2014 Volume 62 Issue 12 Pages 1200-1213
2014 Volume 62 Issue 12 Pages 1200-1213
A novel class of phosphodiesterase 10A (PDE10A) inhibitors with improved metabolic stability in mouse liver microsomes were designed and synthesized starting from 2-({4-[1-methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]phenoxy}methyl)quinoline (MP-10). Replacement of the phenoxymethyl part of MP-10 with an oxymethyl phenyl unit led to the identification of 2-[4-({[1-methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}methyl)phenyl]quinoline (14), which showed moderate PDE10A inhibitory activity with improved metabolic stability in mouse and human liver microsomes over MP-10. Compound 14 showed high concentrations in plasma and brain after intraperitoneal administration and dose-dependently attenuated the hyperlocomotion induced by phencyclidine in mice, and oral administration of 14 (0.1, 0.3 mg/kg) also improved visual-recognition memory impairment in mice.
Schizophrenia is a chronic and debilitating psychiatric disorder affecting approximately 1% of the world’s population.1) However, most current therapeutic treatments primarily address positive symptoms with only limited efficacy on negative symptoms and cognitive dysfunction. In addition, these existing antipsychotics frequently cause undesirable side effects such as extrapyramidal syndrome, weight gain, diabetes and QT prolongation,2–5) highlighting the unmet medical needs for drugs less prone to such side effects.
Cyclic nucleotide phosphodiesterases (PDEs) are enzymes that regulate intracellular signaling by hydrolyzing cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). The PDE superfamily of enzymes is classified into 11 families in mammals (PDE1–PDE11). PDE10A enzyme is a dual substrate (cAMP/cGMP) phosphodiesterase that is highly expressed in the brain, particularly in the medium spiny neurons of the mammalian striatum.6–8) Inhibition of PDE10A may enhance the intracellular second messenger signaling and striatal output suggested to be impaired in schizophrenic patients.9) Further, some selective PDE10A inhibitors have shown potent efficacy in several rodent behavioral models of schizophrenia.10–14) PDE10A inhibitors have therefore garnered attention as a new therapeutic approach for the treatment of schizophrenia.15–25)
Among reported PDE10A inhibitors, TP-10 and MP-10 (Fig. 1) have extremely potent PDE10A inhibitory activity and high selectivity against other PDEs, and both of these compounds have been reported to be active in the mouse behavioral model for positive symptoms.12–14) However, while TP-10 and MP-10 demonstrated good in vitro and in vivo activity as described above, the intrinsic clearance (CLint) of these compounds in mouse liver microsomes (MLM) was extremely high in our assay (CLint>1000 mL/min/kg). Enhancing stability of PDE10A inhibitors such as TP-10 and MP-10 in MLM would improve potency in behavioral models. Taken together, these observations prompted us to modify the structure of TP-10 and MP-10 to enhance its metabolic stability and develop novel PDE10A inhibitors with in vivo efficacy.

Before any attempt at structure modification, we performed metabolite identification of MP-10 to search for clues to enhancing its stability in MLM, revealing two major metabolic pathways which gave compounds 1 and 2 (Fig. 2). Compound 1 was obtained on removal of the quinolinylmethyl part from MP-10, with subsequent demethylation at the pyrazole ring, producing compound 3. Compound 2 was formed via oxidization of quinoline ring in MP-10. Findings for the X-ray co-crystal structure have shown that this quinoline ring occupies the PDE10A selectivity pocket and forms a hydrogen bond to Tyr693 of the PDE10A enzyme,13) suggesting that quinoline of MP-10 may be an important ring for high selectivity against other PDEs. Given these previous findings, we decided to keep quinoline structure and modify “pyrazol-phenoxy-methyl” unit of MP-10 to improve stability in MLM and human liver microsomes (HLM).

The synthesis of target compounds is shown in Charts 1–7. The reaction between an anion of 4-methylpyridine and Weinreb amide 4 achieved ketone 5. Treatment of 5 with N,N-dimethylformamide dimethyl acetal (DMF-DMA) followed by the addition of methylhydrazine yielded pyrazole analogue 6, which was converted to 7 by deprotection of the tert-butoxycarbonyl (Boc) group. The nucleophilic addition of 7 to 2-vinylquinoline gave 8, and the alkylation of piperidine with 2-(chloromethyl)quinoline yielded 9 (Chart 1).

Reagents and conditions: (a) 4-methylpyridine, LDA, THF; (b) DMF-DMA, DMF; (c) methyl hydrazine, acetic acid, EtOH; (d) HCl, dioxane, MeOH; (e) Et3N, MeOH; then 2-vinylquinoline, acetic acid; (f) 2-(chloromethyl)quinoline, iPr2NEt, DMF.

Reagents and conditions: (a) 2-chloroquinoline, Pd(PPh3)4, Na2CO3, 1,2-dimethoxyethane, H2O; (b) DMF-DMA, DMF; (c) methyl hydrazine, acetic acid, EtOH; (d) ADDP, nBu3P, THF.

Reagents and conditions: (a) 13, ADDP, nBu3P, THF; (b) 2-chloroquinoline, K2CO3, DMF; (c) 2-chloroquinoline, Pd(PPh3)4, Na2CO3, 1,2-dimethoxyethane, H2O.

Reagents and conditions: (a) 3-chloroperbenzoic acid, CH2Cl2; (b) 13, ADDP, nBu3P, THF; (c) acetic anhydride; (d) NaOH aq, MeOH; (e) MnO2, CH2Cl2; (f) diethylaminosulfur trifluoride, CH2Cl2.

Reagents and conditions: (a) 2-chloroquinoline, Pd(PPh3)4, Na2CO3, 1,2-dimethoxyethane, H2O; (b) NaBH4, EtOH; (c) SOCl2, CH2Cl2; (d) 13, K2CO3, DMF.

Reagents and conditions: (a) NaOH aq, MeOH, THF then DMF-DMA, DMF; (b) methyl hydrazine, acetic acid, EtOH; (c) 4-(quinolin-2-yl)benzaldehyde, Ti(OiPr)4, 1,2-dichloroethane; then NaBH4, MeOH; (d) formalin, NaBH(OAc)3, acetic acid, CH2Cl2.

Reagents and conditions: (a) SOCl2, CH2Cl2; (b) DMF-DMA, DMF; (c) hydrazine hydrate, acetic acid, EtOH; (d) acetic anhydride, pyridine; (e) K2CO3, DMF; then MeOH, H2O; (f) 1,1,1-trifluoro-2-iodomethane, Cs2CO3, DMF.
Chart 2 shows the synthesis of compound 14. Suzuki-coupling between boronic acid 10 and 2-chloroquinoline gave alcohol 11. Treatment of 12 with DMF-DMA and subsequent reaction with methylhydrazine afforded alcohol 13, which was reacted with the above-obtained 11 under Mitsunobu-type reaction condition producing 14. Pyrazole analogues with various linkers 16a–e were also synthesized in a similar manner from their precursors 15a–e as described in Chart 3.26) Precursors 15c and d were synthesized via ipso-substitution reactions of 2-chloroquinoline with amines 17 and 18 respectively, and 15e was prepared via Suzuki coupling of 19 with 2-chloroquinoline.
Compounds 23a, b, 24, and 25 were prepared using the synthesis depicted in Chart 4. Quinoline analogues 15a and b were oxidized with 3-chloroperbenzoic acid to afford corresponding N-oxide 20a and b, which were reacted with alcohol 13 under Mitsunobu-type reaction conditions to give ethers 21a and b, respectively. The reaction of 21a and b with acetic anhydride yielded esters 22a and b, which after hydrolysis gave alcohols 23a and b respectively. Oxidation of alcohol 23b with manganese dioxide gave ketone 24, and fluorination of 23b with diethylaminosulfur trifluoride produced 25.
Thiophene analogue 29 was prepared in four steps as outlined in Chart 5. Suzuki coupling between 26 and 2-chloroquinoline generated 27, which afforded alcohol 28 on reduction of the aldehyde group. Treatment of 28 with thionyl chloride followed by reaction with compound 13 gave compound 29.
The synthesis of aminoquinoline analogues 32 and 33 is shown in Chart 6. Treatment of 30 with aqueous sodium hydroxide solution and subsequent DMF-DMA followed by the addition of methylhydrazine yielded amino pyrazole analogue 31, which after reductive alkylation with 4-(quinolin-2-yl)benzaldehyde gave compound 32.27) Further reductive alkylation with formaldehyde afforded compound 33.
Chart 7 shows the synthesis of compound 37. Benzyl chloride 34 was prepared from corresponding alcohol 11. Treatment of 12 with DMF-DMA and subsequent reaction with hydrazine hydrate afforded alcohol 35, which was reacted with acetic anhydride producing 36. Alkylation of 36 with 34 and subsequent hydrolysis followed by reaction with 1,1,1-trifluoro-2-iodomethane gave compound 37.
PDE10A inhibitory potencies of synthesized compounds were tested using in vitro inhibition of human recombinant PDE10A catalyzed cAMP hydrolysis. All compounds in Table 1 which altered pyrazole portion of TP-10 and MP-10 have been already reported in a patent, but the exact PDE10A inhibitory activities and metabolic stabilities were not mentioned there.28) We therefore synthesized the compounds listed in Table 1 and examined their PDE10A inhibitory activity and intrinsic clearances. Among pyrazole derivatives, compounds 38 and 40 had potent activity, but in vitro clearance of all pyrazole analogues remained high. Triazole analogue 41 showed no PDE10A inhibitory activity up to 1000 nM. Compared to TP-10 and MP-10, compound 40 showed comparable potency against PDE10A but increased in vitro microsomal clearance in humans. One possible reason for the conversion of the pyrazole ring having little effect on in vitro metabolic stability is that the rate of formation of compound 1 from MP-10 may be much faster than that of compound 3 from 1. We therefore turned our attention to the investigation of a suitable linker alternative to phenoxymethyl unit of MP-10 in an effort to address clearance issues.
 | ||||
|---|---|---|---|---|
| Compd | A | PDE10A IC50 (nM) | Mouse CLint (mL/min/kg) | Human CLint (mL/min/kg) |
| TP-10 |  | 0.80 | >1000 | 218 |
| MP-10 |  | 0.55 | >1000 | 186 |
| 38 |  | 4.4 | 984 | 274 |
| 39 |  | 183 | 841 | 193 |
| 40 |  | 0.50 | >1000 | 721 |
| 41 | 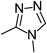 | >1000 | NTa) | NTa) |
a) Not tested.
As shown in Table 2, replacing phenoxymethyl linker of MP-10 with a piperidin-ethyl or piperidine-methyl spacer resulted in a loss of PDE10A inhibitory activity (8 and 9), which indicates that strong basicity of aliphatic amine might have negative impact on in vitro activity. Compound 16a with a non-basic oxy-n-propyl linker showed potent activity in single-digit nanomolar values (IC50=8.9 nM), and increasing the spacer length by one carbon atom maintained PDE10A inhibitory activity (16b). As shown in Fig. 3, superimposition of 16a and b with the conformation of MP-10 binding to PDE10A enzyme derived from Protein Data Bank (PDB code: 3HR1) showed good overlapping of the pyridine and quinoline rings between these three compounds,29) which suggests that phenoxymethyl structure of MP-10 is not essential for PDE10A inhibitory activity and mainly plays a role in assigning pyridine and quinoline rings to the suitable positions. Unfortunately, neither compounds 16a nor b were stable in MLM or HLM. We considered the possibility that a carbon atom adjacent to quinoline ring of compounds 16a and b was metabolized,30) and a hydroxyl group was introduced into this carbon atom of 16a and b to block the possible metabolized position. While compound 23a lost in vitro activity, compound 23b showed only four-fold less potent activity compared to 16b. Although the mouse intrinsic clearance value of 23b was still markedly high, human clearance improved slightly as expected. Conversion of hydroxyl group of 23b into an oxo or fluoro group had a deleterious effect on both in vitro activity and stability in HLM (24 and 25).

Given that the introduction of substituents at carbon atom adjacent to quinoline ring of 16b was not successful with respect to improving metabolic stability and thereby keeping in vitro activity, we next replaced a carbon atom adjacent to quinoline ring of 16b with a nitrogen atom to block possible metabolized part. Although 16c was less active than 16b, slight increase of stability in HLM was observed. We substituted straight alkyl part of 16c with ring structures to further enhance metabolic stability.31) Contrary to our expectation, human CLint of 16d with a piperidine ring was not improved compared to 16c, but moderate PDE10A inhibitory activity of 16d encouraged us to investigate other cyclized linkers. Replacement of piperidine ring of 16d with phenyl ring maintained in vitro activity (14). More importantly, the mouse CLint of 14 was much smaller than those of 16d or MP-10, and the human CLint was also improved over that of MP-10. Decreased electron density of quinoline ring of 14 compared to 16d may prevent metabolic oxidation of the quinoline ring. On comparing MP-10 with 14, differences between linker units were suggested to have little effect on the electron density of the quinoline ring, as Hammett’s substituent constant of phenoxymethyl group differs little from that of the phenyl group,32) which was also supported by the result that atomic charges of quinoline moiety of MP-10 calculated using Molecular Operating Environment (MOE) are little differentiated from that of compound 1433) (Fig. 4). Thus, we assumed that differences in linker units between MP-10 and 14 have little effect on the rate of metabolic oxidation of the quinoline ring in liver microsomes, and the oxymethyl phenyl unit of 14 may be more metabolically stable than phenoxymethyl part of MP-10. Replacement of the phenyl ring with thiophene ring—a bioisostere of the phenyl ring—was well tolerated in terms of PDE10A inhibitory activity (29). Compound 29 also had lower mouse and human CLint values than MP-10. Changing ether linkage of 14 to amine linkage (32 and 33) or changing the substitution position on phenyl ring (16e) resulted in reduced PDE10A inhibitory activity. Replacement of the methyl group on pyrazole ring of 14 with a trifluoroethyl group was investigated in anticipation of showing similar activity to 14. Unlike a relationship between MP-10 and TP-10, compound 37 had less activity than 14 (Table 3). Why 37 had weak activity remains unclear, but 37 might have bound to PDE10A in a different manner from TP-10 and the bulkiness of the trifluoroethyl group may have reduced in vitro activity.

 | ||||
|---|---|---|---|---|
| Compd | Linker | PDE10A IC50 (nM) | Mouse CLint (mL/min/kg) | Human CLint (mL/min/kg) |
| 8 |  | >1000 | NTa) | NTa) |
| 9 |  | >1000 | NTa) | NTa) |
| 16a |  | 8.9 | >1000 | >1000 |
| 16b |  | 8.7 | >1000 | >1000 |
| 23a |  | 719 | NTa) | NTa) |
| 23b |  | 37 | >1000 | 527 |
| 24 |  | 790 | NTa) | NTa) |
| 25 |  | 115 | NTa) | >1000 |
| 16c |  | 102 | NTa) | 660 |
| 16d |  | 19 | >1000 | 589 |
| 14 |  | 29 | 366 | 124 |
| 29 |  | 25 | 556 | 138 |
| 32 |  | 215 | NTa) | NTa) |
| 33 |  | 100 | NTa) | NTa) |
| 16e |  | >100 | NTa) | NTa) |
a) Not tested.
 | ||||
|---|---|---|---|---|
| Compd | R | PDE10A IC50 (nM) | Mouse CLint (mL/min/kg) | Human CLint (mL/min/kg) |
| 14 | Me | 29 | 366 | 124 |
| 37 | –CH2CF3 | 239 | NTa) | NTa) |
a) Not tested.
We obtained compound 14 that showed moderate PDE10A inhibitory activity with improved stability in MLM and HLM. PDE selectivity of 14 was also investigated, with results showing a good profile of greater than 530-fold selectivity over PDE1, 2, 3, 4B, D, 5, and 9 (Table 4). We next examined TP-10 and 14 in a 30 mg/kg single-dose in vivo pharmacokinetic study in mice. As shown in Table 5, compound 14 showed much higher plasma concentrations than those of TP-10 until 2 h after intraperitoneal administration, suggesting that the higher stability of 14 in MLM would contribute to plasma concentration in vivo. In addition, 14 showed better brain permeability in mice (Kp, brain=2.3–3.1) than TP-10. The high blood concentration and good brain permeability of 14 prompted us to assess the in vivo behavioral effect on hyperlocomotion induced by phencyclidine (PCP) in mice, an animal model for positive symptom of schizophrenia. As shown in Fig. 5, compound 14 dose-dependently attenuated the locomotor activity after intraperitoneal administration with an ED50 value of 14 mg/kg. In addition, oral administration of 14 ameliorated hyperlocomotion with an ED50 value of 19 mg/kg, suggesting that 14 had good oral bioavailability in mice.
| Isoform | Selectivity |
|---|---|
| PDE1 | 5700 |
| PDE2 | 2500 |
| PDE3 | 850 |
| PDE4B | 640 |
| PDE4D | 540 |
| PDE5 | 1400 |
| PDE9 | 5700 |
| Time (h) | 0.5 | 1 | 2 | 4 | |
|---|---|---|---|---|---|
| 14 | Plasma conc. (ng/mL) | 9040 | 9100 | 9377 | NTa) |
| Brain conc. (ng/g) | 20383 | 25383 | 29100 | NTa) | |
| TP-10 | Plasma conc. (ng/mL) | NTa) | 4097 | 3263 | 1117 |
| Brain conc. (ng/g) | NTa) | 5807 | 3893 | 1183 |
a) Not tested.

PCP was administered subcutaneously (s.c.). The data represent the mean±S.E.M. (n=6 in each group): ## p<0.01, ### p<0.001 vs. normal group (Student’s t-test); ** p<0.01, *** p<0.001 vs. control group (Dunnett’s test). (A) 14 was administered intraperitoneally (i.p.). (B) 14 was administered orally per os (p.o.).
We next tested the in vivo effect of 14 on novel object recognition task (NORT), which is a model for assessing visual-recognition memory using the natural preference of mice for novelty. While control mice treated neonatally with saline spent nearly 65% of total time exploring the novel object in the test session, mice treated neonatally with PCP spent only approximately 50% of total time exploring the novel object (Fig. 6). This visual-recognition memory impairment in PCP-treated mice was significantly improved by the oral administration of 14 at 0.1 and 0.3 mg/kg, and the effective dose in the NORT was lower than that for the hyperlocomotion model described above, the same tendency as observed following in vivo administration of another PDE10A inhibitor, THPP-1.34) It was reported that PDE10A plays a pivotal role in regulating the tone of dopamine D1 receptor signaling,35) and an “inverted-U” relationship between prefrontal dopamine transmission and cortical efficacy that varies as a function of working memory load was also suggested,36) which may explain the inverted “U-shaped” dose–response curve of 14 in NORT (Fig. 6). In contract, MP-10 administration reportedly induced no significant improvement in NORT in rats, an inefficacy which may be attributed to the administration timing of MP-10 (30 min before training time).12) MP-10 showed high metabolic clearance in rats in our assay (CLint in rat liver microsomes >1000 mL/min/kg). We assumed that brain concentration of MP-10 might be inadequate when visual recognition memory was built in NORT due to high metabolic clearance of MP-10. In our assay, compound 14 was administered 60 min before training time of NORT and showed significant improvement, which suggested that high metabolic stability of 14 contribute an in vivo effect on visual-recognition.

The data represent the mean±S.E.M. (n=12 in each group): ## p<0.01 vs. normal group (Student’s t-test); ** p<0.01 vs. control group (Dunnett’s test).
Here, based on the proposed metabolic pathway of MP-10 in MLM, the phenoxymethyl moiety of MP-10 was modified to increase metabolic stability. Accordingly, the replacement of phenoxymethyl moiety of MP-10 with oxymethyl phenyl unit to afford compound 14 improved metabolic stability in MLM and HLM. Compound 14 had moderate PDE10A inhibitory activity and good selectivity against other PDEs with high plasma concentration and good brain penetration in mice. Additionally, compound 14 dose-dependently attenuated the locomotor activity induced by PCP after intraperitoneal or oral administration in mice, and oral administration of 14 also improved visual-recognition memory impairment in mice at doses of 0.1 and 0.3 mg/kg. These findings suggest that compound 14 therefore may represent a novel lead compound for developing PDE10A inhibitors. Following this study, we next attempted to identify novel compounds with potent activity both in vitro and in vivo, and the results of this research will be reported in due course.
1H-NMR spectra were recorded on a Varian VNS-400, JEOL JNM-LA400, or JEOL JNM-AL400 and the chemical shifts were expressed in δ (ppm) values with tetramethylsilane as an internal reference (s=singlet, d=doublet, t=triplet, m=multiplet, dd=doublet of doublets, tt=triplet of triplets, and br=broad peak). Mass spectra (MS) were recorded on Thermo Electron LCQ Advantage. Elemental analyses were performed using Yanaco MT-6 (C, H, N), Elementar Vario EL III (C, H, X), and Dionex ICS-3000 (S, halogene) and were within ±0.4% of theoretical values. Electrospray ionization (ESI) positive high resolution mass spectrum (HR-MS) was obtained using Waters LCT Premier.
tert-Butyl 4-(Pyridin-4-ylacetyl)piperidine-1-carboxylate (5)Under argon gas atmosphere, to a mixture of 4-methylpyridine (2.63 g, 28.3 mmol) in tetrahydrofuran (THF) (50 mL) cooled with ice-water bath was added lithium diisopropylamide (2.0 M solution in THF/heptane/ethylbenzene; 17.0 mL, 34.0 mmol), and the mixture was stirred at same temperature for 30 min. To the resultant mixture cooled with dryice-acetone bath was slowly added a solution of tert-butyl 4-(N-methoxy-N-methylcarbamoyl)-1-piperidinecarboxylate (4, 5.12 g, 18.8 mmol) in THF (100 mL), and the mixture was stirred at same temperature for 1 h. The mixture was quenched with water and extracted with EtOAc. The organic layer was washed with brine, dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (10–50% EtOAc in CHCl3) to give 5 (4.05 g, 71%) as a yellow oil. 1H-NMR (CDCl3) δ: 1.45 (9H, s), 1.50–1.63 (2H, m), 1.82 (2H, br d, J=12.2 Hz), 2.58 (1H, tt, J=11.2, 3.9 Hz), 2.78 (2H, br t, J=11.2 Hz), 3.76 (2H, s), 4.03–4.21 (2H, m), 7.11–7.14 (2H, m), 8.55–8.58 (2H, m); MS (ESI) m/z 305 [M+H]+.
1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-ol (13)To a solution of ethyl 4-pyridineacetate (12, 25.0 g, 151 mmol) in DMF (100 mL) was added DMF-DMA (50.4 mL, 378 mmol), and the mixture was stirred at 80°C for 2 h. The reaction was cooled at room temperature and concentrated in vacuo. To the residue in ethanol (250 mL) cooled with ice-water bath were added methylhydrazine (15.9 mL, 302 mmol) and acetic acid (60 mL), and the mixture was stirred at room temperature for 16 h. The mixture was concentrated in vacuo, and the residue was purified by silica gel column chromatography (0–10% MeOH in CHCl3) to give crude product, which was washed with EtOAc–hexane to give an orange solid. The orange solid was purified again by silica gel column chromatography (0–10% MeOH in CHCl3) to give 13 (15.3 g, 58%) as a pale yellow solid. 1H-NMR (DMSO-d6) δ: 3.67 (3H, s), 7.55–7.59 (2H, m), 8.09 (1H, s), 8.39–8.44 (2H, m), 10.71 (1H, br s); MS (ESI) m/z 176 [M+H]+.
tert-Butyl 4-[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]piperidine-1-carboxylate (6)Compound 6 was prepared from 5 in a manner similar to that described for compound 13, with a yield of 20% as a colorless solid. 1H-NMR (CDCl3) δ: 1.46 (9H, s), 1.72–1.89 (4H, m), 2.66–2.90 (2H, m), 2.93–3.04 (1H, m), 3.90 (3H, s), 4.19 (2H, br s), 7.22–7.25 (2H, m), 7.47 (1H, s), 8.56–8.61 (2H, m); MS (ESI) m/z 343 [M+H]+.
4-[1-Methyl-3-(piperidin-4-yl)-1H-pyrazol-4-yl]pyridine Dihydrochloride (7)To a mixture of 6 (342 mg, 1.00 mmol) in MeOH (7.3 mL) was added 4 M HCl/dioxane (3.6 mL, 14.4 mmol), and the mixture was stirred at 50°C for 1 h. After cooling at room temperature, the mixture was concentrated in vacuo to give 7 (315 mg, quant) as a pale yellow solid. 1H-NMR (DMSO-d6) δ: 1.88–2.06 (4H, m), 3.00–3.12 (2H, m), 3.26–3.50 (3H, m), 3.90 (3H, s), 8.07 (2H, d, J=6.7 Hz), 8.58 (1H), 8.78 (2H, d, J=6.7 Hz), 8.97 (1H, br s), 9.24 (1H, br s); MS (ESI) m/z 398 [M+H]+.
2-(2-{4-[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]piperidin-1-yl}ethyl)quinoline Trihydrochloride (8)To a solution of 7 (100 mg, 0.32 mmol) in MeOH (30 mL) was added triethylamine (90 µL, 0.65 mmol), and the mixture was concentrated in vacuo. To the residue in ethanol (2.0 mL) were added 2-vinylquinoline (100 mg, 0.65 mmol) and acetic acid (30 µL, 0.53 mmol), and the mixture was stirred at 80°C for 12 h. After cooling at room temperature, the mixture was partitioned between saturated NaHCO3 aqueous solution and EtOAc. The organic layer was washed with brine, dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (1–10% MeOH in CHCl3) to give a pale brown solid, which was dissolved in MeOH (5.0 mL) and 4 M HCl/dioxane (0.25 mL, 1.00 mmol) was added to the mixture. The mixture was concentrated in vacuo, and the residue was washed with diisopropylether to give 8 (21 mg, 13%) as a dark green solid. 1H-NMR (DMSO-d6) δ: 2.04–2.26 (4H, m), 3.27 (2H, br s), 3.45 (1H, br s), 3.69 (6H, br s), 3.91 (3H, s), 7.71–7.80 (2H, m), 7.90–7.97 (1H, m), 8.07 (2H, d, J=6.6 Hz), 8.13 (1H, d, J=8.0 Hz), 8.21 (1H, d, J=8.3 Hz), 8.58 (1H, s), 8.68 (1H, d, J=8.0 Hz), 8.79 (2H, d, J=6.6 Hz); MS (ESI) m/z 398 [M+H]+. Anal. Calcd for C25H27N5·3HCl·4.3H2O·0.3C6H14O: C, 52.34; H, 7.01; N, 11.39; Cl, 17.29. Found: C, 52.44; H, 6.81; N, 11.09; Cl, 16.98.
2-({4-[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]piperidin-1-yl}methyl)quinoline Trihydrochloride (9)To a mixture of 7 (144 mg, 0.46 mmol) and 2-(chloromethyl)quinoline (100 mg, 0.47 mmol) in DMF (8.0 mL) was added N-ethyl-N-isopropylpropan-2-amine (0.33 mL, 1.90 mmol), and the mixture was stirred at 60°C for 4 h. After cooling at room temperature, the mixture was diluted with EtOAc and washed with saturated NaHCO3 aqueous solution and brine, dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (1–9% MeOH in CHCl3) to give a pale yellow solid, which was dissolved in MeOH (5 mL) and 4 M HCl/EtOAc (0.35 mL) was added to the mixture. The mixture was concentrated in vacuo to give 9 (47 mg, 21%) as a beige solid. 1H-NMR (DMSO-d6) δ: 2.04–2.29 (4H, m), 3.28–3.47 (3H, m), 3.53–3.63 (2H, m), 3.99 (3H, s), 4.67 (2H, s), 7.70 (1H, dd, J=7.2, 7.2 Hz), 7.82–7.89 (1H, m), 7.94 (1H, d, J=8.5 Hz), 8.02–8.13 (4H, m), 8.54 (1H, d, J=8.5 Hz), 8.58 (1H, s), 8.77 (2H, d, J=6.7 Hz); MS (ESI) m/z 384 [M+H]+. Anal. Calcd for C24H25N5·3HCl·4.1H2O: C, 50.86; H, 6.44; N, 12.36; Cl, 18.77. Found: C, 51.01; H, 6.41; N, 12.24; Cl, 18.56.
[4-(Quinolin-2-yl)phenyl]methanol (11)Under argon gas atmosphere, to a mixture of 2-chloroquinoline (1.05 g, 6.42 mmol), [4-(hydroxymethyl)phenyl]boronic acid (10, 976 mg, 6.42 mmol) and Pd(PPh3)4 (384 mg, 0.33 mmol) in 1,2-dimethoxyethane (15 mL) and water (5 mL) was added Na2CO3 (1.65 g, 15.6 mmol), and the mixture was stirred at 100°C for 14 h. After cooling at room temperature, the mixture was partitioned between EtOAc and water. The organic layer was dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (10–100% EtOAc in hexane) to give 11 (1.50 g, 99%) as an off-white solid. 1H-NMR (DMSO-d6) δ: 4.60 (2H, d, J=5.7 Hz), 5.28 (1H, t, J=5.7 Hz), 7.50 (2H, d, J=8.2 Hz), 7.56–7.62 (1H, m), 7.75–7.61 (1H, m), 7.99 (1H, d, J=8.1 Hz), 8.07 (1H, d, J=8.5 Hz), 8.14 (1H, d, J=8.7 Hz). 8.25 (2H, d, J=8.2 Hz), 8.44 (1H, d, J=8.7 Hz); MS (ESI) m/z 236 [M+H]+.
[3-(Quinolin-2-yl)phenyl]methanol (15e)Compound 15e was prepared from [3-(hydroxymethyl)phenyl]boronic acid (19) and 2-chloroquinoline in a manner similar to that described for compound 11, with a quantitative yield as a yellow oil. 1H-NMR (CDCl3) δ: 2.13 (1H, br s), 4.81 (2H, br s), 7.43–7.56 (3H, m), 7.71–7.76 (1H, m), 7.83 (1H, d, J=8.1 Hz), 7.87 (1H, d, J=8.6 Hz), 8.03–8.07 (1H, m), 8.16–8.24 (3H, m); MS (ESI) m/z 236 [M+H]+.
2-[3-({[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}methyl)phenyl]quinoline Dihydrochloride (16e)To a mixture of 13 (200 mg, 1.14 mmol), 15e (295 mg, 1.26 mmol) and tributylphosphine (346 mg, 1.71 mmol) in THF (20 mL) was added 1,1′-(azodicarbonyl)dipiperidine (ADDP; 432 mg, 1.71 mmol), and the mixture was stirred at room temperature for 14 h before the mixture was concentrated in vacuo. The residue was suspended in EtOAc, and the insoluble material was removed by filtration. The filtrate was concentrated in vacuo, and the residue was purified by silica gel column chromatography (0–3% MeOH in CHCl3) to give a colorless oil, which was dissolved in EtOAc (20 mL). To the mixture was added 4 M HCl/EtOAc (1.1 mL) and the precipitate was collected by filtration give 16e (289 mg, 54%) as an off-white solid. 1H-NMR (DMSO-d6) δ: 3.85 (3H, s), 5.55 (2H, s), 7.63–7.75 (3H, m), 7.86–7.92 (1H, m), 8.11 (1H, s, J=8.1 Hz), 8.15 (2H, d, J=7.0 Hz), 8.23–8.31 (3H, m), 8.45 (1H, s), 8.67 (1H, d, J=8.7 Hz), 8.70–8.74 (3H, m); MS (ESI) m/z 393 [M+H]+. Anal. Calcd for C25H20N4O·2HCl·0.9H2O: C, 62.35; H, 4.98; N, 11.63; Cl, 14.72. Found: C, 62.70; H, 5.31; N, 11.45; Cl, 14.83.
2-[4-({[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}methyl)phenyl]quinoline Dihydrochloride (14)Compound 14 was prepared from 11 and 13 in a manner similar to that described for compound 16e, with a yield of 39% as an off-white solid. 1H-NMR (DMSO-d6) δ: 3.84 (3H, s), 5.52 (2H, s), 7.66–7.72 (1H, m), 7.74 (2H, d, J=8.3 Hz), 7.85–7.91 (1H, m), 8.10 (1H, d, J=8.1 Hz), 8.15 (2H, d, J=7.0 Hz), 8.20–8.27 (2H, m), 8.34 (2H, d, J=8.3 Hz), 8.64 (1H, d, J=8.7 Hz), 8.71 (1H, s), 8.74 (2H, d, J=7.0 Hz) ; MS (ESI) m/z 393 [M+H]+. Anal. Calcd for C25H20N4O·2HCl·3.1H2O: C, 57.61; H, 5.45; N, 10.75; Cl, 13.60. Found: C, 57.76; H, 5.51; N, 10.76; Cl, 13.54.
[1-(Quinolin-2-yl)piperidin-4-yl]methanol (15d)To a mixture of 2-chloroquinoline (2.50 g, 15.3 mmol) and piperidine-4-ylmethanol (18, 5.28 g, 45.8 mmol) in DMF (20 mL) was added K2CO3 (3.17 g, 22.9 mmmol), and the mixture was stirred at 110°C for 1 d. After cooling at room temperature, the mixture was concentrated in vacuo. The residue was partitioned between EtOAc and water, and the organic layer was concentrated in vacuo. The residue was purified by silica gel column chromatography (20–50% EtOAc in hexane) to give 15e (3.69 g, quant) as a pale yellow solid. 1H-NMR (DMSO-d6) δ: 1.08–1.20 (2H, m), 1.62–1.80 (3H, m), 2.84–2.92 (2H, m), 3.28 (2H, t, J=5.8 Hz), 4.47 (1H, t, J=5.3 Hz), 4.51–4.58 (2H, m), 7.15–7.21 (1H, m), 7.23 (1H, d, J=9.2 Hz), 7.46–7.56 (2H, m), 7.64–7.68 (1H, m), 7.98 (1H, d, J=9.2 Hz); MS (ESI) m/z 243 [M+H]+.
3-[Methyl(Quinolin-2-yl)amino]propan-1-ol (15c)Compound 15c was prepared from 17 and 2-chloroquinoline in a manner similar to that described for compound 15d, with a yield of 68% as a colorless oil. 1H-NMR (DMSO-d6) δ: 1.70–1.78 (2H, m), 3.13 (3H, s), 3.42–3.48 (2H, m), 3.67 (2H, t, J=7.0 Hz), 4.69 (1H, t, J=5.3 Hz), 7.07 (1H, d, J=9.2 Hz), 7.13–7.18 (1H, m), 7.46–7.52 (2H, m), 7.64–7.68 (1H, m), 7.99 (1H, d, J=9.1 Hz); MS (ESI) m/z 217 [M+H]+.
2-(3-{[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}propyl)quinoline Dihydrochloride (16a)Compound 16a was prepared from 13 and 15a in a manner similar to that described for compound 16e, with a yield of 41% as a yellow oil. 1H-NMR (DMSO-d6) δ: 2.40–2.50 (2H, m), 3.47–3.55 (2H, m), 3.79 (3H, s), 4.46 (2H, t, J=5.8 Hz), 7.82 (1H, t, J=7.6 Hz), 7.94–8.09 (4H, m), 8.19 (1H, d, J=8.3 Hz), 8.46 (1H, brd, J=8.3 Hz), 8.59 (1H, s), 8.64 (2H, d, J=7.0 Hz), 8.92 (1H, br d, J=8.3 Hz); MS (ESI) m/z 345 [M+H]+. Anal. Calcd for C21H20N4O·2HCl·3H2O: C, 53.51; H, 5.99; N, 11.89; Cl, 15.04. Found: C, 53.56; H, 6.03; N, 11.82; Cl, 15.22.
2-(4-{[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}butyl)quinoline Dihydrochloride (16b)Compound 16b was prepared from 13 and 15b26) in a manner similar to that described for compound 16e, with a yield of 61% as a pale pink solid. 1H-NMR (DMSO-d6) δ: 1.87–1.98 (2H, m), 2.03–2.14 (2H, m), 3.35 (2H, t, J=7.5 Hz), 3.76 (3H, s), 4.35 (2H, t, J=6.2 Hz), 7.86 (1H, dd, J=7.5, 7.5 Hz), 7.98 (1H, d, J=8.3 Hz), 8.06 (1H, t, J=7.8 Hz), 8.11 (2H, d, J=7.0 Hz), 8.25 (1H, d, J=8.1 Hz), 8.44 (1H, d, J=8.1 Hz), 8.61 (1H, s), 8.73 (2H, d, J=7.0 Hz), 8.96 (1H, br d); MS (ESI) m/z 359 [M+H]+. Anal. Calcd for C22H22N4O·2HCl·3H2O: C, 54.44; H, 6.23; N, 11.54; Cl, 14.61. Found: C, 54.30; H, 5.97; N, 11.51; Cl, 14.71.
N-Methyl-N-(3-{[1-methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}propyl)quinolin-2-amine Dihydrochloride (16c)Compound 16c was prepared from 13 and 15c in a manner similar to that described for compound 16e, with a yield of 23% as an off-white solid. 1H-NMR (DMSO-d6) δ: 2.22–2.32 (2H, m), 3.44 (3H, s), 3.73 (3H, s), 4.02–4.14 (2H, m), 4.46 (2H, t, J=5.9 Hz), 7.47–7.52 (2H, m), 7.73–7.79 (1H, m), 7.88–7.92 (1H, m), 8.01 (2H, d, J=6.6 Hz), 8.16 (1H, d, J=8.3 Hz), 8.35 (1H, d, J=9.8 Hz), 8.55 (1H, s), 8.65 (2H, d, J=6.6 Hz); MS (ESI) m/z 374 [M+H]+. Anal. Calcd for C22H23N5O·2HCl·0.8H2O: C, 57.34; H, 5.82; N, 15.20; Cl, 15.39. Found: C, 57.44; H, 5.76; N, 15.16; Cl, 15.63.
2-[4-({[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}methyl)piperidin-1-yl]quinoline Dihydrochloride (16d)Compound 16d was prepared from 13 and 15d in a manner similar to that described for compound 16e, with a yield of 56% as a colorless solid. 1H-NMR (DMSO-d6) δ: 1.49–1.61 (2H, m), 2.00–2.08 (2H, m), 2.31–2.43 (1H, m), 3.40–3.50 (2H, m), 3.80 (3H, s), 4.24 (2H, d, J=6.6 Hz), 4.76 (2H, br d J=12.4 Hz), 7.47–7.54 (1H, m), 7.65 (1H, d, J=9.8 Hz), 7.75–7.81 (1H, m), 7.93 (1H, dd, J=8.0, 1.2 Hz), 8.11 (2H, d, J=7.0 Hz), 8.41–8.50 (2H, m), 8.69 (1H, s), 8.73 (2H, d, J=7.0 Hz); MS (ESI) m/z 400 [M+H]+. Anal. Calcd for C24H25N5O·2.2HCl·3.1H2O: C, 53.82; H, 6.29; N, 13.08; Cl, 14.56. Found: C, 53.87; H, 6.28; N, 12.96; Cl, 14.78.
4-(1-Oxidoquinolin-2-yl)butan-1-ol (20b)To a mixture of 15b26) (1.49 g, 7.38 mmol) in CH2Cl2 (30 mL) was added 3-chloroperbenzoic acid (75% purity; 2.21 g, 9.59 mmol), and the mixture was stirred at room temperature for 4 h. The mixture was partitioned between CHCl3 and 1 M NaOH aqueous solution, and the organic layer was dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (0–3% MeOH in CHCl3) to give 20b (1.38 g, 86%) as a pale yellow solid. 1H-NMR (DMSO-d6) δ: 1.47–1.56 (2H, m), 1.71–1.82 (2H, m), 3.02 (2H, t, J=7.7 Hz), 3.42–3.48 (2H, m), 4.42 (1H, t, J=5.2 Hz), 7.54 (1H, d, J=8.6 Hz), 7.65–7.72 (1H, m), 7.77–7.83 (1H, m), 7.87 (1H, d, J=8.6 Hz), 8.05 (1H, d, J=8.1 Hz), 8.58 (1H, d, J=8.7 Hz); MS (ESI) m/z 218 [M+H]+.
3-(1-Oxidoquinolin-2-yl)propan-1-ol (20a)Compound 20a was prepared from 15a in a manner similar to that described for compound 20b, with a yield of 99% as a brown solid. 1H-NMR (DMSO-d6) δ: 1.84–1.95 (2H, m), 3.06 (2H, t, J=7.5 Hz), 3.45–3.52 (2H, m), 4.65 (1H, t, J=5.3 Hz), 7.54 (1H, d, J=8.6 Hz), 7.66–7.72 (1H, m), 7.78–7.84 (1H, m), 7.88 (1H, d, J=8.6 Hz), 8.05 (1H, d, J=8.1 Hz), 8.58 (1H, d, J=8.6 Hz); MS (ESI) m/z 204 [M+H]+.
2-(4-{[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}butyl)quinoline 1-Oxide (21b)To a mixture of 13 (930 mg, 5.31 mmol), 20b (1.27 g, 5.84 mmol) and tributylphosphine (2.15 g, 10.6 mmol) in THF (93 mL) was added ADDP (2.68 g, 10.6 mmol), and the mixture was stirred at room temperature for 2 h before the mixture was concentrated in vacuo. The residue was suspended in toluene and the precipitate was removed by filtration. The filtrate was concentrated in vacuo and the residue was purified by silica gel column chromatography (0–50% EtOAc in CHCl3) to give 21b (1.50 g, 76%) as a pale yellow solid. 1H-NMR (DMSO-d6) δ: 1.82–1.98 (4H, m), 3.11 (2H, t, J=7.2 Hz), 3.72 (3H, s), 4.29 (2H, t, J=6.0 Hz), 7.52–7.60 (3H, m), 7.65–7.71 (1H, m), 7.77–7.84 (1H, m), 7.87 (1H, d, J=8.6 Hz), 8.05 (1H, d, J=8.1 Hz), 8.20 (1H, s), 8.40–8.45 (2H, m), 8.57 (1H, d, J=8.6 Hz); MS (ESI) m/z 375 [M+H]+.
2-(3-{[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}propyl)quinoline 1-Oxide (21a)Compound 21a was prepared from 13 and 20a in a manner similar to that described for compound 21b, with a yield of 78% as a pale yellow solid. 1H-NMR (DMSO-d6) δ: 2.25–2.34 (2H, m), 3.21 (2H, t, J=7.4 Hz), 3.72 (3H, s), 4.33 (2H, t, J=6.2 Hz), 7.45–7.50 (2H, m), 7.57 (1H, d, J=8.6 Hz), 7.65–7.71 (1H, m), 7.78–7.84 (1H, m), 7.85 (1H, d, J=8.6 Hz), 8.00–8.05 (1H, m), 8.20 (1H, s), 8.34 (2H, d, J=5.6 Hz), 8.58 (1H, d, J=8.8 Hz); MS (ESI) m/z 361 [M+H]+.
4-{[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}-1-(quinolin-2-yl)butyl Acetate (22b)A mixture of 21b (1.40 g, 3.74 mmol) and acetic anhydride (14.2 mL, 150 mmol) was stirred at 80°C for 4 h. After cooling at room temperature, the mixture was concentrated in vacuo. The residue was purified by NH silica gel column chromatography (0–50% EtOAc in CHCl3) to give 22b (902 mg, 58%) as a yellow oil. 1H-NMR (DMSO-d6) δ: 1.82–1.91 (1H, m), 2.15 (3H, s), 3.26–3.32 (4H, m), 3.70 (3H, s), 4.26 (2H, t, J=6.3 Hz), 7.52–7.64 (4H, m), 7.75–7.80 (1H, m), 7.97–8.01 (2H, m, J=9.3), 8.19 (1H, s), 8.38–8.44 (3H, m); MS (ESI) m/z 417 [M+H]+.
3-{[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}-1-(quinolin-2-yl)propyl Acetate (22a)Compound 22a was prepared from 21a in a manner similar to that described for compound 22b, with a yield of 77% as a yellow oil. 1H-NMR (DMSO-d6) δ: 2.13 (3H, s), 3.27–3.35 (2H, m), 3.71 (3H, s), 4.34–4.40 (2H, m), 6.04–6.10 (1H, m), 7.42–7.46 (2H, m), 7.56–7.63 (2H, m), 7.74–7.80 (1H, m), 7.94–8.03 (2H, m), 8.19 (1H, s), 8.31–8.35 (2H, m), 8.38 (1H, d, J=8.5 Hz); MS (ESI) m/z 403 [M+H]+.
4-{[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}-1-(quinolin-2-yl)butan-1-ol (23b)To a mixture of 22b (895 mg, 2.15 mmol) in MeOH (13 mL) was added 1 M NaOH aqueous solution (6.45 mL, 6.45 mmol), and the mixture was stirred at room temperature for 3 h. The mixture was partitioned between CHCl3 and brine, and the organic layer was dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (1–5% MeOH in EtOAc) to give 23b (444 mg, 55%) as a pale yellow solid. 1H-NMR (DMSO-d6) δ: 1.80–2.07 (4H, m), 3.71 (3H, s), 4.25 (2H, t, J=6.0 Hz), 4.80–4.85 (1H, m), 5.63 (1H, d, J=4.8 Hz), 7.53–7.60 (3H, m), 7.70 (1H, d, J=8.5 Hz), 7.71–7.76 (1H, m), 7.94–7.98 (2H, m), 8.19 (1H, s), 8.36 (1H, d, J=8.5 Hz), 8.41–8.44 (2H, m); MS (ESI) m/z 375 [M+H]+. Anal. Calcd for C22H22N4O2·0.1H2O: C, 70.23; H, 5.95; N, 14.89. Found: C, 70.12; H, 5.90; N, 14.79.
3-{[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}-1-(quinolin-2-yl)propan-1-ol (23a)Compound 23a was prepared from 22a in a manner similar to that described for compound 23b, with a yield of 58% as a pale yellow solid. 1H-NMR (DMSO-d6) δ: 2.17–2.28 (1H, m), 2.35–2.46 (1H, m), 3.72 (3H, s), 4.35–4.45 (2H, m), 4.96–5.02 (1H, m), 5.76 (1H, d, J=4.9 Hz), 7.44–7.48 (2H, m), 7.54–7.60 (1H, m), 7.71–7.77 (2H, m), 7.92–8.00 (2H, m), 8.19 (1H, s), 8.33–8.38 (3H, m); MS (ESI) m/z 361 [M+H]+. Anal. Calcd for C21H20N4O2: C, 69.98; H, 5.59; N, 15.55. Found: C, 69.99; H, 5.61; N, 15.48.
4-{[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}-1-(quinolin-2-yl)butan-1-one (24)To a solution of 23b (312 mg, 0.83 mmol) in CH2Cl2 (31 mL) was added MnO2 (290 mg, 3.33 mmol), and the mixture was stirred at room temperature for 1 d. The mixture was filtered through celite pad and concentrated in vacuo. The residue was purified by silica gel column chromatography (0–5% MeOH in EtOAc) to give a solid, which was triturated with EtOAc to give 24 (138 mg, 45%) as a colorless solid. 1H-NMR (DMSO-d6) δ: 2.20–2.28 (2H, m), 3.56 (2H, t, J=7.1 Hz), 3.70 (3H, s), 4.36 (2H, t, J=6.3 Hz), 7.52–7.55 (2H, m), 7.73–7.78 (1H, m), 7.85–7.90 (1H, m), 8.04–8.11 (2H, m), 8.13 (1H, d, J=8.5 Hz), 8.19 (1H, s), 8.37–8.41 (2H, m), 8.54 (1H, d, J=8.5 Hz); MS (ESI) m/z 373 [M+H]+. Anal. Calcd for C22H20N4O2·0.35H2O: C, 69.77; H, 5.51; N, 14.79. Found: C, 69.85; H, 5.38; N, 14.68.
2-(1-Fluoro-4-{[1-methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}butyl)quinoline Dihydrochloride (25)Under argon gas atmosphere, to a mixture of 23 (128 mg, 0.34 mmol) in CH2Cl2 (5.1 mL) cooled with dryice-acetone bath was added diethylaminosulfur trifluoride (72 mg, 0.44 mmol), and the mixture was stirred at same temperature for 2 h and at 0°C for another 6 h. The reaction was quenched with water and extracted with CHCl3. The organic layer was washed with saturated NaHCO3 aqueous solution and brine, dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (0–5% MeOH in EtOAc) to give a colorless oil (28 mg), which was dissolved in EtOAc (3.8 mL) and 4 M HCl/EtOAc (85 µL, 0.34 mmol) was added to the mixture. The mixture was stirred at room temperature for 2 h, and the precipitate was collected by filtration to give 25 (21 mg, 14%) as a pale yellow solid. 1H-NMR (DMSO-d6) δ: 1.95–2.06 (2H, m), 2.19–2.35 (2H, m), 3.77 (3H, s), 4.38 (2H, t, J=6.5 Hz), 5.79–5.97 (1H, m), 7.63–7.69 (1H, m), 7.72 (1H, d, J=8.4 Hz), 7.79–7.84 (1H, m), 8.03 (2H, dd, J=8.7, 8.7 Hz), 8.09 (2H, d, J=7.0 Hz), 8.52 (1H, d, J=8.6 Hz), 8.62 (1H, s), 8.70 (2H, d, J=7.0 Hz); MS (ESI) m/z 377 [M+H]+; HR-MS Calcd for C22H21FN4O [M+H]+ 377.1778. Found 377.1780.
5-(Quinolin-2-yl)thiophene-2-carbaldehyde (27)Compound 27 was prepared from 5-formyl-2-thienylboronic acid (26) and 2-chloroquinoline in a manner similar to that described for compound 11, with a yield of 43% as a beige solid. 1H-NMR (DMSO-d6) δ: 7.61–7.67 (1H, m), 7.78–7.84 (1H, m), 8.00–8.06 (2H, m), 8.12 (1H, d, J=4.0 Hz), 8.20 (1H, d, J=4.0 Hz), 8.26 (1H, d, J=8.6 Hz), 8.51 (1H, d, J=8.6 Hz), 9.99 (1H, s); MS (EI) m/z 239 [M]+.
[5-(Quinolin-2-yl)-2-thienyl]methanol (28)To a mixture of 27 (172 mg, 0.72 mmol) in EtOH (5.2 mL) cooled with ice-water bath was added NaBH4 (27 mg, 0.72 mmol), and the mixture was stirred at same temperature for 2 h. The mixture was partitioned between EtOAc and a 1 : 1 mixture of brine and water, and the organic layer was dried over MgSO4, filtered and concentrated in vacuo to give 28 (171 mg, 99%) as a pale yellow solid. 1H-NMR (DMSO-d6) δ: 4.68 (2H, dd, J=5.7, 0.8 Hz), 5.57 (1H, t, J=5.7 Hz), 7.03–7.06 (1H, m), 7.52–7.58 (1H, m), 7.71–7.77 (1H, m), 7.83 (1H, d, J=3.7 Hz), 7.91–7.96 (2H, m), 8.06 (1H, d, J=8.7 Hz), 8.37 (1H, d, J=8.5 Hz); MS (ESI) m/z 242 [M+H]+.
2-[5-({[1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-yl]oxy}methyl)-2-thienyl]quinoline Hemisuccinate (29)To a solution of 28 (320 mg, 1.33 mmol) in CH2Cl2 (6.4 mL) was added SOCl2 (0.29 mL, 3.97 mmol), and the mixture was stirred at room temperature for 3 h before the addition of toluene. The precipitate was collected by filtration to give a beige solid (302 mg). To a mixture of above-obtained beige solid and 13 (179 mg, 1.02 mmol) in DMF (6.0 mL) was added K2CO3 (352 mg, 2.55 mmol), and the mixture was stirred at 60°C for 7 h. After cooling at room temperature, the mixture was partitioned between EtOAc and a 1 : 1 mixture of brine and water, and the aqueous layer was extracted with EtOAc. The combined organic layer was dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (0–10% MeOH in CHCl3) to give a beige oil (213 mg), which was dissolved in EtOAc (9.0 mL). To the solution was added succinic acid (35 mg), and the mixture was stirred at room temperature for 1 h. The precipitate was collected by filtration to give 29 (205 mg, 39%) as a beige solid. 1H-NMR (DMSO-d6) δ: 2.42 (2H, s), 3.80 (3H, s), 5.55 (2H, s), 7.34 (1H, d, J=3.7 Hz), 7.53–7.61 (3H, m), 7.72–7.79 (1H, m), 7.92 (1H, d, J=3.7 Hz), 7.93–7.99 (2H, m), 8.11 (1H, d, J=8.7 Hz), 8.29 (1H, s), 8.40 (1H, d, J=8.6 Hz), 8.44–8.50 (2H, m), 12.16 (1H, br s); MS (ESI) m/z 399 [M+H]+. Anal. Calcd for C23H18N4OS·0.5C4H6O4: C, 65.63; H, 4.63; N, 12.25; S, 7.01. Found: C, 65.37; H, 4.75; N, 12.16; S, 6.98.
1-Methyl-4-(pyridin-4-yl)-1H-pyrazol-3-amine (31)To a mixture of pyridin-4-ylacetonitrile hydrochloride (30, 2.01 g, 13.0 mmol) in THF (30 mL) and ethanol (30 mL) was added 1 M NaOH aqueous solution (13.0 mL, 13.0 mmol), and the mixture was filtered and the filtrate was concentrated in vacuo. To the residue dissolved in DMF (19 mL) was added DMF-DMA (3.48 mL, 26.0 mmol), and the mixture was stirred at 80°C for 1 h. After cooling at room temperature, the mixture was concentrated in vacuo. To the residue in MeOH (20 mL) were added acetic acid (780 mg, 13.0 mmol) and methylhydrazine (826 µL, 15.6 mmol), and the mixture was stirred at 60°C for 12 h. After cooling at room temperature, the mixture was concentrated in vacuo. The residue was purified by silica gel column chromatography (1–10% MeOH in CHCl3) to give 31 (400 mg, 18%) as an orange solid. 1H-NMR (DMSO-d6) δ: 3.64 (3H, s), 4,90 (2H, s), 7.44–7.47 (2H, m), 7.96 (1H, s), 8.40–8.43 (2H, m); MS (ESI) m/z 175 [M+H]+.
1-Methyl-4-(pyridin-4-yl)-N-[4-(quinolin-2-yl)benzyl]-1H-pyrazol-3-amine (32)To a mixture of 4-(quinolin-2-yl)benzaldehyde27) (284 mg, 1.22 mmol) and 31 (177 mg, 1.02 mmol) in 1,2-dichloroethane (1.0 mL) was added titanium(IV) isopropoxide (0.45 mL, 1.53 mmol), and the mixture was stirred at 85°C for 2 h. To the resultant mixture cooled with ice-water bath were added MeOH (5 mL) and NaBH4 (270 mg, 7.14 mmol), and the mixture was stirred at room temperature for 5 h. The reaction was quenched with saturated NaHCO3 aqueous solution and diluted with CHCl3. The mixture was filtered through celite pad and the organic layer of the filtrate was washed with brine, dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (1–7% MeOH in CHCl3) to give 32 (138 mg, 35%) as a pale yellow solid. 1H-NMR (DMSO-d6) δ: 3.67 (3H, s), 4.46 (2H, d, J=6.0 Hz), 5.95 (1H, t, J=6.0 Hz), 7.50–7.53 (2H, m), 7.55–7.61 (3H, m), 7.75–7.80 (1H, m), 7.97–8.01 (2H, m), 8.06 (1H, d, J=8.4 Hz), 8.13 (1H, d, J=8.7 Hz), 8.20–8.24 (2H, m), 8.42–8.47 (3H, m); MS (ESI) m/z 392 [M+H]+. Anal. Calcd for C25H21N5·1.3H2O·0.1CHCl3: C, 70.63; H, 5.60; N, 16.41. Found: C, 70.90; H, 5.37; N, 16.17.
N,1-Dimethyl-4-(pyridin-4-yl)-N-[4-(quinolin-2-yl)benzyl]-1H-pyrazol-3-amine Dihydrochloride (33)To a solution of 32 (77 mg, 0.20 mmol) and acetic acid (0.80 mL) in CH2Cl2 (4.0 mL) was added 36% formaldehyde aqueous solution (150 µL, 1.96 mmol), and the mixture was stirred at room temperature for 10 min. To the resultant mixture was added sodium triacetoxyborohydride (167 mg, 0.79 mmol), and the mixture was stirred at room temperature for 2 h. To the resultant mixture were added 36% formaldehyde aqueous solution (150 µL, 1.96 mmol) and sodium triacetoxyborohydride (167 mg, 0.79 mmol), and the mixture was stirred at room temperature overnight. To the resultant mixture were added 36% formaldehyde aqueous solution (150 µL, 1.96 mmol) and sodium triacetoxyborohydride (167 mg, 0.79 mmol) again, and the mixture was stirred at room temperature for 12 h. The reaction was quenched with saturated NaHCO3 aqueous solution and extracted with CHCl3. The organic layer was dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (1–7% MeOH in CHCl3) to give a pale yellow amorphous, which was dissolved in MeOH (5 mL) and 4 M HCl/EtOAc (0.15 mL, 0.60 mmol) was added to the mixture. The mixture was concentrated in vacuo, and the residue was washed with diisopropylether/2-propanol to give a yellow solid. 1H-NMR (DMSO-d6) δ: 2.68 (3H, s), 3.82 (3H, s), 4.32 (2H, s), 7.54 (2H, d, J=8.3 Hz), 7.65–7.70 (1H, m), 7.83–7.89 (1H, m), 8.08 (1H, d, J=8.0 Hz), 8.17–8.27 (6H, m), 8.58–8.63 (m, 2H), 8.78 (2H, d, J=7.0 Hz); MS (ESI) m/z 406 [M+H]+. Anal. Calcd for C26H23N5·2HCl·2.5H2O·0.75C3H8O: C, 59.68; H, 6.38; N, 12.32; Cl, 12.47. Found: C, 59.91; H, 6.42; N, 12.18; Cl, 12.24.
2-[4-(Chloromethyl)phenyl]quinoline Hydrochloride (34)To a mixture of 11 (500 mg, 2.13 mmol) in CH2Cl2 (7.5 mL) was added SOCl2 (758 mg, 6.37 mmol), and the mixture was stirred at r.t for 2 h. To the reaction mixture was added toluene and the precipitate was collected by filtration to give 2-[4-(chloromethyl)phenyl]quinoline hydrochloride (616 mg, quant) as a colorless solid. 1H-NMR (DMSO-d6) δ: 4.88 (2H, s), 7.64–7.71 (3H, m), 7.85–7.90 (1H, m), 8.07–8.11 (1H, m), 8.19 (1H, d, J=8.5 Hz), 8.23 (1H, d, J=8.7 Hz), 8.27–8.31 (2H, m), 8.63 (1H, d, J=8.7 Hz); MS (ESI) m/z 254, 256 [M+H]+.
4-(Pyridin-4-yl)-1H-pyrazol-3-ol (35)Compound 35 was prepared from 12 and hydrazine hydrate in a manner similar to that described for compound 13, with a yield of 80% as a pink solid. 1H-NMR (DMSO-d6) δ: 7.65 (2H, s), 8.12 (1H, s), 8.38 (2H, s), 10.6 (1H, br s), 12.1 (1H, br s); MS (ESI) m/z 162 [M+H]+.
1-[3-Hydroxy-4-(pyridin-4-yl)-1H-pyrazol-1-yl]ethanone (36)To a mixture of 35 (7.84 g, 48.6 mmol) in pyridine (78 mL) was added Ac2O (4.81 mL, 50.9 mmol), and the mixture was stirred at 100°C for 2 h before the mixture was concentrated in vacuo. The residue was washed with water and hexane to give 36 (9.89 g, quant) as yellow solid. 1H-NMR (DMSO-d6) δ: 2.55 (3H, s), 7.80–7.84 (2H, m), 8.53–8.56 (2H, m), 8.92 (1H, s), 12.0 (1H, br s); MS (ESI) m/z 204 [M+H]+.
2-[4-({[4-(Pyridin-4-yl)-1-(2,2,2-trifluoroethyl)-1H-pyrazol-3-yl]oxy}methyl)phenyl]quinoline Hydrochloride (37)To a mixture of 36 (2.03 g, 10.0 mmol), 34 (3.19 g, 11.0 mmol) in DMF (77 mL) was added K2CO3 (4.15 g, 30.0 mmol), and the mixture was stirred at 60°C for 3 h before the mixture was concentrated in vacuo. To the residue were added MeOH (80 mL) and water (20 mL), and the mixture was stirred at 60°C for 3 h before the mixture was concentrated in vacuo. The residue was washed with water and purified by silica gel column chromatography (0–5% MeOH in CHCl3) to give a yellow solid (1.52 g). To a mixture of the yellow solid obtained above (568 mg) and 1,1,1-trifluoro-2-iodomethane (630 mg, 3.00 mmol) in DMF (20 mL) was added Cs2CO3 (1.47 g, 4.50 mmol), and the mixture was stirred at 60°C for 5 h before the mixture was concentrated in vacuo. The residue was partitioned between water and EtOAc, and the organic layer was concentrated in vacuo. The residue was purified by silica gel column chromatography (1–7% MeOH in CHCl3) to give free form of the title compound, which was dissolved in EtOAc (20 mL). To the mixture was added 4 M HCl/EtOAc (1.5 mL) and the mixture was stirred at r.t. for 30 min. The precipitate was collected by filtration and washed with EtOAc to give 37 (650 mg, 27%) as a pale yellow solid. 1H-NMR (DMSO-d6) δ: 5.19 (2H, q, J=8.9 Hz), 5.54 (2H, s), 7.69–7.75 (1H, m), 7.78 (2H, d, J=8.4 Hz), 7.89–7.94 (1H, m), 8.13 (1H, d, J=7.8 Hz), 8.23–8.38 (6H, m), 8.71 (1H, d, J=8.7 Hz), 8.79–8.84 (2H, m), 8.89 (1H, s); MS (ESI) m/z 461 [M+H]+. Anal. Calcd for C26H19F3N4·2.2HCl·0.3C4H8O2·2.2H2O: C, 53.84; H, 4.65; N, 9.23; Cl, 12.86; F, 9.39. Found: C, 54.00; H, 4.61; N, 9.48; Cl, 13.05; F, 8.81.
Molecular ModelingThe geometry of MP-10 binding to PDE10A enzyme was derived from PDB (code: 3HR1). Structure alignment of MP-10, 16a, and b was performed with the Flexible Alignment tool in the MOE program29) with the geometry of MP-10 as a template and the MMFF94x force field.
Calculation of Atomic Charge DistributionMOE was used to build the ligand structures of MP-10 and 14.33) Conformation search of the ligands were done with Conformation Import module in MOE with MMFF94x force field. Potential energy of each conformation were calculated with PM6 method implemented in MOPAC2012. The lowest energy was selected as energy-minimized conformation to calculate partial charge. The electrostatic potential-fitted atomic partial charges were calculated with the AM1 method implemented in the MOPAC7.1.
PDE10A Enzyme Assay ProtocolThe full-length human PDE10A2 was amplified via PCR using the 1st strand cDNA synthesized from the total RNA isolated from human neuroblastoma TGW cell line. The PCR products were cloned into a pCR2.1-TOPO vector (Invitrogen Inc.) to confirm sequences. The confirmed plasmid was digested with restricted enzymes, BamHI/HindIII, and this digested product was inserted into a pFastBac1 vector (Invitrogen Inc.). Human PDE10A2 enzyme protein was expressed in a Spodoptera frugiperda Sf9 insect cell using the Bac-to-Bac Baculovirus Expression System (Invitrogen Inc.). The infected Sf9 cells were collected via centrifuge and the medium was removed. The collected cells were lysed by sonication in lysis buffer (50 mM Tris–HCl [pH 8.0], 150 mM NaCl, 3 mM DTT, 0.1% NP-40, and 20% glycerol with protease inhibitors), after which the lysate was centrifuged and supernatant was collected to obtain PDE10A2 enzyme solution. We confirmed the PDE10A2 expression via Western blot analysis. Inhibitory effects of compounds on human PDE10A enzyme activity was assessed by measuring the amount of cAMP via the Homogeneous Time-Resolved Fluorescence (HTRF) detection method. The assay was performed in 12 µL samples containing a optimal amount of the PDE10A enzyme, buffer (40 mM Tris–HCl pH 7.5; 5 mM MgCl2), 0.1 µM cAMP and various concentrations of compounds (0.1 nM to 10 µM). After compounds were preincubated for 30 min with the enzyme, the reaction was initiated by adding the substrate cAMP and the mixture was incubated for 60 min at room temperature with agitation. The reaction was terminated by the addition of the fluorescence acceptor (cAMP labeled with the dye d2) and the fluorescence donor (anti-cAMP antibody labeled with Cryptate, Cisbio). After 60 min, the fluorescence transfer corresponding to the amount of residual cAMP was measured at lex. 320 nm, lem. 620 nm and lem. 665 nm using an Envision plate reader (PerkinElmer, Inc.) and signal ratio (665/620) was calculated. The ratio determined in the absence of enzyme was subtracted from all data. The obtained results were converted to activity relative to an uninhibited control (100%) and IC50 values were calculated using Prism software (GraphPad Software, Inc.).
In Vitro Enzyme Assays for Profiling PDE SelectivityPhosphodiesterases 4B, D, 9A, and 10A were generated from full-length human recombinant clones. PDE2A was isolated from rat. while PDE3 and 5 were isolated from rabbit. PDE activity was measured with the preferred substrates using a scintillation proximity assay. For PDE2A, 3, 4B, D, 10A, cAMP was used, and for PDE5, and 9A, cGMP was used. The effect of PDE inhibitors was investigated by assaying a fixed amount of enzyme and varying inhibitor concentrations. For determination of IC50 values, the Hill-plot two-parameter model was used.
In Vivo Behavioral Assay in MiceTwo behavioral tests described below were conducted for mice. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Astellas Pharma Inc. Further, the Astellas Pharma Inc. Tsukuba Research Center was awarded Accreditation Status by the AAALAC International. All efforts were made to minimize the number of animals used and to avoid suffering and distress.
Phencyclidine-Induced Hyperlocomotion: ICR mice aged 5–6 weeks were used to evaluate the effect of PDE10A inhibitor on hyper-locomotion induced by the N-methyl-D-aspartate (NMDA) antagonist phencyclidine (PCP). Immediately after oral administration of either vehicle or agent as pre-treatment, mice were placed into individual plastic test cages (30×35×17.5 cm) of a SUPERMEX system (PAT.P; Muromachi Kikai Co., Ltd.), and measurement of locomotor activity was started. After 1 h, the mice were injected with a post-treatment of saline or PCP (2.5 mg/10 mL/kg, subcutaneously (s.c.)), and locomotor activity was measured for a further 60 min. Total locomotor activity for 60 min post-treatment was calculated.
NORT in Neonatally PCP-Treated Mice: Three-day-old male ddY mice were housed 10–12 per cage with a stepmother. Saline or PCP (15 mg/kg) was administered subcutaneously once daily on days 7, 9, and 11 after birth. The mice were separated from their mother at 3 weeks of age, and used for NORT at 8–9 weeks old. Neonatal mice were treated with PCP, and the NORT was conducted as previously described.37)
In Vitro Metabolic Pathway in Mouse Liver MicrosomesThe incubation mixture consisted of the following: 10 µM MP-10, 100 mM phosphate buffer (pH 7.4), liver microsomes (0.2 mg protein), and a reduced nicotinamide adenine dinucleotide phosphate (NADPH)-generating system (15 mM glucose 6-phosphate, 3 mM NADP+, 5 mM magnesium chloride, and 0.3 U glucose 6-phosphate dehydrogenase) in a total volume of 500 µL. The mixture was preincubated for 5 min at 37°C. A reaction was started by the addition of MP-10 (added in acetonitrile–water, 50 : 50, v/v; final solvent concentration not exceeding 0.5% [v/v]). The samples were incubated at 37°C for 15 and 60 min, and the reaction was stopped by the addition of 1 mL cold MeCN. The reaction mixture was centrifuged at 16000×g for 5 min, and the supernatant was evaporated under a steam of nitrogen gas at 40°C. The dried extracts containing the parent drug and metabolites were dissolved in 200 µL of acetonitrile–water (50 : 50, v/v), and 800 µL of acetonitrile was added. Centrifugation and evaporation of the supernatant was repeated once again. The dried extracts were then dissolved in 200 µL of MeCN–water (50 : 50, v/v), and filtered through a membrane filter, and 5 µL of the filtrate was analyzed using LC-MS/MS with Waters Ultra Performance Liquid Chromatography (UPLC) system and Thermo Scientific LTQ-Orbitrap mass spectrometer.
Mouse and Human Liver Microsomal AssaysPooled mouse or human liver microsomes (Xenotech LLC.) were diluted in 100 mM KH2PO4/K2HPO4 buffer (pH 7.4) containing 0.1 mM ethylenediaminetetraacetic acid (EDTA). The incubation mixtures (270 µL total volume), which contained 0.2 mg/mL of microsomal proteins, and 1 mM NADPH (30 µL) were pre-incubated for 5 min at 37°C. Reactions were initiated by the addition of 0.2 µM of substrates. After the appropriate incubation time (0, 15, 30, 45 min), 50 µL of incubation mixture was transferred into 80% acetonitrile containing internal standard (50 ng/mL diazepam, 250 µL) and centrifuged for 10 min at 2800 rpm. The supernatant (200 µL) was prepared and analyzed via LC-MS/MS with a Surveyor HPCL system (Thermo Fisher Scientific Inc.) and TSQ Quantum ultra tandem triple quadrupole mass spectrometer (Thermo Fisher Scientific Inc.). The in vitro intrinsic clearance (CLint, vitro) was calculated using Eq. 1, which is based on the time course of the residual ratio of the compounds.38)
 | (1) |
Compound 14 or TP-10 was administered to ICR mice as a 30 mg/kg solution of saline containing 5% dimethylsulfoxide (DMSO) and 5% Cremophor. Blood samples were collected using syringes containing heparin sodium at 0.5, 1, 2, and 4 h after intraperitoneal administration. Blood samples were kept on ice and centrifuged within 0.5 h of collection at 16000×g, for 2 min at 4°C to obtain plasma, which was then stored at −20°C prior to analysis. Whole brain samples were also removed from the same animals as blood samples just after the collection of blood samples,and stored at −20°C and homogenized in 4-fold volume of phosphate buffered saline (pH 7.4) before extraction processing. Extraction and analysis of compound concentrations were performed via LC-MS/MS with a Prominence HPLC (Shimadzu Corporation) and API4000 Triple Quadrupole Mass Spectrometer (AB SCIEX).
The authors wish to thank Ms. Ayako Moritomo for her helpful support in preparing this manuscript, Dr. Atsushi Suzuki and Ms. Junko Yarimizu for performing pharmacological evaluations, and the staff of Astellas Research Technologies Co., Ltd., for conducting mouse and human liver microsomal assays, elemental analysis, and spectral measurements.