2014 年 62 巻 4 号 p. 336-342
2014 年 62 巻 4 号 p. 336-342
A series of 1H-pyrrolo[2,3-c]pyridine-7-amine derivatives were designed and synthesized based on our docking model as potassium-competitive acid blockers (P-CABs). Molecular modeling of these derivatives led us to introduce a substituent at the 1-position to access two lipophilic sites and polar residues. We identified potent P-CABs that exhibit excellent inhibitory activity in vitro and in vivo. These results indicate that the 1H-pyrrolo[2,3-c]pyridine-7-amine derivatives are promising lead compounds as P-CABs.
Current therapies for gastroesophageal reflux disease (GERD), peptic ulcer, and other acid-related diseases either prevent the stimulation of parietal cells (e.g., H2 receptor antagonists, H2RAs) or inhibit gastric H+/K+-ATPase (e.g., proton pump inhibitors, PPIs).1) Despite their potent inhibitory activities against acid secretion and their worldwide clinical application, it is suggested that the current treatment could be improved in several aspects.2) Potassium-competitive acid blockers (P-CABs), a new class of acid suppressants with a different mode of action from the existing therapies, are expected to offer some therapeutic benefits such as better symptom control and faster healing of GERD as well as other acid-related diseases.
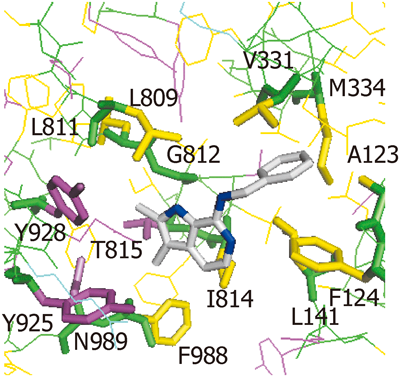
Over the past 30 years, various companies have focused on developing P-CABs as alternative acid suppressants.3) From our efforts to develop new P-CABs, we found a 1H-pyrrolo[2,3-c]pyridine-7-amine derivative 1 (Fig. 1, Left) has potent H+/K+-ATPase inhibitory activity (IC50 27 nM). Recently, Panchal et al. also reported this compound and its derivatives as P-CABs.4) The acid secretion inhibitory activity in rats of this compound was 62% at the dose of 1 mg/kg on intravenous (i.v.) administration. This result encouraged us to explore more potent compounds in this derivative. In order to design potent inhibitors, we created the homology model of the luminal region of H+/K+-ATPase from the crystal structure of Ca2+-ATPase (PDB ID, 1IWO)5) using SCWRL ver. 2.9.6) We identified two lipophilic cavities (LP-1 and LP-2 sites) and two polar residues (Tyr925 and Tyr928) in the enzyme that could be used to improve inhibitory activity.7,8) Figure 1 displays the docking model of compound 1 and also the target LP-1, LP-2 sites and two Tyr residues. This model suggested that this compound binds to the enzyme mainly via the LP-1 site (mode A).

The LP-1 site is surrounded by Val331, Met334, Ala123, and Phe124, whereas the LP-2 site consists of Leu809, Thr815, Tyr925, Tyr928, and Asn989 residues.
In order to improve the in vitro inhibitory activity, we used the following two strategies. First, the 7-position of P-CABs has been considered as a possible target for improving inhibitory activity because the orthogonal conformation of benzyl substituents has a major impact on inhibitory activity in a series of imidazo[1,2-a]pyridine compounds.9) Next, we anticipated that the inhibitory activity could be improved via additional lipophilic and polar interactions with the unused LP-2 site and the Tyr residues. The docking model (mode A) suggested that a substituent at the 5-position may occupy the LP-2 cavity. However, according to Panchal et al., introduction of a heteroaromatic and heterocyclic group at the 5-position results in decreased activity.4) In contrast, compound 1 was calculated to have an ability to bind to the enzyme with another binding mode (mode B), as described in Fig. 2, at a lower priority. In the mode B, the LP-1 site is similarly occupied by a benzylamino group; however, the 1H-pyrrolo[2,3-c]pyridine ring is flipped.
From this docking model (mode B), the 1- or 2-position seemed to be a possible target for substitution to access the LP-2 site. The 1- or 2-propyl substituted compounds 2a and 2b were designed as model compounds and their plausible binding models are shown in Fig. 3. In these docking models, the LP-1 site is similarly occupied by the benzylamino group, and the introduced propyl group could occupy the LP-2 site. These observations led us to explore the appropriate substituent at the 1-position because synthetic feasibility of the 1-position was much higher. Furthermore, in the binding model of this 1-substitued derivatives, 3-position of the 1H-pyrrolo[2,3-c]pyridine is placed close to Tyr925 or Tyr928 enough to allow for polar interactions. This indicated that the 1H-pyrrolo[2,3-c]pyridine derivatives could acquire three desired interactions with the LP-1, LP-2, and the polar residues and that demonstrate potent inhibitory activity. We herein report the design, synthesis, and in vitro/in vivo pharmacological activities of 1H-pyrrolo[2,3-c]pyridine-7-amine derivatives with various substituents at the 7-, 1-, and 3-positions.

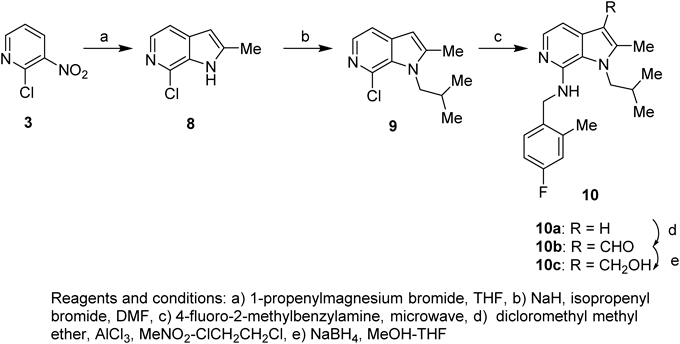
The synthesis of 1H-pyrrolo[2,3-c]pyridine derivatives with various substituents at the 7-position was achieved using the methods outlined in Chart 1. 7-Chloro-2,3-dimethyl-1H-pyrrolo[2,3-c]pyridine (4) was prepared by the Bartoli reaction using 2-chloro-3-nitropyridine 3 and 3 eq of 1-methyl-1-propenylmagnesium bromide.10) Condensation with various benzyl amines gave the desired compounds 5a and 5c–g. Introduction of the benzoylamino group (5b) was achieved using a palladium-catalyzed reaction in a similar manner as that reported previously.11,12) The synthesis of 1-substituted derivative 7 is described in Chart 2. Alkylation of compound 4 was performed using an alkyl halide in the presence of sodium hydride to give the corresponding compound 6, which was converted into compound 7 using the same method as that described above. The synthetic method of the 3-hydroxymethyl derivative is shown in Chart 3. The 3-unsubstituted compound 10a was prepared in a similar manner as described above. Formulation was conducted using aluminum chloride and dichloromethyl methyl ether, following sodium borohydride reduction gave the desired 3-hydroxymethyl derivative 10c.


H+/K+-ATPase inhibitory activities of the compounds with a substituent at the 7-position are summarized in Table 1. N-Methylbenzyl (5a) and N-benzoyl (5b) derivatives showed decreased inhibitory activity. According to Yoon et al., replacement of the benzylamino group in compound 1 with a benzyloxy group resulted in decreased inhibitory activity.13) These results suggest that basicity and the hydrogen atom on the nitrogen atom at the 7-position are important for potent H+/K+-ATPase inhibitory activity. In the binding model of compound 1 (mode A), considering that the distance between NH and Phe124 is calculated to be approximately 4 Å, electrostatic interaction between δ+ of NH and δ− of π electron of Phe124 is feasible. Next, we investigated the effects of the substituents on the benzylamino group. Introduction of the 2-methyl group (5c) did not have significant effects on the activity; however, 2,6-dimethyl (5d) and 4-fluoro-2-methyl (5e) derivatives demonstrated 2–3-fold more potent activity than the benzylamino derivative (1). When the benzyl carbon and the ortho position of the phenyl ring was cyclized, the indan derivative (5f) exhibited similar activity to compound 5e; however, the tetrahydronaphthyl derivative (5g) exhibited less potency. This reason is considered that in the case of compounds 5d–f, the angle of each phenyl ring and 1H-pyrrolo[2,3-c]pyridine is kept preferred angle, while tetrahydronaphthyl derivative 5g can no longer hold the favored angle. Based on these results, we chose benzyl and 4-fluoro-2-methylbenzyl derivatives (1 and 5e, respectively) as scaffolds and introduced an alkyl group at their 1-positions to improve inhibitory activity.
 | ||
|---|---|---|
| Compound | R | H+/K+-ATPase (IC50, nM) |
| 1 | NHCH2Ph | 27 |
| 5a | NMeCH2Ph | >1000 |
| 5b | NHCOPh | 1800 |
| 5c | NHCH2(2-Me-Ph) | 25 |
| 5d | NHCH2(2,6-Me2-Ph) | 14 |
| 5e | NHCH2(4-F-2-Me-Ph) | 8.4 |
| 5f | NH(1-Indanyl) | 9.8 |
| 5g | NH(1-Tetrahydronaphthyl) | 38 |
H+/K+-ATPase inhibitory activities of compounds with alkyl substituents at the 1-position are summarized in Table 2. Methylation of the 1-position (7a) resulted in a significant reduction in activity, whereas elongation of the alkyl chain improved potency (7b, 2a). We speculated that the 1-methyl derivative 7a binds to H+/K+-ATPase in the mode A and that undesired steric repulsion around the methyl group decreases inhibitory activity. In contrast, ethyl and propyl derivatives (7b, 2a) could bind to the enzyme in mode B and the alkyl groups could be accessible to the LP-2 site as previously shown in Fig. 3. This is considered to be a reason for the increasing tendency of inhibitory activity. In a series of the 4-fluoro-2-methylbenzylamine derivatives 7c–e, the same tendency was observed as that observed in benzyl derivatives. Although compounds 2a and 7d are considered to occupy both LP-1 and LP-2 pockets, their H+/K+-ATPase inhibitory activities were 2.9 and 4.4 folds lower than those of compounds 1 and 5e, respectively, which do not have LP-2 interaction. The reason is considered to be that the binding mode of compounds 2a and 7d may be less preferable in terms of the electronic interaction between NH and Phe124 owing to the direction change of the NH group, which is caused by the flip of the core 1H-pyrrolo[2,3-c]pyridine ring. This factor may offset binding affinity delivered by the additional lipophilic interaction between the LP-2 site and the propyl group. The isobutyl derivative 7e demonstrated comparable inhibitory activity with compound 5e that indicates lipophilic interaction between the isobutyl group and the LP-2 site can compensate for the electronic interaction between NH and Phe124. More importantly, 1-isobutyl-3-hydroxymethyl derivative 10c demonstrated over 2.5-fold potent inhibitory activity compared to compound 7e. The plausible binding mode of compound 10c is shown in Fig. 4. This compound is considered to bind in a similar manner with compound 2a and the introduced hydroxyl group could additionally make a polar interaction with Tyr925. These three desired interactions with LP-1, LP-2, and Tyr925 in the enzyme are considered to enable this compound to show potent inhibitory activity.
 | |||
|---|---|---|---|
| Compound | R1 | R2 | H+/K+-ATPase (IC50, nM) |
| 7a | Me | H | >1000 |
| 7b | Et | H | 240 |
| 2a | Pr | H | 120 |
| 7c | Et | 4-F-2-Me | 28 |
| 7d | Pr | 4-F-2-Me | 24 |
| 7e | iBu | 4-F-2-Me | 8.6 |
| 10c | — | — | 3.2 |
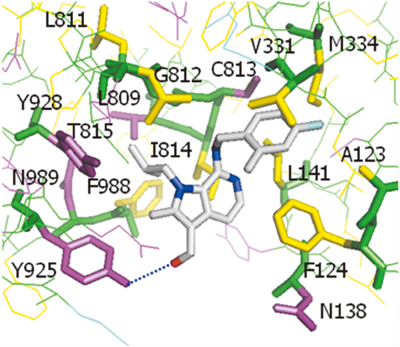
The indicated polar interaction is shown in line.
Some potent in vitro activity compounds (5d–f, 7e, 10c) were examined for their inhibitory activities against histamine-induced gastric acid secretion in anesthetized rats at the dose of 1 mg/kg, i.v.; the results are summarized in Table 3. As expected from their potent in vitro inhibitory activity, most of them effectively inhibited gastric acid secretion. Their effects were more potent than that of lansoprazole (90% inhibition).14) From these results, the 1H-pyrrolo[2,3-c]pyridine-7-amine derivatives are revealed to show potent gastric acid secretion inhibitory activity in rats.
| Compound | H+/K+-ATPase (IC50, nM) | In vivo (% inh.) |
|---|---|---|
| 5d | 14 | 98 |
| 5e | 8.4 | 96 |
| 5f | 9.8 | 94 |
| 7e | 8.6 | 90 |
| 10c | 3.2 | 45 |
In summary, we designed and synthesized a series of 1H-pyrrolo[2,3-c]pyridine-7-amines based on our docking model. The detailed analysis of the binding mode led us to introduce the alkyl group at the 1-position. This conversion enabled these derivatives to access two lipophilic sites and the polar residues. In particular, compound 10c demonstrated potent inhibitory activity in vitro. Although this compound resulted in lower acid secretion inhibitory activity in rats, other potent P-CABs which exhibit excellent inhibitory activity were identified such as compounds 5d and 5e. These results indicate that the 1H-pyrrolo[2,3-c]pyridine-7-amine derivatives are promising lead compounds as P-CABs.
Melting points were determined on a Yanagimoto micro melting point apparatus or Büchi B-545 and are uncorrected. Proton and carbon nuclear magnetic resonance (NMR) spectra were recorded on a Varian Mercury-300 or a Bruker AV-300M spectrometer. Chemical shifts are given in δ values (ppm) using tetramethylsilane as the internal standard. Coupling constants (J) are reported in hertz (Hz). Spectral splitting patterns are designated as follows: s, singlet; br, broad; d, doublet; t, triplet; m, multiplet. Fourier transform infrared (FT-IR) spectra were made on Thermo 5700 Nicolet sprectrometer and were expressed in wave number (cm−1) using attenuated total reflectance (ATR) accessory. Microwave was irradiated with CEM Discover microwave focused chemical synthesis reactor. TLC analyses were carried out on Merck Kieselgel 60 F254 plates or FUJI SILYSIA Chemical Ltd. Chromatorex NH-TLC plates. Silica gel column chromatography was performed using Merck 0.063–0.200 mm silica gel 60, and FUJI SILYSIA Chemical Ltd. 100–200 mesh Chromatorex NH silica DM1020. Elemental analyses and high resolution (HR)-MS analyses were carried out by Takeda Analytical Research Laboratories, Ltd.
7-Chloro-2,3-dimethyl-1H-pyrrolo[2,3-c]pyridine (4)A solution of 2-chloro-3-nitropyridine (3) (3.5 g, 22 mmol) in tetrahydrofuran (THF) (100 mL) was cooled to −78°C, and a 0.5 M 1-methyl-1-propenylmagnesium bromide in THF (100 mL, 50 mmol) was added. The reaction mixture was stirred at −20°C for 18 h, warmed to room temperature, and concentrated under reduced pressure to a liquid amount of about 40 mL. Twenty percent aqueous ammonium chloride solution (200 mL) was added, and the mixture was extracted with EtOAc. The extract was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane–EtOAc=1 : 1). The collected fraction was concentrated and unreacted compound 3 was filtered off. The mother liquid was concentrated and the residue was washed with isopropyl ether (IPE) to give the title compound as a pale-yellow solid (580 mg, 3.2 mmol, 15%). mp 170–171°C. 1H-NMR (CDCl3) δ: 2.21 (3H, s), 2.44 (3H, s), 7.30 (1H, d, J=5.7 Hz), 7.98 (1H, d, J=5.7 Hz), 8.20 (1H, br).
N-Benzyl-2,3-dimethyl-1H-pyrrolo[2,3-c]pyridine-7-amine (1)A mixture of 4 (200 mg, 1.1 mmol) and benzylamine (1 mL, 9.2 mmol) was stirred at 180°C for 30 min under microwave irradiation. The mixture was cooled to room temperature (rt). A saturated aqueous sodium hydrogencarbonate solution was added to the reaction mixture which was then extracted with EtOAc. The extract was washed successively with a saturated aqueous sodium hydrogencarbonate solution, and brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane–EtOAc=2 : 1) and the obtained solid was crystallized from IPE to give the title compound as a colorless solid (154 mg, 0.61 mmol, 56%). mp 180–182°C. 1H-NMR (CDCl3) δ: 2.17 (3H, s), 2.29 (3H, s), 4.51 (1H, br), 4.67 (2H, d, J=5.2 Hz), 6.85 (1H, d, J=5.2 Hz), 7.20–7.40 (5H, m), 7.78 (1H, d, J=5.2 Hz), 8.30 (1H, br). 13C-NMR (CDCl3) δ: 8.5, 11.4, 46.1, 105.2, 107.8, 119.4, 126.9, 127.6, 128.3, 133.3, 133.8, 135.6, 139.4, 145.2. IR (ATR) cm−1: 1632, 1570, 1555, 1476, 1452, 1406. Anal. Calcd for C16H17N3: C, 76.46; H, 6.82; N, 16.72. Found: C, 76.51; H, 6.94; N, 16.55.
Following compounds 5a and 5c–g were prepared in the similar manner with compound 1.
N-Benzyl-N,2,3-trimethyl-1H-pyrrolo[2,3-c]pyridine-7-amine (5a): Colorless solid (79%). mp 106–107°C. 1H-NMR (CDCl3) δ: 2.16 (3H, s), 2.24 (3H, s), 3.12 (3H, s), 4.69 (2H, s), 6.93–6.95 (1H, m), 7.26–7.46 (5H, m), 7.64 (1H, br s), 7.88 (1H, d, J=5.4 Hz). 13C-NMR (CDCl3) δ: 8.4, 11.6, 38.0, 56.3, 106.6, 107.7, 121.5, 127.1, 127.3, 128.9, 132.7, 135.4, 136.5, 139.3, 148.2. IR (ATR) cm−1: 1552, 1494, 1451, 1392. Anal. Calcd for C17H19N3: C, 76.95; H, 7.22; N, 15.84. Found: C, 76.82; H, 7.13; N, 15.82.
2,3-Dimethyl-N-(2-methylbenzyl)-1H-pyrrolo[2,3-c]pyridine-7-amine (5c): Colorless solid (90%). mp 225–226°C. 1H-NMR (CDCl3) δ: 2.16 (3H, s), 2.24 (3H, s), 2.28 (3H, s), 4.37 (1H, br), 4.60 (2H, d, J=4.8 Hz), 6.82 (1H, d, J=5.7 Hz), 7.01–7.14 (3H, m), 7.20–7.25 (1H, m), 7.75 (1H, d, J=5.7 Hz), 8.68 (1H, br). 13C-NMR (CDCl3) δ: 8.5, 11.5, 18.8, 44.2, 105.2, 108.0, 119.4, 125.9, 127.3, 128.6, 130.2, 132.9, 133.8, 136.0, 136.7, 137.3, 145.0. IR (ATR) cm−1: 1633, 1570, 1556, 1468, 1407. Anal. Calcd for C17H19N3: C, 76.95; H, 7.22; N, 15.84. Found: C, 76.84; H, 7.16; N, 15.86.
N-(2,6-Dimethylbenzyl)-2,3-dimethyl-1H-pyrrolo[2,3-c]pyridine-7-amine (5d): Colorless solid (84%). mp 279–280°C. 1H-NMR (CDCl3) δ: 2.17 (3H, s), 2.32 (3H, s), 2.34 (6H, s), 3.91 (1H, br), 4.66 (2H, d, J=4.2 Hz), 6.83 (1H, d, J=5.7 Hz), 6.98–7.01 (2H, m), 7.05–7.10 (1H, m), 7.80 (1H, d, J=5.7 Hz), 8.19 (1H, br). 13C-NMR (CDCl3) δ: 8.5, 11.6, 19.6, 40.5, 105.1, 108.2, 119.2, 127.5, 128.2, 132.5, 133.6, 135.5, 136.3, 137.8, 145.0. IR (ATR) cm−1: 1631, 1571, 1556, 1468, 1407. Anal. Calcd for C18H21N3: C, 77.38; H, 7.58; N, 15.04. Found: C, 77.22; H, 7.44; N, 15.02.
N-(4-Fluoro-2-methylbenzyl)-2,3-dimethyl-1H-pyrrolo[2,3-c]pyridine-7-amine (5e): Colorless solid (48%). mp 215–218°C. 1H-NMR (CDCl3) δ: 2.17 (3H, s), 2.18 (3H, s), 2.29 (3H, s), 4.45 (1H, br s), 4.52–5.54 (2H, m), 6.62–6.75 (2H, m), 6.83 (1H, d, J=5.7 Hz), 7.08–7.12 (1H, m), 7.72 (1H, d, J=5.7 Hz), 9.01 (1H, br s). 13C-NMR (CDCl3) δ: 8.5, 11.5, 18.9, 43.5, 105.3, 108.1, 112.1, 112.4, 116.6, 116.9, 119.2, 129.8, 130.0, 132.68, 132.73, 133.3, 133.7, 135.4, 139.0, 139.1, 144.8, 160.3, 163.5. IR (ATR) cm−1: 1635, 1569, 1554, 1470, 1412. Anal. Calcd for C17H18N3F: C, 72.06; H, 6.40; N, 14.83. Found: C, 71.85; H, 6.40; N, 14.46.
N-(2,3-Dihydro-1H-inden-1-yl)-2,3-dimethyl-1H-pyrrolo[2,3-c]pyridine-7-amine (5f): Colorless solid (46%). mp 245–247°C. 1H-NMR (CDCl3) δ: 1.62–1.92 (1H, m), 2.17 (3H, s), 2.32 (3H, s), 2.58–2.69 (1H, m), 2.78–2.98 (2H, m), 4.44 (1H, br), 5.72–5.79 (1H, m), 6.89 (1H, d, J=5.9 Hz), 7.06–7.11 (1H, m), 7.15–7.28 (3H, m), 7.78 (1H, d, J=5.9 Hz), 8.30 (1H, br). 13C-NMR (CDCl3) δ: 8.5, 11.6, 30.2, 34.6, 56.5, 105.2, 108.2, 119.4, 124.3, 124.6, 124.7, 126.4, 127.5, 132.9, 133.9, 136.1, 143.7, 144.7. IR (ATR) cm−1: 1633, 1572, 1555, 1471, 1406. Anal. Calcd for C18H19N3: C, 77.95; H, 6.90; N, 15.15. Found: C, 77.80; H, 6.86; N, 15.10.
2,3-Dimethyl-N-(1,2,3,4-tetrahydronaphthalen-1-yl)-1H-pyrrolo[2,3-c]pyridine-7-amine (5g): Colorless solid (34%). mp 248–250°C. 1H-NMR (CDCl3) δ: 1.76–1.86 (2H, m), 1.95–2.06 (2H, m), 2.17 (3H, s), 2.32 (3H, s), 2.72–2.76 (2H, m), 4.35 (1H, br), 5.43–5.49 (1H, m), 6.81 (1H, d, J=5.9 Hz), 7.03–7.16 (3H, m), 7.34–7.37 (1H, m), 7.77 (1H, d, J=5.9 Hz), 8.18 (1H, br). 13C-NMR (CDCl3) δ: 8.5, 11.6, 19.6, 29.3, 29.9, 48.6, 104.9, 108.2, 119.2, 126.0, 126.8, 128.9, 129.4, 132.7, 133.9, 136.2, 137.7, 138.4, 144.4. IR (ATR) cm−1: 1632, 1571, 1555, 1470, 1406. Anal. Calcd for C19H21N3: C, 78.32; H, 7.26; N, 14.42. Found: C, 78.08; H, 7.31; N, 14.47.
N-[7-(2,3-Dimethyl-1H-pyrrolo[2,3-c]pyridyl)]benzamide (5b)To a solution of 4 (306 mg, 1.7 mmol) in toluene (20 mL) were added Tris(dibenzylideneacetone) dipalladium (15.8 mg, 0.017 mmol), 9,9-dimethyl-4,5-bis(diphenylphosphino)xanthine (30.1 mg, 0.052 mmol), cesium carbonate (775 mg, 2.4 mmol) and benzamide (291 mg, 2.4 mmol), and the reaction mixture was stirred under an argon atmosphere at 120°C for 14 h. After cooling to room temperature, a saturated aqueous sodium hydrogencarbonate solution was added to the reaction mixture and the mixture was extracted with EtOAc. The extract was washed with a saturated aqueous sodium hydrogencarbonate solution, water and saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane–EtOAc=3 : 1) to give the title compound as colorless crystals (117 mg, 0.44 mmol, 26%). mp 163–165°C. 1H-NMR (CDCl3) δ: 2.22 (3H, s), 2.43 (3H, s), 7.20 (1H, d, J=5.7 Hz), 7.45–7.53 (2H, m), 7.54–7.62 (1H, m), 7.78 (1H, d, J=5.7 Hz), 7.97–8.05 (2H, m), 9.04 (1H, br s), 10.50 (1H, br s). 13C-NMR (CDCl3) δ: 8.4, 11.8, 107.4, 110.3, 122.0, 127.7, 128.7, 132.2, 133.8, 134.4, 136.0, 136.9, 137.3, 167.3. IR (ATR) cm−1: 1663, 1645, 1581, 1520, 1489, 1331. Electrospray ionization (ESI)-HR-MS m/z: 266.1287 (Calcd for C16H15N3O2S: 266.1288 [M+H]+).
7-Chloro-1,2,3-trimethyl-1H-pyrrolo[2,3-c]pyridine (6a)Sodium hydride (60% in oil, 329 mg, 8.2 mmol) was washed twice with hexane and suspended in N,N-dimethylformamide (DMF) (15 mL). A solution of 4 (1.19 g, 6.6 mmol) in DMF (5 mL) was added dropwise at 0°C. After stirring at the same temperature for 15 min, a solution of iodomethane (0.50 mL, 8.0 mmol) in DMF (5 mL) was added dropwise at 0°C and the mixture was stirred at room temperature for 14 h. Saturated aqueous sodium hydrogencarbonate solution was added to the reaction mixture and the mixture was extracted with EtOAc. The extract was washed with saturated aqueous sodium hydrogencarbonate solution, water and saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was crystallized from IPE to give the title compound as colorless crystals (0.93 g, 4.8 mmol, 72%). mp 126–127°C. 1H-NMR (CDCl3) δ: 2.22 (3H, s), 2.37 (3H, s), 4.04 (3H, s), 7.28 (1H, d, J=5.4 Hz), 7.91 (1H, d, J=5.4 Hz).
Following compounds 6b–d were prepared in the similar manner with compound 6a.
7-Chloro-1-ethyl-2,3-dimethyl-1H-pyrrolo[2,3-c]pyridine (6b): Colorless crystals (73%). mp 65–67°C. 1H-NMR (CDCl3) δ: 1.36 (3H, t, J=7.2 Hz), 2.21 (3H, s), 2.37 (3H, s), 4.51 (2H, q, J=7.2 Hz), 7.28 (1H, d, J=5.4 Hz), 7.91 (1H, d, J=5.4 Hz).
7-Chloro-2,3-dimethyl-1-propyl-1H-pyrrolo[2,3-c]pyridine (6c): Colorless crystals (89%). mp 72–73°C. 1H-NMR (CDCl3) δ: 0.97 (3H, t, J=6.9 Hz), 1.71–1.83 (2H, m), 2.21 (3H, s), 2.37 (3H, s), 4.35–4.41 (2H, m), 7.27 (1H, d, J=5.1 Hz), 7.91 (1H, d, J=5.1 Hz).
7-Chloro-1-isobutyl-2,3-dimethyl-1H-pyrrolo[2,3-c]pyridine (6d): Colorless crystals (83%). mp 56–57°C. 1H-NMR (CDCl3) δ: 0.90 (6H, d, J=6.6 Hz), 2.21–2.30 (m, 1H), 2.45 (3H, d, J=0.9 Hz), 4.23 (2H, br d, J=6.9 Hz), 6.29 (1H, d, J=0.9 Hz), 7.31 (1H, d, J=5.4 Hz), 7.92 (1H, d, J=5.4 Hz).
Following compounds 2a and 7a–e were prepared in the similar manner with compound 1.
N-Benzyl-2,3-dimethyl-1-propyl-1H-pyrrolo[2,3-c]pyridine-7-amine (2a): Pale-yellow crystals (33%). mp 51–52°C. 1H-NMR (CDCl3) δ: 0.83 (3H, t, J=7.5 Hz), 1.67–1.80 (2H, m), 2.17 (3H, s), 2.29 (3H, s), 4.04–4.09 (2H, m), 4.50 (1H, br), 4.74 (2H, d, J=5.4 Hz), 6.85 (1H, d, J=5.7 Hz), 7.26–7.37 (3H, m), 7.41–7.45 (2H, m), 7.77 (1H, d, J=5.7 Hz). 13C-NMR (CDCl3) δ: 8.9, 10.3, 10.9, 25.9, 46.5, 47.0, 105.5, 107.3, 120.6, 127.2, 127.9, 128.6, 133.9, 134.9, 135.9, 140.2, 145.3. IR (ATR) cm−1: 1607, 1554, 1473, 1368. Anal. Calcd for C19H23N3: C, 77.78; H, 7.90; N, 14.32. Found: C, 77.69; H, 7.83; N, 14.43.
N-Benzyl-1,2,3-trimethyl-1H-pyrrolo[2,3-c]pyridine-7-amine (7a): Colorless solid (56%). mp 112–113°C. 1H-NMR (CDCl3) δ: 2.17 (3H, s), 2.29 (3H, s), 3.90 (3H, s), 4.67 (1H, br d, J=5.4 Hz), 4.74 (2H, d, J=5.4 Hz), 6.84 (1H, d, J=5.7 Hz), 7.26–7.37 (3H, m), 7.42–7.57 (2H, m), 7.75 (1H, d, J=5.7 Hz). 13C-NMR (CDCl3) δ: 8.9, 10.3, 32.5, 46.4, 105.6, 107.1, 121.4, 127.1, 127.9, 128.6, 133.5, 135.2, 135.9, 140.3, 145.8. IR (ATR) cm−1: 1608, 1553, 1473, 1451, 1368. Anal. Calcd for C17H19N3: C, 76.95; H, 7.22; N, 15.84. Found: C, 77.05; H, 7.28; N, 16.02.
N-Benzyl-1-ethyl-2,3-dimethyl-1H-pyrrolo[2,3-c]pyridine-7-amine Hydrochloride (7b)The obtained free base was dissolved in methanol (5 mL), 10% hydrogen chloride methanol solution (2 mL) was added, and the mixture was concentrated under reduced pressure. The residue was crystallized from ethanol to give the title compound as a colorless solid (24%). mp 232–235°C. 1H-NMR (DMSO-d6) δ: 1.24 (3H, t, J=7.4 Hz), 2.17 (3H, s), 2.42 (3H, s), 4.53 (2H, q, J=7.4 Hz), 4.89 (2H, d, J=6.2 Hz), 7.10 (1H, d, J=7.0 Hz), 7.28–7.46 (6H, m), 8.00–8.20 (1H, m), 12.62 (1H, br s). 13C-NMR (DMSO-d6) δ: 8.3, 10.2, 17.0, 40.2, 44.8, 105.3, 109.6, 116.1, 124.5, 127.0, 127.3, 128.5, 134.2, 137.3, 141.0, 142.5. IR (ATR) cm−1: 1643, 1603. ESI-HR-MS m/z: 280.1811 (Calcd for C18H21N3: 280.1808 [M+H]+).
1-Ethyl-N-(4-fluoro-2-methylbenzyl)-2,3-dimethyl-1H-pyrrolo[2,3-c]pyridine-7-amine (7c): Colorless solid (75%). mp 102–104°C. 1H-NMR (CDCl3) δ: 1.29 (3H, t, J=7.2 Hz), 2.18 (3H, s), 2.30 (3H, s), 2.41 (3H, s), 4.16 (2H, q, J=7.2 Hz), 4.35 (1H, br t, J=5.1 Hz), 4.68 (2H, d, J=5.1 Hz), 6.82–6.90 (3H, m), 7.29–7.34 (1H, m), 7.78 (1H, d, J=5.4 Hz). 13C-NMR (CDCl3) δ: 8.9, 10.0, 17.4, 19.2, 40.1, 43.7, 105.5, 107.6, 112.5, 112.7, 116.9, 117.2, 120.2, 130.0, 130.2, 133.65, 133.69, 133.9, 134.5, 135.9, 139.0, 139.1, 145.1, 160.4, 163.6. IR (ATR) cm−1: 1608, 1555, 1496, 1474, 1367. Anal. Calcd for C19H22FN3: C, 73.28; H, 7.12; N, 13.49. Found: C, 73.06; H, 7.29; N, 13.48.
N-(4-Fluoro-2-methylbenzyl)-2,3-dimethyl-1-propyl-1H-pyrrolo[2,3-c]pyridine-7-amine (7d): Colorless solid (33%). mp 88–89°C. 1H-NMR (CDCl3) δ: 0.78 (3H, t, J=7.8 Hz), 1.66–1.74 (2H, m), 2.17 (3H, s), 2.29 (3H, s), 2.41 (3H, s), 3.99–4.04 (2H, m), 4.28 (1H, br t, J=5.1 Hz), 4.65 (2H, d, J=5.1 Hz), 6.85–6.94 (3H, m), 7.29–7.34 (1H, m), 7.78 (1H, d, J=5.4 Hz). 13C-NMR (CDCl3) δ: 8.9, 10.3, 10.8, 19.1, 25.9, 43.8, 47.0, 105.5, 107.3, 112.5, 112.8, 116.9, 117.2, 120.4, 130.2, 130.3, 133.66, 133.70, 133.8, 134.9, 135.9, 139.1, 139.2, 145.2, 160.4, 163.7. IR (ATR) cm−1: 1607, 1554, 1496, 1471, 1367. Anal. Calcd for C20H24FN3: C, 73.82; H, 7.43; N, 12.91. Found: C, 73.76; H, 7.42; N, 12.55.
N-(4-Fluoro-2-methylbenzyl)-1-isobutyl-2,3-dimethyl-1H-pyrrolo[2,3-c]pyridine-7-amine (7e): Colorless solid (65%). mp 102–103°C. 1H-NMR (CDCl3) δ: 0.74 (6H, d, J=6.9 Hz), 2.01–2.10 (1H, m), 2.17 (3H, s), 2.27 (3H, s), 2.41 (3H, s), 3.83 (2H, d, J=7.5 Hz), 4.24 (1H, br t, J=4.5 Hz), 4.64 (2H, d, J=4.5 Hz), 6.83–6.94 (3H, m), 7.29–7.34 (1H, m), 7.78 (1H, d, J=5.7 Hz). 13C-NMR (CDCl3) δ: 9.0, 10.9, 19.1, 19.6, 32.0, 43.9, 52.8, 105.4, 107.2, 112.5, 112.8, 116.9, 117.2, 120.6, 130.4, 130.5, 133.6, 133.7, 134.0, 135.4, 136.0, 139.2, 139.3, 145.3, 160.5, 163.7. IR (ATR) cm−1: 1606, 1554, 1496, 1469, 1369. Anal. Calcd for C21H26FN3: C, 74.30; H, 7.72; N, 12.38. Found: C, 74.13; H, 7.65; N, 12.19.
The following compounds 8, 9, and 10a were prepared in a similar manner with compounds 4, 6, and 7, respectively.
7-Chloro-2-methyl-1H-pyrrolo[2,3-c]pyridine (8): A pale-yellow solid (28%). mp 160–161°C. 1H-NMR (CDCl3) δ: 2.52 (3H, s), 6.30 (1H, s), 7.34 (1H, d, J=5.6 Hz), 7.98 (1H, d, J=5.6 Hz), 8.46 (1H, br).
7-Chloro-1-isobutyl-2-methyl-1H-pyrrolo[2,3-c]pyridine (9): An oil (81%). 1H-NMR (CDCl3) δ: 0.90 (6H, d, J=6.6 Hz), 2.21–2.30 (1H, m), 2.45 (3H, d, J=0.9 Hz), 4.23 (2H, br d, J=6.9 Hz), 6.29 (1H, d, J=0.9 Hz), 7.31 (1H, d, J=5.4 Hz), 7.92 (1H, d, J=5.4 Hz).
N-(4-Fluoro-2-methylbenzyl)-1-isobutyl-2-methyl-1H-pyrrolo[2,3-c]pyridine-7-amine (10a): A colorless solid (88%). mp 132–133°C. 1H-NMR (CDCl3) δ: 0.76 (6H, d, J=6.9 Hz), 2.04–2.18 (1H, m), 2.36 (3H, d, J=0.9 Hz), 2.42 (3H, s), 3.84 (2H, d, J=7.5 Hz), 4.25 (1H, br t, J=4.8 Hz), 4.65 (2H, d, J=4.8 Hz), 6.16 (1H, d, J=0.9 Hz), 6.85–6.96 (3H, m), 7.31–7.35 (1H, m), 7.78 (1H, d, J=5.4 Hz).
7-[(4-Fluoro-2-methylbenzyl)amino]-1-isobutyl-2-methyl-1H-pyrrolo[2,3-c]pyridine-3-carbaldehyde (10b)10a (339 mg, 1.0 mmol) was dissolved in nitromethane (4 mL) and 1,2-dichloroethane (4 mL), and aluminum(III) chloride (145 mg, 1.1 mmol) and dichloromethyl methyl ether (0.1 mL, 1.1 mmol) were added at 0°C. After stirring at the same temperature for 30 min, the same amount of aluminum(III) chloride and dichloromethyl methyl ether was added twice, and the mixture was stirred for 1 h. The reaction mixture was basified with 8 mol/L aqueous sodium hydroxide solution, and the mixture was extracted with EtOAc. The extract was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane–EtOAc=1 : 1) to give the title compound as a colorless solid (259 mg, 0.73 mmol, 73%). mp 129–130°C. 1H-NMR (CDCl3) δ: 0.81 (6H, d, J=6.6 Hz), 2.11–2.20 (1H, m), 2.41 (3H, s), 2.66 (3H, s), 3.90 (2H, d, J=6.9 Hz), 4.26 (1H, br t, J=4.5 Hz), 4.65 (2H, d, J=4.5 Hz), 6.86–6.97 (2H, m), 7.30–7.34 (1H, m), 7.57 (1H, d, J=5.7 Hz), 7.98 (1H, d, J=5.7 Hz), 10.18 (1H, s).
{7-[(4-Fluoro-2-methylbenzyl)amino]-1-isobutyl-2-methyl-1H-pyrrolo[2,3-c]pyridin-3-yl}methanol (10c)10b (216 mg, 0.61 mmol) was dissolved in methanol (5 mL) and THF (5 mL), and sodium borohydride (41 mg, 1.1 mmol) was added at 0°C. After stirring at room temperature for 1 h, several drops of acetic acid were added for a treatment, and the solvent was evaporated under reduced pressure. A 6% aqueous sodium hydrogen carbonate solution was added to the residue and the mixture was extracted with EtOAc. The extract was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was recrystallized from EtOAc–hexane to give the title compound as a yellow powder (145 mg, 0.41 mmol, 67%). mp 156–157°C. 1H-NMR (CDCl3) δ: 0.76 (6H, d, J=6.3 Hz), 1.25 (1H, t, J=5.1 Hz), 2.03–2.14 (1H, m), 2.39 (3H, s), 2.41 (3H, s), 3.86 (2H, d, J=6.9 Hz), 4.28 (1H, br t, J=4.8 Hz), 4.65 (2H, d, J=4.8 Hz), 4.77 (2H, d, J=5.1 Hz), 6.85–6.96 (2H, m), 7.01 (1H, d, J=5.4 Hz), 7.30–7.35 (1H, m), 7.84 (1H, d, J=5.4 Hz). 13C-NMR (CDCl3) δ: 10.9, 19.1, 19.6, 31.9, 43.8, 52.8, 55.8, 105.0, 111.8, 112.6, 112.9, 117.0, 117.3, 120.7, 130.5, 130.6, 133.1, 133.40, 133.44, 136.9, 137.0, 139.2, 139.3, 145.4, 160.5, 163.8. IR (ATR) cm−1: 1604, 1554, 1497, 1472, 1373. Anal. Calcd for C21H26FN3O: C, 70.96; H, 7.37; N, 11.82. Found: C, 70.72; H, 7.31; N, 11.68.
Proton Potassium—Adenosine Triphosphatase (H+/K+-ATPase) Inhibitory Activity TestAccording to the method of Wallmark et al.,15) a gastric mucosal membrane microsomal fraction was prepared from the stomach of swine. First, the stomach was removed, washed with tap water, immersed in 3 mol/L brine, and the surface of the mucosal membrane was wiped with a paper towel. The gastric mucosal membrane was detached, chopped, and homogenized in a 0.25 mol/L saccharose solution (pH 6.8) containing 1 mmol/L ethylenediaminetetraacetic acid (EDTA) and 10 mmol/L Tris-hydrochloric acid using polytron (Kinematica). The obtained homogenate was centrifuged at 20000×g for 30 min and the supernatant was centrifuged at 100000×g for 90 min. The precipitate was suspended in 0.25 mol/L saccharose solution, superimposed on a 0.25 mol/L saccharose solution containing 7.5% Ficoll, and centrifuged at 100000×g for 5 h. The fraction containing the interface between the both layers was recovered, and centrifugally washed with 0.25 mol/L saccharose solution. The obtained microsomal fraction was used as a proton, potassium–adenosine triphosphatase standard product. To 40 mL of a 50 mmol/L N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES)-Tris buffer (5 mmol/L magnesium chloride, 10 mmol/L potassium chloride, 10 µmol/L valinomycin, pH=6.5) containing 2.5 µg/mL (based on the protein concentration) of the enzyme standard product was added a test compound (5 mL) dissolved in a 10% aqueous dimethyl sulfoxide solution, and the mixture was incubated at 37°C for 30 min. The enzyme reaction was started by adding 5 mL of a 2 mmol/L adenosine triphosphate tris salt solution (50 mmol/L HEPES-Tris buffer (5 mmol/L magnesium chloride, pH 6.5)). The enzyme reaction was carried out at 37°C for 20 min, and 15 mL of a malachite green solution (0.12% malachite green solution in sulfuric acid (2.5 mol/L), 7.5% ammonium molybdate and 11% Tween 20 were mixed at a ratio of 100 : 25 : 2) was added to quench the reaction. After allowing to stand at room temperature for 15 min, the resulting reaction product of inorganic phosphorus with malachite green was colorimetrically determined at a wavelength of 610 nm. In addition, the amount of the inorganic phosphoric acid in the reaction solution free of potassium chloride was measured in the same manner, which was subtracted from the inorganic phosphoric acid amount in the presence of potassium chloride to determine the H+/K+-ATPase activity. The inhibitory rate (%) was determined from the activity value of the control and the activity values of various concentrations of the test compound, and the 50% inhibitory concentration (IC50) of the H+/K+-ATPase activity was determined.
Inhibiton of Histamine-Stimulated Acid Secretion in Anesthetized Rats (i.v.)Animal experiments were carried out in accordance with ethical guidelines established by the Animal Care and Use Committee at Takeda Pharmaceutical Company, Ltd. Seven-week-old male Jcl: Sprague-Dawley (SD) rats were used. The animals were fasted for 24 h but had free access to water before the experiment. The pylorus was ligated after anesthetization with urethane (1.2 g/kg, intraperitoneally (i.p.)) and the abdomen was closed. Drugs and the vehicle were given intravenously just after the pylorus ligation. Three minutes later, histamine 2HCl (30 mg/kg/10 mL) was injected subcutaneously. Three hours after histamine administration, the rats were sacrificed by CO2 asphyxiation and the stomachs were removed. The gastric contents were collected and centrifuged at 3000 rpm for 10 min. The volume of each sample was measured and the acid concentration was determined by automatic titration to pH 7.0 with 0.1 mol/L NaOH (COM-555SC; Hiranuma Sangyo Co., Ltd., Japan), and the total acid output during the 3 h period (mEq/3 h) was calculated.
We would like to thank Dr. Satoshi Endo for his helpful instructions.