of the isolates (1–6) in the lipopolysaccharide-stimulated murine microglia BV-2 cell line by measuring nitric oxide (NO) levels. The new lignans (1–3) significantly inhibited NO production with IC50 values of 12.10, 9.48, and 9.87 µM, respectively, without cytotoxicity.
Lindera glauca (SIEB. et ZUCC.) BLUME is a deciduous shrub in the Lauraceae family that is widely distributed in the mountainous areas of Korea, Japan, and China.1) L. glauca has been used to treat various diseases as a Korean traditional medicine since ancient times.2) L. glauca fruit has been used to treat symptoms of paralysis including abdominal pain and speech disorders. Its roots have been traditionally applied as a remedy for extravasation, contusion, and pain due to rheumatoid arthritis. Additionally, its leaves have been used as a folk medicine to counteract the effect of poison and to arrest bleeding. Importantly, this tree is well-known as a Korean traditional medicine to treat cancers such as stomach, lung, and uterine cancers without any side effects.2) In this context, our preliminary studies confirmed that the MeOH extract of L. glauca twigs had excellent cytotoxic activity against A549, SK-OV-3, SK-MEL-2, and HCT-15 cells in a sulforhodamine B (SRB) bioassay. Interestingly, the nitrogen-containing compounds and monoterpenes from L. glauca showed anti-tumor metastatic activities for tumor-inhibiting drugs in a recent research.3) However, only few constituents associated with the anti-tumor activity from L. glauca have been reported. Previous phytochemical investigations of the aerial parts of L. glauca revealed the presence of diverse chemical components including sterols, alkaloids, butanolides, sesquiterpenoids, diarylpropanoids, flavonoids, and phenolic compounds.4–10) These results prompted our research on structurally interesting constituents from L. glauca with antitumor activity. Using the bioactivity-guided isolation techniques, 6 lignans including three new lignan derivatives (1–3) were isolated from the most active CHCl3-soluble fraction. In the present study, we report the isolation and structural elucidation of compounds 1–6 (Fig. 1) and their antitumor and anti-inflammatory activities.

Linderuca A (1) was isolated as a light yellowish gum with positive optical rotation ([α]D25+37.2). The molecular formula of 1 was determined to be C33H38O11 by high-resolution (HR)-electrospray ionization (ESI) MS data at m/z 633.2314 [M+Na]+ (Calcd for C33H38NaO11, 633.2312). The IR spectrum showed the presence of hydroxyl (3422 cm−1), carbonyl (1711 cm−1), and aromatic groups (1604 and 1515 cm−1). The 1H-NMR spectrum of 1 (Table 1) showed signals for a trans-substituted double bond at δH 7.38 (1H, d, J=16.0 Hz) and 6.23 (1H, d, J=16.0 Hz). In addition, signals at δH 7.09 (1H, d, J=2.0 Hz), 6.99 (1H, dd, J=8.5, 2.0 Hz), and 6.80 (1H, d, J=8.5 Hz) were observed in the 1H-NMR spectrum of 1, which was indicative of a typical 1,3,4-trisubstitued aromatic ring. The 1H–1H correlation spectroscopy (COSY) and heteronuclear multiple bond correlation (HMBC) analysis of these peaks suggested the existence of a trans-feruloyl moiety in this molecule (Fig. 2). Furthermore, the 1H-NMR data of 1 showed signals for two 1,3,4,5-tetrasubstituted aromatic rings at δH 6.66 (2H, s, H-2′, 6′) and 6.58 (2H, s, H-2, 6), respectively. Overall, inspection of the 1H- and 13C-NMR data of 1 revealed that the data were very similar to those of (+)-9′-O-(E)-feruloyl-5,5′-dimethoxylariciresinol (4),11) except for the presence of an additional methoxy group (δH 3.78; δC 60.4) in 1. HMBC correlation of the methoxy group (δH 3.78) with C-4 (δC 136.9) indicated that the methoxy group was linked to C-4 in 1 (Fig. 2). This partial structure, a 3,4,5-trimethoxyphenyl group, was also supported by the comparison with the corresponding 13C-NMR chemical shifts of (+)-yangambin.12) Finally, the location of the trans-feruloyl group on the 9′-hydroxy group in 1 was confirmed by the HMBC cross-peak of H-9′ (δH 4.52 and 4.32)/C-9″ (δC 167.7).
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 1 | 136.8 | 136.8 | 136.8 | |||
| 2 | 6.58 s | 106.0 | 6.58 s | 106.0 | 6.58 s | 106.0 |
| 3 | 153.3 | 153.3 | 153.3 | |||
| 4 | 136.9 | 136.8 | 136.9 | |||
| 5 | 153.3 | 153.3 | 153.3 | |||
| 6 | 6.58 s | 106.0 | 6.58 s | 106.0 | 6.58 s | 106.0 |
| 7a7b | 2.91 dd (13.5, 5.0)2.58 dd (13.5, 11.0) | 33.9 | 2.91 dd (13.5, 5.0)2.57 dd (13.5, 11.0) | 33.7 | 2.92 dd (13.5, 5.0)2.57 dd (13.5, 11.0) | 33.8 |
| 8 | 2.85 m | 42.7 | 2.84 m | 42.7 | 2.87 m | 42.8 |
| 9a9b | 4.10 dd (9.0, 7.0)3.80 dd (9.0, 6.5) | 72.8 | 4.10 dd (9.0, 7.0)3.80 dd (9.0, 6.5) | 72.6 | 4.12 dd (8.0, 7.0)3.78 dd (8.0, 6.5) | 73.0 |
| 3,5-OCH3 | 3.81 s | 55.5 | 3.81 s | 55.5 | 3.81 s | 55.6 |
| 4-OCH3 | 3.78 s | 60.4 | 3.78 s | 60.4 | 3.78 s | 60.4 |
| 1′ | 134.6 | 134.6 | 135.3 | |||
| 2′ | 6.66 s | 103.5 | 6.66 s | 103.5 | 6.90 d (2.0) | 110.3 |
| 3′ | 148.1 | 148.1 | 149.2 | |||
| 4′ | 135.1 | 135.1 | 147.3 | |||
| 5′ | 148.1 | 148.1 | 6.76 d (8.0) | 116.2 | ||
| 6′ | 6.66 s | 103.5 | 6.66 s | 103.5 | 6.81 dd (8.0, 2.0) | 120.4 |
| 7′ | 4.82 d (7.0) | 84.0 | 4.82 d (7.0) | 84.1 | 4.80 d (7.0) | 84.1 |
| 8′ | 2.64 m | 49.4 | 2.64 m | 49.4 | 2.66 m | 49.5 |
| 9′a9′b | 4.52 dd (11.5, 7.0)4.32 dd (11.5, 7.5) | 62.7 | 4.52 dd (11.5, 7.0)4.31 dd (11.5, 7.5) | 62.7 | 4.50 dd (12.0, 6.0)4.29 dd (12.0, 8.0) | 62.8 |
| 3′,5′-OCH3 | 3.81 s | 55.6 | 3.81 s | 55.6 | 3.81 s | 55.6 |
| 1″ | 126.2 | 127.0 | 126.3 | |||
| 2″ | 7.09 d (2.0) | 110.6 | 7.64 d (1.5) | 111.3 | 7.09 d (2.0) | 110.6 |
| 3″ | 147.7 | 147.7 | 147.7 | |||
| 4″ | 148.1 | 148.5 | 148.1 | |||
| 5″ | 6.80 d (8.5) | 115.3 | 6.78 d (8.5) | 114.0 | 6.79 d (8.5) | 115.3 |
| 6″ | 6.99 dd (8.5, 2.0) | 122.9 | 7.11 dd (8.5, 1.5) | 125.1 | 6.99 dd (8.5, 2.0) | 122.9 |
| 7″ | 7.38 d (16.0) | 145.8 | 6.83 d (13.0) | 144.1 | 7.38 d (16.0) | 145.8 |
| 8″ | 6.23 d (16.0) | 113.9 | 5.71 d (13.0) | 114.5 | 6.23 d (16.0) | 114.0 |
| 9″ | 167.7 | 167.6 | 167.6 | |||
| 3″-OCH3 | 3.89 s | 55.6 | 3.89 s | 55.6 | 3.89 s | 55.6 |
a) 1H- and 13C-NMR data of 1–3 in CD3OD; The assignments were based on 1H–1H-COSY, HMQC, and HMBC experiments.

 ) and HMBC (
) and HMBC (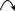 ) Correlations of 1 and 3
) Correlations of 1 and 3The relative configuration of 1 was established by analysis of the nuclear Overhauser effect spectroscopy (NOESY) spectrum (Fig. 3). The chemical shift of H-7′ (δH 4.82) of 1 was in agreement with the assignment of the trans configuration when compared with literature data.13,14) The NOESY spectrum displayed correlations of H-7a/H-7′, H-7b/H-9′b, H-8/H-8′, and H-7′/H-9′a (Fig. 3). This led to assignments of a trans orientation for H-7′/H-8′ and a cis orientation for H-8/H-8′. In turn, the absolute configuration of 1 was assigned as 8R,7′S,8′R by the analysis of circular dichroism (CD) spectrum of 1 showing a negative Cotton effect at 239 nm (Δε=−1.1).11,15) The absolute configuration was also supported by the positive optical rotation ([α]D25+37.2) of 1.11,15) Finally, the structure of 1 was confirmed by the alkaline methanolysis of 1 with 3% NaOMe in MeOH, affording (+)-4,5,5′-trimethoxylariciresinol, which was identified by comparing its 1H-NMR, MS, [α]D value, and CD data.15,16) According to these data, the structure of 1 was elucidated as (+)-9′-O-(E)-feruloyl-4,5,5′-trimethoxylariciresinol.

 ) Correlations of 1
) Correlations of 1Linderuca B (2) was obtained as a light yellowish gum ([α]D25+42.3). The molecular formula of 2 was established as C33H38O11 using positive HR-ESI-MS, which showed a positive ion [M+Na]+ at m/z 633.2311 (Calcd for C33H38NaO11, 633.2312). The 1H- and 13C-NMR data of 2 were similar to those of 1, except that a 9′-O-(Z)-feruloyl moiety of 2 replaced a 9′-O-(E)-feruloyl moiety of 1. This was supported by the cis-coupling constant (J=13.0 Hz) and characteristic chemical shifts (δH 6.83 and 5.71) for H-7″ and H-8″ of 2.11) The structure of 2 was further confirmed by the 1H–1H COSY, heteronuclear multiple quantum coherence (HMQC), HMBC, and NOESY techniques. A comparison of the CD data (λmax 237 nm; Δε=−2.5) of 2 with that of 1 suggested that compound 2 also possessed the same 8R,7′S,8′R configuration.11,15) The structure of 2 was thus determined as (+)-9′-O-(Z)-feruloyl-4,5,5′-trimethoxylariciresinol.
Linderuca C (3), was obtained as a light yellowish gum ([α]D25+23.4) with the molecular formula C32H36O10, as determined by HR-ESI-MS data at m/z 603.2208 [M+Na]+ (Calcd for C32H36NaO10, 603.2206). The 1H- and 13C-NMR spectra of 3 were similar to those of 1, except for the proton and carbon resonances attributable to the aromatic ring located at C-7′. Signals at δH 6.90 (1H, d, J=2.0 Hz), 6.81 (1H, dd, J=8.0, 2.0 Hz), and 6.76 (1H, d, J=8.0 Hz) were assigned discriminatively to the aromatic ring in the 1H-NMR spectrum of 3, suggesting a typical 1,3,4-trisubstitued aromatic ring, instead of the 1,3,4,5-tetrasubstitued one in 1. Similarly, the structure of 3 was further confirmed by the 1H–1H COSY, HMQC, HMBC, and NOESY spectroscopic data (Fig. 2). The absolute configuration of 3 was determined based on its CD data showing a negative Cotton effect at 238 nm (Δε=−1.3) in combination with the positive optical rotation ([α]D25+23.4) of 3,11,15) which proved the 8R,7′S,8′R configuration of 3. In conclusion, the structure of 3 was established as (+)-9′-O-(E)-feruloyl-4,5-dimethoxylariciresinol.
The known compounds were identified as (+)-9′-O-(E)-feruloyl-5,5′-dimethoxylariciresinol (4),11) (+)-9′-O-(Z)-feruloyl-5,5′-dimethoxylariciresinol (5),11) and (+)-5,5′-dimethoxylariciresinol (6)17) by comparison of their spectroscopic and physical data with previously reported values. The absolute configurations of the known lignans (4–6) were established based on their CD and optical rotation values.11,17) To the best of our knowledge, it is the first time that the known compounds 4–6 have been isolated from this genus Lindera.
In this study, the cytotoxic activities of the isolates 1-6 were evaluated by determining their inhibitory effects on four human tumor cell lines including A549, SK-OV-3, SK-MEL-2, and HCT-15 using the SRB bioassay.18) The results (Table 2) showed that the tested isolates 1-5 had antiproliferative activities against above tested cell lines with IC50 values of 7.79–29.42 µM. These results suggest that the presence of a feruloyl moiety located at C-9′ in this lignan skeleton may be critical for exerting the antiproliferative effect against the tested cell lines, because compound 6 without the moiety was inactive against the tested cell lines (IC50 >30.0 µM). Recently, it has been also reported that the feruloyl moiety of the small molecule plays a key role in the antiproliferative effect against the cancer cell lines.19,20) According to their data, it was found that generally, the more the feruloyl units a compound has, the better antiproliferative effect it shows. In the same context, the presence of the methoxy group at C-4 may influence the activity positively, considering the results of compounds 1/4 and 2/5. This is likely associated with the lipophilicity on the compounds. Increased lipophilicity of molecules was reported to be responsible for enhanced cytotoxicity.21) Of these compounds, compound 3 possessing two feruloyl groups and the methoxy group at C-4, showed the most potent cytotoxicity against A549, SK-OV-3, SK-MEL-2, and HCT-15 cell lines with IC50 values of 21.72, 9.83, 14.36, and 7.79 µM, respectively.
| Compounds | IC50 (µM)a) | |||
|---|---|---|---|---|
| A549 | SK-OV-3 | SK-MEL-2 | HCT-15 | |
| 1 | >30.0 | 17.43±0.85b) | 23.59±1.78 | 21.61±2.13 |
| 2 | 22.61±0.92 | 12.70±0.50 | 17.82±1.81 | 14.58±0.88 |
| 3 | 21.72±2.11 | 9.83±1.63 | 14.36±0.28 | 7.79±1.19 |
| 4 | >30.0 | 28.90±2.95 | 27.41±1.35 | >30.0 |
| 5 | 29.42±1.48 | 20.64±1.29 | 18.03±1.27 | 19.58±0.73 |
| 6 | >30.0 | >30.0 | >30.0 | >30.0 |
| Doxorubicinc) | 0.01±0.002 | 0.02±0.001 | 0.01±0.004 | 0.04±0.008 |
a) IC50 value of compounds against each cancer cell line. IC50 value was defined as the concentration (μM) that caused 50% inhibition of cell growth in vitro. b) Data are expressed as mean±S.E.M. of three independent experiments. c) Doxorubicin as a positive control.
On the basis of the expanded understanding that inflammation plays a crucial role in tumor progression, we also evaluated anti-inflammatory activities of the isolates 1–6 in the medium using murine microglia BV-2 cells. Because nitric oxide (NO) is the main pro-inflammatory mediator produced by activated microglia,22) the anti-inflammatory activities of the isolates were determined by measuring NO levels in the medium of lipopolysaccharide (LPS)-stimulated BV-2 cells. As shown in Table 3, compounds 1–3 significantly inhibited NO levels with IC50 values of 12.10, 9.48, and 9.87 µM, respectively without influencing on cell viability. This activity of 1–3 was more potent than that of positive control, NG-monomethyl-L-arginine (L-NMMA), in inhibiting NO production with an IC50 of 18.35 µM. Compounds 4 and 5 also significantly inhibited NO production, but were toxic at a concentration of 20 µM, while compound 6 (IC50 value, 20.29 µM) was less active than the positive control, L-NMMA. These results suggested that the presence of the feruloyl group at C-9′ in this lignan-skeleton might also be a positive influence on the anti-inflammatory activity, similar to cytotoxicity.
| Compounds | IC50 (μM)a) | Cell viability (%)b) |
|---|---|---|
| 1 | 12.10 | 106.2±4.7 |
| 2 | 9.48 | 98.7±2.6 |
| 3 | 9.87 | 107.9±4.5 |
| 4 | 9.24 | 82.0±3.5* |
| 5 | 8.96 | 61.2±2.4* |
| 6 | 20.29 | 108.2±6.9 |
| NMMAc) | 18.35 | 98.2±4.5 |
a) IC50 value of each compound was defined as the concentration (μM) that caused 50% inhibition of NO production in LPS-activated BV-2 cells. b) Cell viability after treatment with 20 µM of each compound is expressed as a percentage (%) of the LPS only treatment group. Results are averages of three independent experiments, and the data are expressed as mean±S.D. Statistical comparisons were performed using a one-way ANOVA test with Student’s t-test. Only * p-value <0.05 was indicated as statistically significant. c) NMMA as a positive control.
Optical rotations were measured on a Jasco P-1020 polarimeter using methanol as a solvent. IR spectra were recorded on a Bruker IFS-66/S FT-IR spectrometer using methanol as a solvent. CD spectra were measured on a Jasco J-715 spectropolarimeter using methanol as a solvent. UV spectra were recorded with a Shimadzu UV-1601 UV-Visible spectrophotometer using methanol as a solvent. HR-ESI-MS and ESI-MS spectra were recorded on a Micromass QTOF2-MS. NMR spectra, including 1H–1H COSY, HMQC, HMBC, NOESY experiments, were recorded on a Varian UNITY INOVA 500 NMR spectrometer operating at 500 MHz (1H) and 125 MHz (13C), with chemical shifts given in ppm (δ). Semi-preparative HPLC used a Gilson 306 pump with a Shodex refractive index detector. Silica gel 60 (Merck, 230–400 mesh) and RP-C18 silica gel (Merck, 230–400 mesh) were used for column chromatography. The packing material for molecular sieve column chromatography was Sephadex LH-20 (Pharmacia Co., Ltd.). Merck precoated silica gel F254 plates and RP-18 F254s plates were used for TLC. Spots were detected on TLC under UV light or by heating after spraying with anisaldehyde–sulfuric acid.
Plant MaterialsThe twigs of L. glauca (SIEB. et ZUCC.) BLUME were collected from Hongcheon, Chungcheongbuk-do, Korea, in March 2010. Samples of plant material were identified by one of the authors (K.R. Lee). A voucher specimen (SKKU 2010-3B) has been deposited in the herbarium of the School of Pharmacy, Sungkyunkwan University, Suwon, Korea.
Extraction and IsolationThe pulverized twigs of L. glauca (6 kg) were extracted twice with 80% aqueous MeOH (2×4 h) under reflux, and then filtered. The filtrate was evaporated under vacuum to obtain a MeOH extract (120 g), which was suspended in distilled water (2 L) and successively partitioned with n-hexane, CHCl3, EtOAc, and n-BuOH, yielding 2.5, 13.3, 7.6, and 17.5 g of residues, respectively. Each fraction was evaluated for its cytotoxicity against A549, SK-OV-3, SK-MEL-2, and HCT-15 cells using the SRB bioassay. The most active fraction was the CHCl3-soluble fraction, which we selected for phytochemical investigation. The CHCl3-soluble fraction (13.3 g) was separated over RP-C18 silica gel (230–400 mesh, 300 g) column chromatography using with a gradient of increasing MeOH in H2O from 50% to 100% to give four fractions (C1–C4). Fraction C1 (4.5 g) was applied to a Sephadex LH-20 column using a solvent system of CH2Cl2–MeOH (1 : 1) to give five subfractions (C11–C15). Subfraction C13 (1.4 g) was separated over RP-C18 silica gel (230–400 mesh, 100 g) column chromatography using a solvent system of 60% aqueous MeOH and further purified by semi-preparative normal-phase HPLC using a 250 mm×10 mm i.d., 5 µm, Apollo Silica column (Alltech, Nicholasville, KY, U.S.A.) with a solvent system of CHCl3–MeOH (50 : 1, flow rate; 2 mL/min) to yield 6 (30 mg). Fraction C2 (5.2 g) was applied to a Sephadex LH-20 column with a solvent system of CH2Cl2–MeOH (1 : 1) to give two subfractions (C21–C22). Subfraction C21 (3.1 g) was separated over a silica gel (230–400 mesh, 150 g) column chromatography using a solvent system of CHCl3–MeOH (20 : 1) to give four subfractions (C211–C214). Subfraction C211 (1.2 g) was separated over RP-C18 silica gel (230–400 mesh, 50 g) column chromatography using a solvent system of 40% aqueous MeOH, and further purified by semi-preparative reversed-phase HPLC using a 250 mm×10 mm i.d., 10 µm, Econosil RP-18 column (Alltech) with a solvent system of 65% aqueous MeOH (flow rate; 2 mL/min) to obtain 1 (6 mg). Subfraction C22 (2.1 g) was applied to a silica gel (230–400 mesh, 100 g) column chromatography using a solvent system of CHCl3–MeOH (20 : 1) to give four subfractions (C221–C224). Subfraction C221 (310 mg) was separated over RP-C18 silica gel (230–400 mesh, 15 g) column chromatography using 50% aqueous MeOH and 100% MeOH, and finally compounds 2 (10 mg), 3 (10 mg), 4 (10 mg), and 5 (4 mg) were isolated from the 100% MeOH eluted fraction by semi-preparative reversed-phase HPLC with 65% aqueous MeOH.
Linderuca A (1): Light yellowish gum; [α]D25+37.2 (c=0.30, MeOH); UV (MeOH) λmax (log ε) 324 (4.5), 298 (3.3), 286 (4.0), 230 (3.7) nm; CD (MeOH) λmax (Δε) 239 (−1.1) nm; IR (KBr) νmax 3422, 2940, 1711, 1604, 1515, 1447, 1270, 1167, 1032 cm−1; 1H-(500 MHz) and 13C-(125 MHz) NMR data, see Table 1; ESI-MS (positive-ion mode) m/z: 633 [M+Na]+; HR-ESI-MS (positive-ion mode) m/z: 633.2314 [M+Na]+ (Calcd for C33H38NaO11, 633.2312).
Linderuca B (2): Light yellowish gum; [α]D25+42.3 (c=0.50, MeOH); UV (MeOH) λmax (log ε) 325 (4.1), 299 (3.6), 287 (3.8), 230 (3.8) nm; CD (MeOH) λmax (Δε) 237 (−2.5) nm; IR (KBr) νmax 3417, 2942, 1709, 1602, 1513, 1447, 1270, 1166, 1032 cm−1; 1H- (500 MHz) and 13C- (125 MHz) NMR data, see Table 1; ESIMS (positive-ion mode) m/z: 633 [M+Na]+; HR-ESI-MS (positive-ion mode) m/z: 633.2311 [M+Na]+ (Calcd for C33H38NaO11, 633.2312).
Linderuca C (3): Light yellowish gum; [α]D25+23.4 (c=0.50, MeOH); UV (MeOH) λmax (log ε) 324 (4.0), 298 (3.2), 286 (3.9), 230 (3.8) nm; CD (MeOH) λmax (Δε) 238 (−1.3) nm; IR (KBr) νmax 3421, 2938, 1710, 1608, 1510, 1443, 1270, 1167, 1030 cm−1; 1H- (500 MHz) and 13C- (125 MHz) NMR data, see Table 1; ESIMS (positive-ion mode) m/z: 633 [M+Na]+; HR-ESI-MS (positive-ion mode) m/z: 603.2208 [M+Na]+ (Calcd for C32H36NaO10, 603.2206).
Alkaline Methanolysis of 1Compound 1 (2.0 mg) was treated with 3% NaOMe in MeOH at room temperature for 2 h. The reaction mixture was then passed through an Amberlite IRA-67 column (Rohm and Haas, Philadelphia, PA, U.S.A.) and chromatographed on a Sephadex LH-20 column with MeOH to give (+)-4,5,5′-trimethoxylariciresinol (1a; 0.7 mg) as a colorless gum. Compound 1a, (+)-4,5,5′-trimethoxylariciresinol was identified by its 1H-NMR, MS data, specific rotation, and CD data.15,16) 1a: [α]D25+16.1 (c 0.03, MeOH); CD (MeOH) λmax (Δε) 235 (−1.5) nm.
Cytotoxicity AssayA sulforhodamine B (SRB) bioassay was used to determine the cytotoxicity of each compound isolated against four cultured human tumor cell lines.18) The assays were performed at the Korea Research Institute of Chemical Technology. The cell lines used were A549 (non-small cell lung carcinoma), SK-OV-3 (ovary malignant ascites), SK-MEL-2 (skin melanoma), and HCT-15 (colon adenocarcinoma). Doxorubicin was used as a positive control. The cytotoxicities of doxorubicin against the A549, SK-OV-3, SK-MEL-2, and HCT-15 cell lines were IC50 0.01, 0.02, 0.01, and 0.04 µM, respectively.
Measurement of NO Production and Cell ViabilityInhibition of NO production was evaluated in LPS-activated murine microglia BV-2 cells. Cells were stimulated with 100 ng/mL of LPS in the presence or absence of samples for 24 h. Nitrite in the culture media, a soluble oxidation product of NO, was determined using the Griess reaction. Cell viability was measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. L-NMMA (Sigma), a NOS inhibitor, was tested as a positive control.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2013R1A1A2A10005315). We thank Drs. E. J. Bang, S. G. Kim, and J. J. Seo at the Korea Basic Science Institute for their aid in the NMR and MS spectra measurements.