2014 Volume 62 Issue 2 Pages 182-184
2014 Volume 62 Issue 2 Pages 182-184
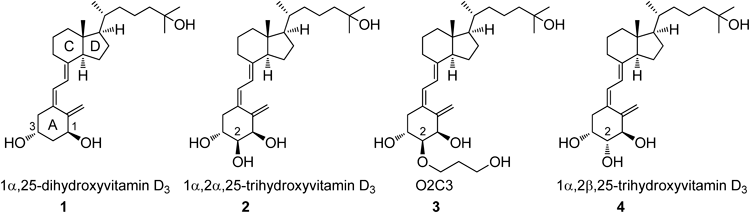
Our previous studies revealed that recombinant human CYP3A4 converted 2α-(3-hydroxypropoxy)-1α,25-dihydroxyvitamin D3 (O2C3), which was a more potent binder to vitamin D receptor (VDR) than the natural hormone, 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3, 1), to 1α,2α,25-trihydroxyvitamin D3 (2). Here, we synthesized 2 using the Trost Pd-mediated coupling reaction between an A-ring precursor and a CD-ring bromoolefin and evaluated its preliminary biological activity. We found that metabolite 2 from O2C3 was still active as a VDR ligand while maintaining human VDR binding affinity (27.3% of 1α,25(OH)2D3) and HL-60 cell differentiation activity (62% of 1α,25(OH)2D3).
The active metabolite of vitamin D3, 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3, 1], plays important roles in cellular growth, differentiation, apoptosis, and immune responses, in addition to its classical major roles in calcium homeostasis and bone mineralization.1–4) Actually, 1 and several synthetic analogs of 1 have been used clinically in the treatment of bone diseases, secondary hyperparathyroidism, psoriasis, and osteoporosis.1,5) Although 1 is inactivated by CYP24A1-dependent catabolism via C-24 hydroxylation to calcitroic acid for excretion from the body,1) it was found that some 2α-substituted active vitamin D analogs were highly resistant to CYP24A1, for example, the kcat/Km value of 2α-(3-hydroxypropoxy)-1α,25-dihydroxyvitamin D3 (O2C3), which showed 1.8-times greater binding affinity for vitamin D receptor (VDR) than 1,6–10) was only 3% of that for 1.11) CYP24A1 is the specific enzyme induced by the VDR-ligand (1 or its analog) complex in the target tissue and inactivates 1 and its analogs; therefore, CYP24A1-resistant ligands would have long-term biological effects on the target tissues.12) On the other hand, CYP3A4 is a broad-spectrum drug-metabolizing P450 enzyme,13) but 1 and its analog 2α-(3-hydroxypropyl)-1α,25-dihydroxyvitamin D3 (O1C3) are not primary substrates for CYP3A4.12) Recently, however, we demonstrated that O2C3 was metabolized by CYP3A4 and converted to 1α,2α,25-trihydroxyvitamin D312) (2, Fig. 1). Its 2β-isomer (4) is a known compound and shows potent 1α,25(OH)2D3-like activities,14) and we report here the synthesis of a new 2α-hydroxylated analog 2 using the Trost Pd-mediated coupling reaction between an A-ring precursor 12 and a CD-ring bromoolefin 13 to evaluate its preliminary biological activity.15–18)
The A-ring precursor 12 for Trost coupling was prepared from the known epoxide 519) in 11 steps (Chart 1). Briefly, treatment of 5 with p-methoxybenzyl alkoxide with heating gave methyl 3-O-(p-methoxybenzyl)altropyranoside 6, which had the 2α-hydorxy group (steroidal numbering) required in target molecule 2. After 2-O-silylation, the p-methoxybenzyl (PMB) protecting group was removed by 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) to give 7, and the methoxymethyl (MOM) group was introduced instead for the next bromination reaction using N-bromosuccinimide (NBS). NBS treatment for the resulting O-MOM-protected bezylidene acetal gave bromide 8. Activated Zn-reduction in the presence of NaBH3CN produced alcohol 9, which was converted to epoxide 10 via tosylation followed by tetrabutylammonium fluoride (TBAF) treatment. Trimethylsilyl (TMS)–ethynylation of 10 afforded enyne 11, and subsequent solvolysis in K2CO3–MeOH and O-silylation provided enyne 12 (Chart 1).

The CD-ring bromoolefin 1320) and enyne 12 obtained above were connected using Pd-catalyst to give the coupling product 14.21) Desilylation by TBAF and deacetalization under acidic conditions gave the target molecule 222) (Chart 2). The isolated product was re-purified by HPLC to test biological activity.

The binding affinity of the new analog 2 for the human vitamin D receptor (hVDR)23) and induction activity of HL-60 cell differentiation24) are shown in Table 1. The new analog 2 was still active, like its 2β-diastereoisomer 4,14) and these results were different from 4-OH analogs of 1α,4α,25-trihydroxyvitamin D3 and 1α,4β,25-trihydroxyvitamin D3, which were very weak agonistic ligands for hVDR (for hVDR binding affinity: 0.9% and 2.9% of the natural hormone 1, respectively).25) The 2α-OH analog 2 showed lower binding affinity for hVDR than that of the natural hormone 1, and X-ray cocrystallographic analysis of 2α-methyl-1α,25-dihydroxyvitamin D3, which was a better binder for hVDR than 1, in the ligand binding domain (LBD) of hVDR explained the 2α-methyl group of 2 was fitted in the hydrophobic pocket formed by Phe150, Leu233, and Ser23710); therefore, the 2α-OH group of 2 at the same position as the 2α-methyl group would not be suitable for the pocket to bind the LBD of hVDR. The 2β-OH group with the different direction in the LBD may not be disturbed in binding.14)
| Compound | hVDR binding affinitya) | HL-60 cell differentiationa,b) |
|---|---|---|
| 1α,25(OH)2D3 (1) | 100 | 100 |
| 1α,2α,25(OH)3D3 (2) | 27.3 | 62 |
| O2C3 (3) | 180c) | 55 |
| 1α,2β,25(OH)3D3 (4) | 110d) | 44d) |
We synthesized a new analog of active vitamin D3, 1α,2α,25-trihydroxyvitamin D3 (2), which was the major metabolite of O2C3 by CYP3A4, to study its biological activity. 2 showed moderate hVDR binding affinity and potent HL-60 cell differentiation activity (EC50 68 nM: 62% activity of 1). The data demonstrate that 2 and 4 with 2α- and 2β-stereochemistries of the 2-OH group on the active vitamin D skeleton maintain VDR binding and HL-60 cell differentiation activity. This is a totally different effect from that of the 4-OH group.25) Although O2C3 was resistant to CYP24A1, its CYP3A4 metabolite 2 was further metabolized by CYP24A1, similar to 1. The kcat/Km value of hCYP24A1 for 2 was 60% of that for 1.12) Further biological testing is underway in our laboratories.
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 25860011 to M.T.) as well as a Grant-in-Aid from the Japan Society for the Promotion of Science (No. 24590021 to A.K.).