2014 年 62 巻 11 号 p. 1146-1150
2014 年 62 巻 11 号 p. 1146-1150
We studied the specific labeling of streptavidin using the modular method for affinity labeling (MoAL) that we developed based on a catalytic amide-forming reaction using 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) and a tertiary amine catalyst. The primary structures of avidin and streptavidin are significantly different from each other, and streptavidin does not possess an acidic amino acid equivalent to Asp108 of avidin, which is the target acidic amino acid that was labeled using MoAL. However, using biotinylated modular ligand catalysts (MLC) originally designed for labeling avidin, the labeling of streptavidin was found to successfully proceed at Glu51, which is located in a different region. The present study indicates that MoAL is readily applicable to protein labeling without a precise design for MLC. The most important factor for the design of MLC is to ensure that the linker is of sufficient length to connect the ligands to a catalytic site.
Affinity labeling (AL) is a powerful tool for the identification of target proteins of drugs.1–3) Recently, we established a novel modular method for AL (MoAL).4) In this method, the three essential elements constituting affinity probes, i.e., a ligand with specific affinity for the target biomolecule, a labeling tag to facilitate the isolation and identification of the labeled biomolecule, and a reactive group to form a covalent bond at the binding site, are present as separate molecules and function as individual modules. It is easy to design and synthesize the simplified modules, i.e., a modular ligand catalyst (MLC), a labeling module, and 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) as a reactive module (Fig. 1). In addition, protein labeling can be achieved just by mixing the target protein with these modules. Because MoAL was designed on the basis of the catalytic dehydrocondensation that proceeds in aqueous solution through the combination of CDMT and a tertiary amine catalyst,5,6) the target functional group is the carboxy group of an Asp or Glu residue located near the ligand-binding site. This is in contrast to the fact that nucleophilic amino acid residues, such as Cys, His, Lys, Arg, Ser, and Tyr, have been the most common target amino acids in the bioconjugation techniques used for protein modification. Because polar amino acids are normally located in hydrophilic protein regions, the probability of the occurrence of carboxy groups on the protein surface should be higher than their average occurrence (11.6%) in proteins.7) Therefore, when the catalytic site of an MLC comes out to the protein surface during formation of a ligand–protein complex, a labeling reaction can occur. In fact, our previous study on the specific labeling of avidin showed that the linker length of the MLCs used in MoAL need not to be precisely designed.8) This implies that if a chemically modified ligand moiety retains its specific affinity for the target protein, and a catalytic site is connected to the ligand with a linker of sufficient length so that it can reach the protein surface, the ligand module can activate Asp or Glu residues of the target proteins. This is the great advantage of MoAL for protein labeling, particularly for labeling of unknown target proteins. On the basis of this consideration, we investigated whether streptavidin,9,10) an alternative target protein of biotin, can be specifically labeled using biotin-containing MLCs designed for avidin labeling. In spite of their functional similarities, the primary structures of avidin and streptavidin are not well conserved (30% amino acid identity).11,12) Importantly, the labeling site of avidin (Asp108) is replaced by a neutral residue (Asn118) in streptavidin (vide infra).
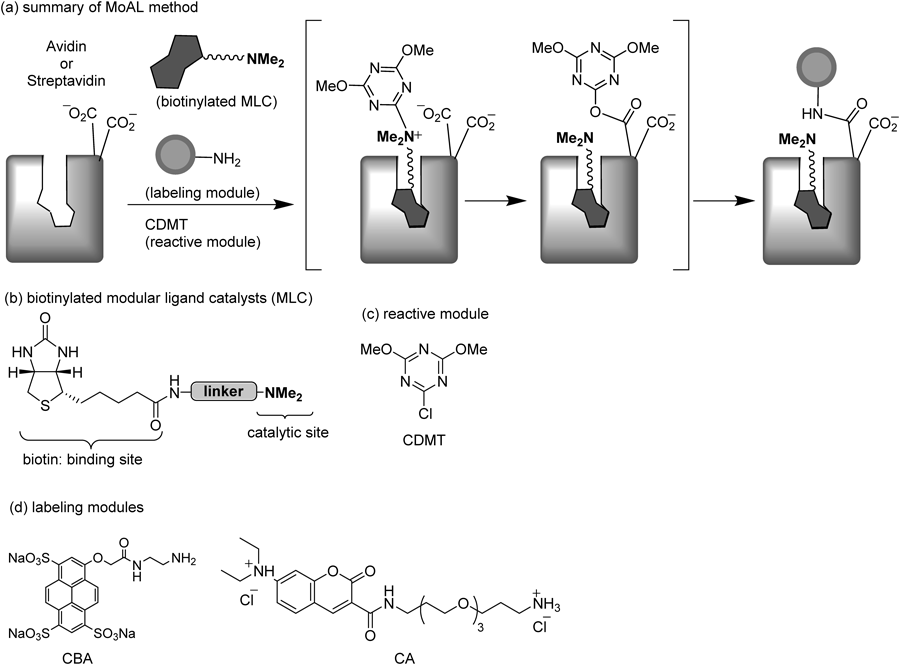
(a) Summary of MoAL. A biotinylated MLC makes a complex with avidin or streptavidin, and it then reacts with 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT, reactive module) to form a triazinylammonium salt. The triazinylammonium moiety can reach a carboxy group of aspartic acid or glutamic acid at the protein surface to form an activated ester. Finally, reaction of the activated ester with a primary amino group of a labeling module affords a labeled protein; (b) Biotinylated modular ligand catalysts (biotinylated MLC); (c) Reactive module (CDMT); and (d) Labeling modules.
We employed four different types of biotinylated MLC (Fig. 2, 1a–d) that were found to have successfully achieved avidin labeling in good to high yields. The catalysts 1a and b possess N,N-dimethylglycine as a catalytic site,4,13) and the catalyst 1c and d possess N,N-dimethyl-N-triazolylmethylamine, which was shown to be a more effective catalytic site, in our recent report.13) The linker lengths in 1b–d have been optimized for the specific labeling of avidin.8) Attempts at labeling streptavidin with Cascade Blue® ethylenediamine (CBA in Fig. 1) using these ligand catalysts were performed under the same conditions as those used for avidin labeling.

1a–1f: Biotinylated modular ligand catalysts (MLC); 2: N,N-Dimethylglycine ethyl ester; 3a: Biotinylated dehydrocondensing reagent.
Table 1 shows the labeling yields per monomeric subunit of streptavidin, along with those of avidin for comparison.14) As expected, the labeling of streptavidin occurred with all the catalysts, albeit in yields lower than those of avidin labeling. In a control reaction using dimethylglycine ethyl ester 2, which has no affinity to streptavidin, no labeling signal was observed. Thus, the labeling of streptavidin with MLC 1a–d is attributable to the specific affinity between streptavidin and the biotin moiety of MLC. Interestingly, despite the similar linker lengths of the catalysts, the labeling yields with 1c (59%) and d (63%) were almost two times those with 1a and b. To examine whether the catalytic site structure (N,N-dimethyl-N-triazolylmethylamine) or the polyethylene glycol (PEG) linker of 1d is primarily responsible for its highest labeling yield among the ligand catalysts, the reaction was conducted using 1e, which was composed of a PEG linker and an N,N-dimethylglycyl group. The labeling yield was found to be the same as that of 1b, which has an alkyl linker, indicating that the 1,2,3-triazolyl-type tertiary amine structure is more important for labeling than the PEG linker or the N,N-dimethylglycyl catalyst.
| Catalyst | Labeling yield (%) | |
|---|---|---|
| Streptavidina) | Avidina) | |
| 1a | 30 | 46b) (53c)) |
| 1b | 36 | 60b) (52c)) |
| 1c | 59 | 86b) |
| 1d | 63 | 83b) |
| 2 | 0 | |
| 1e | 36 | |
| 1f | 38 | |
a) The labeling reaction was performed with protein (10 µM), ligand catalyst (40 µM), CBA (800 µM), and CDMT (800 µM) in 50 mM phosphate buffer (pH 8.0) for 24 h at rt. The labeling yield is represented per monomeric subunit. b) Data are cited from ref. 13. c) Previous results obtained under different conditions (ref. 8).
Based on both the primary and X-ray crystal structures of avidin (PDB ID: 1 LEL) and streptavidin (PDB ID: 1LCZ),15) it is obvious that the acidic amino acid residues are located in different places in these two proteins. In particular, the labeling site of avidin (Asp108) is missing in streptavidin (Fig. 3). Thus, the observed results indicate that a different site on streptavidin must be labeled. We conducted a MS analysis to identify which amino acid residues are modified in labeled streptavidin by using 1d as a ligand catalyst. The labeling module employed was a coumarin fluorophore-containing amine (CA in Fig. 1), rather than CBA, because the three anionic sulfonato groups of CBA make the MS analysis difficult. Because the labeling reagent is totally separate from the ligand in MoAL, it can be readily exchanged with others. All of the acidic amino acids (four Asp and five Glu) contained in core streptavidin were detected during the MS analysis, and only Glu51 was found to be specifically modified with CA (sequence coverage 73%).16)

The cleavage sites producing core streptavidin are indicated by arrows. Acidic amino acid residues (D, E) are underlined. The acidic amino acid residues observed in the MS analysis are indicated in boldface. The acidic amino acid residues that could not be observed in the MS analysis are indicated by strikethrough. The acidic amino acid residues labeled with CA and the lysyl residues modified by the triazinyl group are indicated against a black background. The data for streptavidin was obtained using 1d and CA (see Experimental section in MALDI-MS Analysis for Determination of Modified Amino Acid), and the data for avidin is from ref. 4.
The region from residues 45 to 52 of streptavidin is known to form a flexible loop structure at the surface of the biotin binding site (Fig. 4).11,17,18) This domain usually exists in an open conformation, and changes to a closed conformation when biotin binds to streptavidin.17) This conformational change in the loop structure can occur after a biotinylated ligand catalyst binds to streptavidin, and may allow the carboxy group of Glu51 to get close to the catalytic site of the MLC. Thus, the MLC binding would promote the specific labeling of Glu51.
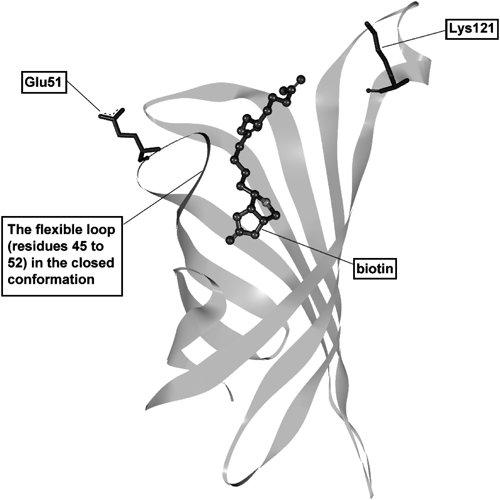
To increase the labeling yield by optimizing the ligand catalyst, we prepare the triazolyl-type MLC 1f, which has a linker length approximately equal to the distance between the biotin binding site and Glu51 in the closed conformation of the X-ray crystal structure (PDB ID: 1 LCZ; supplementary Figure S2). However, the labeling yield using 1f decreased (38%), indicating that employing a linker with a sufficiently longer chain length may be preferable to employing a linker with an adjusted length in the present system. From this perspective, a similar tendency can be observed in the results of the avidin labeling experiments.8)
The reaction of streptavidin with the ligand condensing reagent 3a, prospectively prepared from 1a and 2,4-dimethoxy-6-trifluoromethanesulfonyloxy-1,3,5-triazine, for 8 h resulted in no significant labeling (ca. 0%). However, the yield increased to 20% upon treatment of the resulting mixture with CDMT for another 8 h. This result is in accordance with our observations with avidin8); the triazinyl group of a ligand condensing reagent bound to the biotin binding site preferably reacted with Lys111 over Asp108. In the present case, the MS analysis of the labeled streptavidin showed that Lys121, which is equivalent to Lys111 of avidin (Figs. 3, 4), underwent N-triazinylation at high frequency. Thus, Lys121 would react faster than Glu51 when 3a is bound to streptavidin, and after transferring the triazinyl group to Lys121, the tert-amine moiety (N,N-dimethylamino group) of the regenerated 1a, tightly bound to the streptavidin, could then catalyze the intended dehydrocondensation of the carboxy group of Glu51 with labeling modules. Therefore, the labeling reaction proceeded only in the presence of CDMT.
In conclusion, the specific labeling of streptavidin was shown to proceed in significant yields when using ligand catalysts designed for labeling avidin using MoAL. Because of the low sequence homology between the two proteins, an alternative amino acid (Glu51) located in a different loop was labeled in streptavidin. Because the MLC linker length does not need to be precisely designed, MoAL will be useful for searching for the unknown target proteins of bioactive compounds. For the labeling of known proteins, it is preferable to employ a linker with a length longer than the distance between binding site and a target acidic amino acid. Both the avidin– and streptavidin–biotin systems have been widely utilized in a number of applications in life sciences. For this purpose, conjugation of these proteins with enzyme, fluorescent tag, radioisotope tag, etc., are necessary in many cases. Therefore, our method can be efficiently applicable to the so-called (strept)avidin technologies.18)
Melting points are uncorrected. 1H-NMR spectra were recorded on a JEOL JNM-ECS400 and a JEOL JNM-ECS600 spectrometer. Chemical shifts are reported as δ values relative to tetramethylsilane as internal standard. Infrared spectra were recorded on a HORIBA FT-720 FREEXACT-II spectrometer. Mass spectra were measured on a Micromass Zq2000 spectrometer (electrospray ionization (ESI)-MS), a Bruker Daltonics micrOTOFQ II (ESI-MS), a JEOL JMS-T100TD (ESI-MS and direct analysis in real time (DART)-MS), and a 4800 Plus MALDI TOF/TOFTM Analyzer, ABsciex. UV-visible spectra were recorded on a Shimadzu Vis recording spectrophotometer UV-2400 PC. Cascade Blue® ethylenediamine trisodium salt (CBA),19) biotinylated ligand catalyst 1a–d, condensing reagent 3a,8) 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM),20) and t-butyl N-(13-amino-4,7,10-trioxatridecyl)carbamate21) were prepared according to the literature. Other chemicals were obtained from commercial sources and used as received unless otherwise noted.
Preparation of the Biotinylated MLCs (1e, f) and the Labeling Module (CA)17-Azido-3,6,9,12,15-pentaoxa-1-heptadecyl N,N-DimethylglycinateTo a solution of 17-azido-3,6,9,12,15-pentaoxa-1-heptadecanol (1.00 g, 3.25 mmol), N,N-dimethylglycine (437 mg, 4.24 mmol) and 4-dimethylaminopyridine (199 mg, 1.63 mmol) in dry CH2Cl2 (8.1 mL) was added 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (1.87 g, 9.76 mmol) under nitorogen atmosphere at 0°C. After being stirred overnight at rt, the reaction mixture was poured into water, and extracted with CH2Cl2. The organic layer was washed with 1 M Na2CO3aq (20 mL) and brine, and then dried over MgSO4, filtered, and evaporated. The crude mixture was purified by silica gel column chromatography (CHCl3/MeOH=10 : 1) to give 0.937 g of the desired compound as a yellow oil (73.5% yield). 1H-NMR (CDCl3) δ: 2.36 (6H, s), 3.21 (2H, s), 3.39 (2H, t, J=5.0 Hz), 3.72–3.64 (20H, m), 4.29 (2H, t, J=5.0 Hz). 13C-NMR (CDCl3) δ: 45.2, 50.6, 60.2, 69.0, 70.0, 70.4, 70.5, 70.5, 70.6, 70.6, 170.6. IR (CHCl3) cm−1: 2996, 2875, 2107, 1743, 1453, 1286, 1261, 1128, 1101. DART-MS m/z: 393.2364 (Calcd for C25H46N7O2S1: 393.2349).
Biotinylated MLC (1e)17-Azido-3,6,9,12,15-pentaoxa-1-heptadecyl N,N-dimethylglycinate (300 mg, 0.764 mmol) was dissolved in a mixture of methanol (7.6 mL) and 4 M HClaq in ethyl acetate (764 µL) at 0°C, and hydrogenated using Pd(C) (24.5 mg) at 0°C under a H2 atmosphere. After being stirred at 0°C for 2.5 h, the mixture was filtered through celite and concentrated to afford a residue. To this residue, a MeOH (4 mL)/water (3 mL) solution of (+)-biotin (224 mg, 0.917 mmol) and 4-methylmorpholine (336 µL, 3.05 mmol) was added at 0°C, and then DMT-MM (253.7 mg, 0.917 mmol) was added at rt. After being stirred for 3 h, the solution was evaporated and lyophilized under vacuum. The crude mixture was purified by silica gel column chromatography (CHCl3/MeOH=6 : 1) to give 261 mg of the desired compound as a yellow oil (57.7% yield). 1H-NMR (methanol-d4) δ: 1.40–1.79 (6H, m), 2.22 (2H, t, J=7.3 Hz), 2.71 (1H, d, J=12.8 Hz), 2.93 (1H, dd, J=4.8, 12.8 Hz), 2.98 (6H, s), 3.18–3.24 (1H, m), 3.36 (2H, t, J=5.5 Hz), 3.54 (2H, t, J=5.5 Hz), 3.64 (16H, m), 3.75 (2H, t, J=4.6 Hz), 4.18 (2H, s), 4.31 (1H, dd, J=4.8, 7.8 Hz), 4.41 (2H, d, J=4.6 Hz), 4.50 (1H, dd, J=5.0, 8.0 Hz). 13C-NMR (methanol-d4) δ: 26.9, 29.5, 29.8, 36.7, 40.3, 41.0, 44.6, 57.0, 58.1, 61.6, 63.4, 66.5, 69.6, 70.5, 71.3, 71.3, 71.5, 71.5, 71.5, 166.1, 167.0, 176.1. IR (CHCl3) cm−1: 3465, 2993, 2929, 2875, 1755, 1700, 1655, 1456, 1263, 1105. DART-MS m/z: 593.3219 (Calcd for C26H49N4O9S1: 593.3220).
N-(10-Azidodecyl)biotinamideTo a solution of (+)-biotin (135.3 mg, 0.554 mmol) and 10-azidodecylamine (100.0 mg, 0.504 mmol) in MeOH (5 mL) was added DMT-MM (153.2 mg, 0.554 mmol) at rt. After being stirred for 2.5 h at rt, the solvent was removed under vacuum. The crude mixture was dissolved in CHCl3, and the organic layer was washed with 1 M HClaq, saturated Na2CO3aq, and brine, dried over MgSO4, filtered, and concentrated in vacuo. The crude mixture was purified by silica gel column chromatography (CHCl3/MeOH=9 : 1) to give the desired compound as white powder (44.8 mg, 20% yield). mp: 175–177°C. 1H-NMR (CDCl3) δ: 1.28–1.69 (22H, m), 2.20 (2H, t, J=7.3 Hz), 2.74 (1H, d, J=12.8 Hz), 2.92 (1H, dd, J=5.0, 12.8 Hz), 3.13–3.18 (1H, m), 3.21 (2H, t, J=6.9 Hz), 3.26 (2H, t, J=6.9 Hz), 4.32 (1H, dd, J=5.0, 7.4 Hz), 4.52 (1H, dd, J=5.0, 7.4 Hz), 5.20 (1H, s), 5.78 (1H, br s), 6.00 (1H, s). 13C-NMR (CDCl3) δ: 25.7, 26.7, 26.9, 28.1, 28.1, 28.8, 29.1, 29.3, 29.4, 29.4, 29.7, 36.1, 39.5, 40.6, 51.5, 55.5, 60.2, 61.8, 163.6, 173.0. IR (KBr) cm−1: 3295, 2927, 2854, 2096, 1704, 1643, 1550, 1463. ESI-MS m/z: 447.2501 (Calcd for C20H36N6Na1O2S1: 447.2518). MS (ESI) m/z: 447 (M+Na)+.
Biotinylated MLC (1f)To a solution of N-(10-azidodecyl)biotinamide (44.8 mg, 0.106 mmol), N,N-dimethylpropargylamine (22.9 mg, 0.276 mmol), and CuSO4·5H2O (5.3 mg, 0.021 mmol) in CHCl3/MeOH was added sodium ascorbate (10.5 mg, 0.0530 mmol) at rt. After stirring for 19 h at rt, the solvent was removed under vacuum. The crude mixture was purified by silica gel column chromatography (CHCl3/MeOH=9 : 1 containing 0.5% Et3N) to give the desired compound as white powder (18 mg, 33% yield). mp: 135–136°C. 1H-NMR (methanol-d4) δ: 1.30–1.76 (20H, m), 1.90 (2H, q, J=6.9 Hz,), 2.19 (2H, t, J=7.3 Hz), 2.30 (6H, s), 2.70 (1H, d, J=12.8 Hz), 2.92 (1H, dd, J=4.8, 12.8 Hz), 3.15 (2H, t, J=6.9 Hz), 3.17–3.22 (1H, m), 3.68 (2H, s), 4.30 (1H, dd, J=4.8, 7.8 Hz), 4.40 (2H, t, J=6.9 Hz), 4.49 (1H, dd, J=4.6, 7.8 Hz), 7.92 (1H, s). 13C-NMR (methanol-d4) δ: 27.0, 27.4, 28.0, 29.5, 29.8, 30.0, 30.4, 30.4, 30.5, 30.5, 31.3, 36.8, 40.3, 41.1, 44.7, 44.7, 51.4, 54.2, 57.0, 61.6, 63.4, 125.4, 144.0, 166.1, 175.9. IR (KBr) cm−1: 3301, 2925, 2852, 1693, 1633, 1556, 1543, 1471. ESI-MS m/z: 508.3424 (Calcd for C25H46N7O2S1: 508.3434).
7-(Diethylamino)coumarin-3-carboxylic Acid HydrochlorideTo a solution of ethyl 7-(diethylamino)coumarin-3-carboxylate (100.0 mg, 0.346 mmol) in EtOH (1.4 mL) was added NaOH (20.7 mg, 0.518 mmol) in H2O (0.35 mL) at rt. After being stirred for 5 h at rt, the reaction was quenched with conc. HCl (1 mL), and the solvent was removed under vacuum. The residue was dissolved in EtOH followed by filtering to remove precipitation, and the filtrate was concentrated under vacuum to give 97.2 mg of the desired compound as a yellow solid (95% yield). mp: 231–232°C. 1H-NMR (methanol-d4) δ: 1.24 (6H, t, J=7.3 Hz), 3.56 (4H, q, J=7.3 Hz), 6.62 (1H, d, J=2.7 Hz), 6.86 (1H, dd, J=2.7, 9.2 Hz), 7.58 (1H, d, J=9.2 Hz), 8.65 (1H, s). IR (CHCl3) cm−1: 3032, 3006, 1736, 1684, 1614, 1581, 1516. MS (ESI) m/z: 262 (M+H)+.
Labeling Module (CA)To a solution of t-butyl N-(13-amino-4,7,10-trioxatridecyl)carbamate (85.1 mg, 0.266 mmol), 7-(diethylamino)coumarin-3-carboxylic acid hydrochloride (70.0 mg, 0.235 mmol), and 4-methylmorpholine (26.0 µL, 0.235 mmol) in MeOH (1 mL) was added DMT-MM (77.5 mg, 0.280 mmol) at rt. After being stirred for 3 h at rt, the solvent was removed under vacuum. The residue was loaded on a silica gel column and eluted with CHCl3/MeOH (97 : 3) to give a crude product (173.5 mg, inseparable amine adduct of dimethoxytriazine was contained), which was dissolved in dioxane (0.5 mL), and then treated with conc. HCl (0.5 mL) at rt for 3 h. The solvent of the resulting mixture was removed under vacuum. The crude compound was purified by decantation with MeOH (containing 0.5 M HCl)–Et2O at rt to give the desired compound as a yellow solid (105.1 mg, 83% over 2 steps). mp: 251–252°C. 1H-NMR (methanol-d4) δ: 1.23 (6H, t, J=7.0 Hz), 1.82–1.97 (4H, m), 3.12 (2H, t, J=6.4 Hz), 3.46–3.74 (18H, m), 6.58 (1H, d, J=2.5 Hz), 6.83 (1H, dd, J=2.5, 9.2 Hz), 7.56 (1H, d, J=9.2 Hz), 8.63 (1H, s). 13C-NMR (methanol-d4) δ: 12.7, 28.0, 30.4, 38.3, 40.3, 46.0, 70.1, 70.5, 71.0, 71.1, 71.2, 71.4, 97.3, 109.5, 110.1, 111.7, 132.7, 149.3, 151.6, 159.2, 164.0, 165.4. IR (CHCl3) cm−1: 3007, 1697, 1616, 1540, 1511, 1134. FAB-MS m/z: 464.2755 (Calcd for C24H38N3O6: 464.2761). MS (ESI) m/z: 464 (M–72)+.
Labeling Experiment of Streptavidin by MoALGeneral Procedure for LabelingStreptavidin (100 µL of 0.015 mM solution in 50 mM phosphate buffer, pH 8.0) and the ligand catalyst (5 µL of 1.2 mM solution in 50 mM phosphate buffer containing 25% MeOH, pH 8.0) were mixed and allowed to stand for 30 min at rt. To the solution was added CBA (20 µL of 6.0 mM solution in 50 mM phosphate buffer, pH 8.0), CDMT (10 µL of 12 mM solution in 50 mM phosphate buffer containing 5% MeOH, pH 8.0), and 50 mM phosphate buffer (15 µL, pH 8.0), and the resulting solution was shaken by Vortex and allowed to stand for 24 h at rt. The concentrations of the solutes in the resulting solution (150 µL) were as follows: streptavidin: 10 µM; ligand catalyst: 40 µM; CBA: 800 µM; CDMT: 800 µM. The solution was loaded on a gel filtration column (Sephadex G-50 medium, 0.7×35 cm), and eluted with 50 mM phosphate buffer (pH 8.0) containing 0.1 M NaCl at rt. The labeling yield was determined by UV-visible spectral analysis.4)
Matrix Assisted Laser Desorption/Ionization (MALDI)-MS Analysis for Determination of Modified Amino AcidStreptavidin was labeled with CA by MoAL using 1d according to the method described above. After quenching the mixture with N,N-dimethylethylenediamine and denaturation with sample buffer at 100°C for 1 h, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) separation was performed (14% polyacrylamide gel). The band containing the monomeric subunit of CA-labeled streptavidin was subjected to in-gel chymotryptic digestion, and then analyzed on HPLC–MALDI-MS/MS under the following conditions.
Nano-LC: DiNa-AM system, KYA technologies, column: HiQ sil C18-3 (0.5 mm i.d.×1 mmL), HiQ sil C18w-3 (0.15 mm i.d.×50 mmL), solvent A: 0.1% trifluoroacetic acid (TFA)/water; solvent B: 0.1% TFA/MeCN, flow rate: 0.3 µL/min, gradient: 0 min/(A : B=100 : 0)→10 min/(A : B=100 : 0)→12 min/(A : B=95 : 5)→75 min/(A : B=50 : 50)→80 min/(A : B=0 : 100)→90 min/(A : B=0 : 100).
This work was supported partially by a Grant-in-Aid for Science Research (No. 20390007) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.