2014 年 62 巻 4 号 p. 343-349
2014 年 62 巻 4 号 p. 343-349
Novel hybrids 8a–j and 9a–j were designed and synthesized by coupling the carboxyl group of hydroxylcinnamic acids with tetrahydro-β-carboline alkaloids which were linked with different substituted nitrogen-containing heterocycles at the positions-N9, and their in vitro biological activities were evaluated. It was found that most hybrids showed good to moderate anti-tumor activities. Especially, compound 9j had a great potency superior to 5-fluorouracil (5-FU) and comparable to adriamycin in human cancer cells, and could selectively inhibit tumor cells, but not inhibit non-tumor cell proliferation in vitro. More importantly, apoptosis assay indicated that 9j could significantly induce tumor cell apoptosis in a dose-dependent manner. Therefore, our novel findings may provide a new framework for the design of new hybrids for the intervention of human cancers.
Cancer is a major health problem worldwide and is the leading cause of human mortality exceeded only by cardiovascular diseases.1) Therefore, development of new anticancer drugs and more effective treatment strategies for cancer are of utmost importance when traditionally prescribed chemotherapeutic agents have problems with toxicity and drug resistance.2)
The tetrahydro-β-carboline alkaloids, a large group of synthetic and naturally occurring polycyclic indolic compounds, have attracted a great deal of interest amongst medicinal chemists and pharmacologists over the years, partially due to their widespread potent biological and pharmaceutical properties including antimalarial, antitumor, anti-human immunodeficiency virus (HIV), anti-thrombotic, and antimicrobial activities.3–8) Recently, more attention has been focused on the study of the tetrahydro-β-carboline derivatives about their potential antitumor activities.4,5,9,10) Current issues in cancer research show that many tricyclic ring carboline compounds which display potent cytotoxicity against numerous cancer cell lines are harman and norharman derivatives,11) and more complex structures such as manzanine,12) azatoxin,13) eudistomine K,14) fascaplysine,15) and picrasidine L.16) Therefore, the structural fragment of tetrahydro-β-carboline seems to be essential for many biologically important indole alkaloids and represents an important lead structure for the development of novel anticancer agents.
In an ongoing study on discovering new anti-tumor agents for human use, we were inspired by the fact that hydroxylcinnamic acids, such as ferulic acid and p-hydroxy-cinnamic acid, are known as phenolic compounds occurring in natural plant product, and their derivatives displayed selective antiproliferative activity against some types of cancer cells,17–19) which attracts considerable attention from medicinal chemists. There is an increased interest in the use of hybrid molecules for drug discovery against a multitude of disease indications.20,21) Many tetrahydro-β-carboline hybrids bearing cinnamoyl or aroyl group at the N2 position, reported in recent literatures, exhibit excellent biological acitivities.22,23) With these ideas in mind, we conjugated tetrahydro-β-carboline alkaloid with hydroxylcinnamic acid and different nitrogen-containing heterocycle was introduced to positions-N9 of tetrahydro-β-carboline through carbonchain with the purpose for the improvement of water-solubility. Thus, we hypothesized that the novel types of tetrahydro-β-carboline/hydroxylcinnamic acid hybrids will be more efficacious and selectively inhibit tumor cell proliferation. Therefore, a total of twenty target compounds (8a–j, 9a–j) were designed and synthesized, and their in vitro antitumor effects were investigated. Herein, the synthesis and preliminary biological evaluation of these compounds were reported.
The synthesis of 8a–j and 9a–j was described in Chart 1. The 1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid 2 was prepared by the condensation of L-tryptophan 1 with aqueous 37% formaldehyde according to the Pictet–Spengler reaction. Then the carboxyl group of compound 2 was subsequently methyl-esterified through SOCl2 in MeOH solution to afford 3. In addition, the phenolic hydroxyl of hydroxylcinnamic acid 4a or 4b was protected by acetic anhydride to form acetylated product 5a or 5b, which was further reacted with intermediate 3 to obtain compound 6 in the presence of N-methylmorpholine and ethyl chloroformate. The aromatic secondary amine of compound 6 was substituted with dibromopropane to yield compound 7, which was then transformed to 8a–j after treatment with nitrogen-containing heterocycles. Finally, compounds 8a–j were hydrolyzed to gain the target compounds 9a–j. The products 8a–j and 9a–j were purified by column chromatography, and their structures were characterized by IR, 1H-NMR, 13C-NMR, MS, and elemental analyses.

Reaction conditions and reagents: a) 1 N NaOH, HCHO, r.t. to 38°C, 3 h, 82%; b) SOCl2, MeOH, 0°C-reflux, 6 h, 94%; c) Ac2O, NaOH, 0°C, 1 h, 88%; d) ethyl chloroformate, N-methylmorpholine, THF, 0°C to r.t., 1 h; e) 3, TEA, THF, 0°C, 1 h, 56–75%; f) NaH, Br(CH2)3Br, DMF, 0°C, 1 h; g) KI, K2CO3, nitrogen-containing heterocycles, CH3CN, 50°C, 3–6 h, 52–69%; h) 2 N NaOH, MeOH, 65°C, 2–6 h, 81–90%.
The inhibitory activities of synthetic compounds 8a–j, 9a–j against six human cancer cells SMMC-7721, Hep G2 (human hepatoma cells), SGC7901 (human gastric cancer cells), HCT-116 (human colon carcinoma cells), MCF-7 (human breast adenocarcinoma cells), SKOV-3 (human ovarian cancer cells) were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays in vitro, and 5-fluorouracil (5-FU) and adriamycin were used as positive controls. The results (shown in Table 1) illustrated the IC50 values of target compounds against each tumor cell line. The antitumor activities of intermediates 8a–j were slightly weaker than target compounds 9a–j. Compounds 8g, 8i–j, and 9g–j showed good to moderate cytotoxic activities, which were significantly superior to 5-FU. Among these compounds, compound 9j (IC50=6.93–9.32 µM) exhibited higher antiproliferative activities than the other target compounds against six tumor cells, and its IC50 values were especially 3–6-fold less than those of 5-FU (IC50=19.36–43.52 µM), and comparable to or slightly weaker than those of adriamycin (IC50=1.92–4.72 µM).
| Compound | In vitro inhibition of human cancer cells proliferation (IC50a), µM) | |||||
|---|---|---|---|---|---|---|
| SMMC-7721 | SGC-7901 | HCT116 | Hep G2 | MCF-7 | SKOV-3 | |
| 5-FU | 39.03 | 41.35 | 32.84 | 43.52 | 19.36 | 41.65 |
| Adriamycin | 1.92 | 3.39 | 4.72 | 3.48 | 2.78 | 4.63 |
| 8a | >50 | >50 | >50 | 37.01 | >50 | 43.11 |
| 8b | 29.12 | >50 | 32.46 | 26.65 | 25.28 | 27.82 |
| 8c | 23.71 | 32.53 | >50 | 30.98 | 35.13 | 30.02 |
| 8d | 23.26 | 15.27 | 26.58 | 28.00 | 17.88 | 15.21 |
| 8e | 26.00 | 18.09 | 23.22 | 25.38 | 21.14 | 18.62 |
| 8f | >50 | 41.34 | >50 | 38.98 | >50 | 33.77 |
| 8g | 10.12 | 9.53 | 9.18 | 8.72 | 9.24 | 10.03 |
| 8h | 14.56 | 11.50 | 18.66 | 19.56 | 20.10 | 13.98 |
| 8i | 7.22 | 9.26 | 11.89 | 8.36 | 9.01 | 11.35 |
| 8j | 7.02 | 8.44 | 10.75 | 7.71 | 7.93 | 9.74 |
| 9a | >50 | 32.09 | 28.36 | 30.08 | >50 | 35.91 |
| 9b | 16.73 | 21.87 | 25.09 | 22.90 | 19.25 | 29.38 |
| 9c | 26.15 | 29.91 | 18.45 | 24.34 | 20.62 | 22.38 |
| 9d | 21.16 | 17.55 | 15.23 | 15.23 | 15.22 | 13.35 |
| 9e | 18.25 | 18.42 | 14.85 | 12.58 | 16.75 | 11.33 |
| 9f | 31.69 | 22.29 | >50 | 26.32 | 24.37 | 25.99 |
| 9g | 12.28 | 8.25 | 11.83 | 9.78 | 9.86 | 9.18 |
| 9h | 11.09 | 9.46 | 13.95 | 10.16 | 12.11 | 11.26 |
| 9i | 7.98 | 7.25 | 10.07 | 6.68 | 9.17 | 8.21 |
| 9j | 6.45 | 6.63 | 9.32 | 5.93 | 7.76 | 9.14 |
a) The inhibitory effects of individual compounds on the proliferation of cancer cell lines were determined by the MTT assay. The data are the mean values of IC50 from at least three independent experiments.
Given that most of tetrahydro-β-carboline/hydroxylcinnamic acid hybrids show strong anticancer activities, we wonder to know whether those compounds were toxic to the normal cells or not. So compound 9j with the highest anticancer activities was further determined to compare the inhibitory activities of hepatocellular carcinoma cells (SMMC-7721, Hep G2) with those of human normal cell (LO2) by MTT assay. As shown in Fig. 1, it was found that compound 9j (1.5–12.5 µM) inhibited hepatocellular carcinoma cell proliferation in a concentration-dependent manner, and treatment with 12.5 µM 9j promoted nearly 90% of the inhibition in tumor cells, but the same treatment had no significant inhibitory effect on non-tumor liver LO2 cell proliferation (only 20% of inhibition). Apparently, these results indicated that compound 9j had strong cytotoxicity selectively to human cancer cells in vitro.

SMMC-7721, Hep G2, and LO2 cells were incubated with the indicated concentrations of 9j for 48 h. Cell proliferation was assessed using the MTT assay. Data are means±S.E.M. of the inhibition (%) from three independent experiments.
To determine whether the inhibitory activities on tumor cells of 9j were due to cell apoptosis, apoptosis assay was further assessed employing Hep G2 cells which were incubated with vehicle alone or with different concentrations of 9j (3.0, 6.0 or 12 µM) for 48 h. The percentages of the apoptotic Hep G2 cells, presented in Fig. 2, were determined by fluorescein isothiocyanate (FITC)-Annexin V/propidium iodide (PI) staining and flow cytometry analysis. In the untreated group, the frequency of Hep G2 cell apoptosis was unobvious. However, the percentage of apoptotic Hep G2 cells was gradually increased for those cells exposed to increasing concentrations of 9j (23.4% for 3.0 µM; 45.8% for 6.0 µM; and 84.5% for 12 µM), which demonstrated that incubation with 9j could induce tumor cell apoptosis in a dose-dependent manner.
Structure–Activity Relationship (SAR)Analysis of SAR revealed that the antiproliferative activities of tetrahydro-β-carboline/ferulic acid hybrids 8f–j and 9f–j were more potent than that of tetrahydro-β-carboline/p-hydroxy-cinnamic acid hybrids 8a–d and 9a–d, which suggested that the electron-donating substitutions (OCH3) on the ferulic acid derivatives were able to confer antitumor activities to these molecules. Secondary, different substituted nitrogen-containing heterocycles connected with tetrahydro-β-carboline were also crucial for their anticancer activities in vitro. These hybrid molecules linked with piperazine ring, especially for N-methylpiperazine, exibited the outstanding antiprofication effects while the hybrids linked with tetrahydropyrrole group showed extremely weak activities. The relative antitumor activities of tetrahydro-β-carboline/hydroxylcinnamic acid hybrids with varying heterocycles were as following: N-methylpiperazine>piperazine>piperide>morpholine>tetrahydropyrrole. Finally, the antitumor activities of intermediates 8a–j containing ester groups were slightly weaker than their hydrolyzates 9a–j containing carboxyl and phenolic hydroxyl groups. However, further investigation about the precise SAR of these compounds is ongoing.
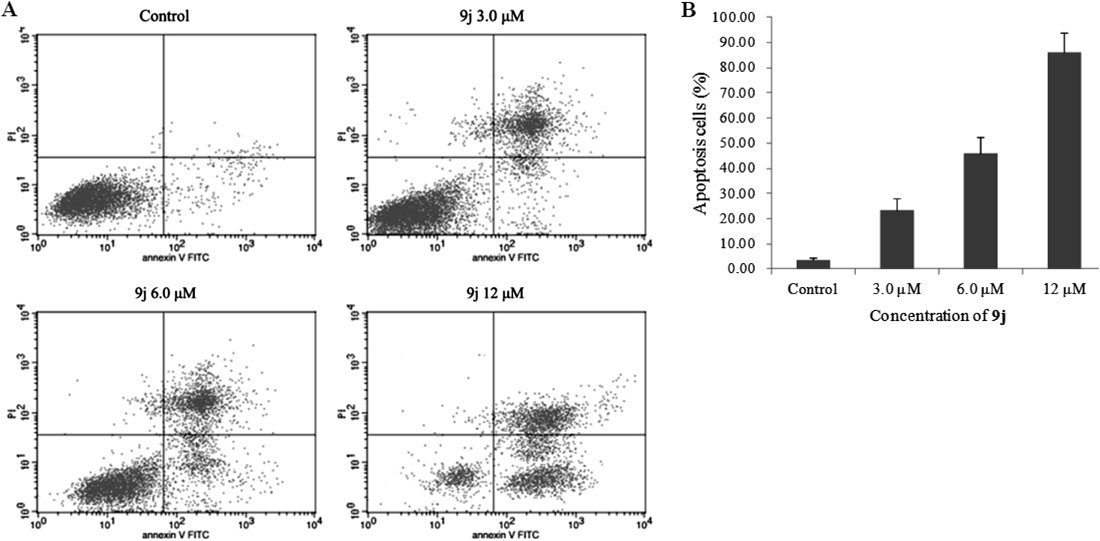
Hep G2 cells were incubated with the indicated concentrations of 9j for 48 h, and the cells were stained with FITC-Annexin V and PI, followed by flow cytometry analysis. (A) Flow cytometry analysis. (B) Quantitative analysis of apoptotic cells. Data are expressed as means±S.E.M. of the percentages of apoptotic cells from three independent experiments.
In conclusion, a novel series of hybrids from tetrahydro-β-carboline and hydroxylcinnamic acid were designed and synthesized, and their in vitro antitumor effects were investigated. It was found that most hybrids displayed strong antitumor activities against six human cancer cells in vitro. The hybrid 9j containing ferulic acid and N-methylpiperazine ring was the most active, with IC50s of 5.93–9.14 µM against all tested cancer cells, which were even more potent than 5-FU and comparable to adriamycin. Furthermore, compound 9j could selectively inhibit tumor cells, but not inhibit non-tumor cell proliferation. More importantly, apoptosis assay indicated that 9j could significantly induce cell apoptosis in a dose-dependent manner. Our findings suggest that the tetrahydro-β-carboline/hydroxylcinnamic acid hybrids may hold greater promise as therapeutic agents for the intervention of human cancers.
Melting points were determined on Yanaco MP-J3 microscope melting point apparatus. Infrared (IR) spectra (KBr) were recorded on a Nicolet Impact 410 instrument (KBr pellet). Proton and carbon nuclear magnetic resonance (1H- and 13C-NMR) spectra were recorded with a Bruker Avance 300 MHz spectrometer at 300 K, with chemical shift in parts per million (ppm, δ) downfield from TMS as an internal standard. MS spectra were recorded on a Mariner Mass Spectrum (electrospray ionization (ESI)). Element analysis was performed on an Eager 300 instrument. All compounds were routinely checked by TLC and 1H-NMR. TLCs and preparative thin-layer chromatography were performed on silica gel GF/UV 254, and the chromatograms were conducted on silica gel (200–300 mesh, Merck) and visualized under UV light at 254 and 365 nm. L-Tryptophan 1 and compounds 4a, b were commercially available, and compounds 2, 3, and 5a, b were prepared according the literatures.24,25) All solvents were reagent grade and, when necessary, were purified and dried by standards methods. Solutions after reactions and extractions were concentrated using a rotary evaporator operating at a reduced pressure of ca. 20 Torr. Organic solutions were dried over anhydrous sodium sulfate.
General Procedure for the Synthesis of 6a, bTo a solution of 5 (12 mmol) in 50 mL anhydrous tetrahydrofuran (THF) at 0°C were added N-methylmorpholine (1.57 g, 18 mmol) and ethyl carbonochloridate (1.30 g, 12 mmol), and the mixture was stirred at room temperature for 1–2 h, which was then added dropwise to a solution of 3 (2.76 g, 12 mmol) in 20 mL anhydrous THF. After the reaction was completed, the resulting mixture was allowed to pour into ice-water, and extracted with ethyl acetate (30 mL×3). The organic phase was washed with water and brine, then dried over anhydrous sodiumsulfate, filtered and evaporated to afford the crude product. The crude was purified by column chromatography on silica gel to give compound 6.
(S,E)-Methyl 2-(3-(4-Acetoxyphenyl)acryloyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (6a): The title compound was obtained starting from 5a and 3. White solid, mp 191–194°C; yield: 73%. MS (ESI) m/z=419 [M+H]+.
(S,E)-Methyl 2-(3-(4-Acetoxy-3-methoxyphenyl)acryloyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (6b): The title compound was obtained starting from 5b and 3. White solid, mp 176–179°C; yield: 70%. MS (ESI) m/z=449 [M+H]+.
General Procedure for the Synthesis of 8a–jCompound 6 (2.0 mmol) was dissolved in 25 mL anhydrous N,N-dimethylformamide (DMF) until clear, and then 60% NaH (0.083 g, 2.1 mmol) and 1,3-dibromopropane (0.43 g, 2.0 mmol) were slowly added in sequence at 0°C. The mixture was stirred at room temperature for 1 h, and then nitrogen-containing heterocycles (2.0 mmol), KI (0.033 g, 0.2 mmol), and K2CO3 (0.42 g, 3.0 mmol) were added to the reaction solution and continuously stirred at 50°C for 3–6 h. After the reaction was completed, the resulting mixture was filtered and evaporated to obtain the crude product. The obtained was purified by silica column chromatography (CH2Cl2–MeOH=10 : 1–5 : 1, v/v as the eluent) to gain compound 8a–j.
(S,E)-Methyl 2-(3-(4-Acetoxyphenyl)acryloyl)-9-(3-(pyrrolidin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (8a): The title compound was obtained starting from 6a, 1,3-dibromopropane, and pyrrolidine. White solid, mp 118–121°C; yield: 62%; MS (ESI) m/z=530 [M+H]+.
(S,E)-Methyl 2-(3-(4-Acetoxyphenyl)acryloyl)-9-(3-(piperidin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (8b): The title compound was obtained starting from 6a, 1,3-dibromopropane, and piperidine. Pale yellow solid, mp 97–100°C; yield: 69%; MS (ESI) m/z=544 [M+H]+.
(S,E)-Methyl 2-(3-(4-Acetoxyphenyl)acryloyl)-9-(3-morpholinopropyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (8c): The title compound was obtained starting from 6a, 1,3-dibromopropane, and morpholine. Pale yellow solid, mp 106–109°C; yield: 58%; MS (ESI) m/z=546 [M+H]+.
(S,E)-Methyl 2-(3-(4-Acetoxyphenyl)acryloyl)-9-(3-(piperazin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (8d): The title compound was obtained starting from 6a, 1,3-dibromopropane, and piperazine. Pale yellow solid, mp 123–125°C; yield: 53%; MS (ESI) m/z=545 [M+H]+.
(S,E)-Methyl 2-(3-(4-Acetoxyphenyl)acryloyl)-9-(3-(4-methylpiperazin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (8e): The title compound was obtained starting from 6a, 1,3-dibromopropane, and N-methylpiperazine. Pale yellow solid, mp 127–130°C; yield: 53%; MS (ESI) m/z=559 [M+H]+.
(S,E)-Methyl 2-(3-(4-Acetoxy-3-methoxyphenyl)acryloyl)-9-(3-(pyrrolidin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (8f): The title compound was obtained starting from 6b, 1,3-dibromopropane, and pyrrolidine. Pale yellow solid, mp 110–112°C; yield: 65%; MS (ESI) m/z=560 [M+H]+.
(S,E)-Methyl 2-(3-(4-Acetoxy-3-methoxyphenyl)acryloyl)-9-(3-(piperidin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (8g): The title compound was obtained starting from 6b, 1,3-dibromopropane, and piperidine. Pale yellow solid, mp 102–105°C; yield: 61%; MS (ESI) m/z=574 [M+H]+.
(S,E)-Methyl 2-(3-(4-Acetoxy-3-methoxyphenyl)acryloyl)-9-(3-morpholinopropyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (8h): The title compound was obtained starting from 6b, 1,3-dibromopropane, and morpholine. Yellow solid, mp 104–106°C; yield: 56%; MS (ESI) m/z=576 [M+H]+.
(S,E)-Methyl 2-(3-(4-Acetoxy-3-methoxyphenyl)acryloyl)-9-(3-(piperazin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (8i): The title compound was obtained starting from 6b, 1,3-dibromopropane, and piperazine. Pale yellow solid, mp 119–122°C; yield: 52%; MS (ESI) m/z=575 [M+H]+.
(S,E)-Methyl 2-(3-(4-Acetoxy-3-methoxyphenyl)acryloyl)-9-(3-(4-methylpiperazin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (8j): The title compound was obtained starting from 6b, 1,3-dibromopropane, and N-methylpiperazine. Pale yellow solid, mp 127–130°C; yield: 54%; MS (ESI) m/z=589 [M+H]+.
General Procedure for the Synthesis of 9a–jA suspension of 8 (1 mmol) in 5 mL methanol containing 1.5 mL 2 N NaOH was stirred and refluxed for 2–6 h, and then cooled. The solvent was evaporated and the residue was dissolved in 1 M HCl solution. The solvent was evaporated, the residue was neutralized to pH=5 with 1 M HCl. The precipitate was filtered, washed with water, and dried in vacuum to afford the target compound 9a–j.
(S,E)-2-(3-(4-Hydroxyphenyl)acryloyl)-9-(3-(pyrrolidin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic Acid (9a): The title compound was obtained starting from 8a. Pale yellow solid, mp 136–139°C; yield: 85%. Analytical data for 9a: IR (KBr, cm−1): 3432, 2944, 1719, 1610, 1432, 1215, 1021; 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.64 (m, 2H, Ar-H), 7.59 (d, 1H, J=16.2 Hz, CH), 7.30 (m, 1H, Ar-H), 7.18–7.24 (m, 3H, Ar-H), 7.00 (d, J=7.5 Hz, 1H, Ar-H), 6.88 (d, J=7.5 Hz, 1H, Ar-H), 6.41 (d, 1H, J=16.2 Hz, CH), 4.57 (m, 1H, NCH), 4.46 (m, 1H, NCH2Ar), 4.28 (m, 1H, NCH2Ar), 4.10 (t, J=6.9 Hz, 2H, NCH2), 2.97 (m, 1H, ArCH2), 2.62 (d, J=7.5 Hz, 1H, ArCH2), 2.22–2.35 (m, 6H, 3×NCH2), 1.80–1.88 (m, 2H, NCH2CH2), 1.60–1.65 (m, 4H, CH2CH2); 13C-NMR (DMSO-d6, 75 MHz, δ ppm): 174.6, 163.2, 153.7, 138.1, 136.2, 130.3, 128.5, 127.4, 125.3, 120.9, 118.5, 117.8, 115.6, 111.5, 105.4, 60.5, 56.3, 54.6, 51.2, 45.8, 28.5, 26.2, 23.5; MS (ESI) m/z=474 [M+H]+; Anal. Calcd for C28H31N3O4: C, 71.01; H, 6.60; N, 8.87; Found: C, 70.83; H, 6.72; N, 8.98.
(S,E)-2-(3-(4-Hydroxyphenyl)acryloyl)-9-(3-(piperidin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic Acid (9b): The title compound was obtained starting from 8b. Pale yellow solid, mp 128–131°C; yield: 88%. Analytical data for 9b: IR (KBr, cm−1): 3446, 2951, 1723, 1618, 1436, 1219, 1028; 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.65–7.69 (m, 2H, Ar-H), 7.62 (d, 1H, J=16.2 Hz, CH), 7.33 (m, 1H, Ar-H), 7.15–7.22 (m, 3H, Ar-H), 7.03 (d, J=7.5 Hz, 1H, Ar-H), 6.90 (m, 1H, Ar-H), 6.45 (d, 1H, J=16.2 Hz, CH), 4.48–4.56 (m, 2H, NCH, NCH2Ar), 4.28 (m, 1H, NCH2Ar), 4.07 (t, J=6.9 Hz, 2H, NCH2), 2.95 (m, 1H, ArCH2), 2.59 (d, J=7.5 Hz, 1H, ArCH2), 2.23–2.38 (m, 6H, 3×NCH2), 1.83–1.92 (m, 2H, NCH2CH2), 1.57–1.63 (m, 4H, 2×NCH2CH2), 1.38–1.48 (m, 2H, CH2); 13C-NMR (DMSO-d6, 75 MHz, δ ppm): 174.2, 161.9, 153.5, 138.3, 136.5, 130.4, 128.5, 127.4, 125.5, 121.2, 118.8, 117.7, 115.9, 111.7, 105.2, 61.1, 56.8, 54.4, 51.5, 45.7, 28.8, 26.3, 24.3, 22.9; MS (ESI) m/z=488 [M+H]+; Anal. Calcd for C29H33N3O4: C, 71.44; H, 6.82; N, 8.62; Found: C, 71.52; H, 6.93; N, 8.51.
(S,E)-2-(3-(4-Hydroxyphenyl)acryloyl)-9-(3-morpholinopropyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic Acid (9c): The title compound was obtained starting from 8c. Pale yellow solid, mp 141–144°C; yield: 85%. Analytical data for 9c: IR (KBr, cm−1): 3440, 2943, 1710, 1609, 1425, 1207, 1020; 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.67 (m, 2H, Ar-H), 7.58 (d, 1H, J=16.2 Hz, CH), 7.17–7.29 (m, 4H, Ar-H), 7.06 (d, J=7.5 Hz, 1H, Ar-H), 6.91 (m, 1H, Ar-H), 6.41 (d, 1H, J=16.2 Hz, CH), 4.45–4.57 (m, 2H, NCH, NCH2Ar), 4.24 (m, 1H, NCH2Ar), 4.03 (t, J=6.9 Hz, 2H, NCH2), 3.72 (t, J=4.2 Hz, 4H, 2×OCH2), 2.92 (m, 1H, ArCH2), 2.55 (m, 1H, ArCH2), 2.26–2.41 (m, 6H, 3×NCH2), 1.85–1.93 (m, 2H, NCH2CH2); 13C-NMR (DMSO-d6, 75 MHz, δ ppm): 174.7, 164.7, 153.8, 138.1, 135.9, 130.9, 128.6, 127.7, 125.8, 121.6, 118.7, 115.7, 111.3, 105.9, 66.1, 61.2, 59.2, 56.5, 55.1, 51.6, 46.0, 29.2, 26.9; MS (ESI) m/z=490 [M+H]+; Anal. Calcd for C28H31N3O5: C, 68.69; H, 6.38; N, 8.58; Found: C, 68.82; H, 6.54; N, 8.37.
(S,E)-2-(3-(4-Hydroxyphenyl)acryloyl)-9-(3-(piperazin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic Acid (9d): The title compound was obtained starting from 8d. Pale yellow solid, mp 175–177°C; yield: 81%. Analytical data for 9d: IR (KBr, cm−1): 3465, 2954, 1733, 1626, 1434, 1223, 1117, 1032; 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.66–7.71 (m, 2H, Ar-H), 7.60 (d, 1H, J=16.2 Hz, CH), 7.33 (m, 1H), 7.15–7.26 (m, 3H, Ar-H), 7.02 (d, J=7.5 Hz, 1H, Ar-H), 6.89 (d, J=7.5 Hz, 1H, Ar-H), 6.37 (d, 1H, J=16.2 Hz, CH), 4.59 (m, 1H, NCH), 4.47 (m, 1H, NCH2Ar), 4.27 (m, 1H, NCH2Ar), 4.08 (t, J=6.9 Hz, 2H, NCH2), 3.05 (m, 4H, 2×NCH2), 2.92 (m, 1H, ArCH2), 2.31–2.67 (m, 7H, ArCH2, 3×NCH2), 1.83–1.94 (m, 2H, NCH2CH2); 13C-NMR (DMSO-d6, 75 MHz, δ ppm): 173.5, 161.8, 153.2, 137.7, 135.6, 130.1, 127.9, 126.8, 125.3, 120.2, 118.3, 117.4, 115.3, 111.8, 106.2, 60.8, 56.1, 54.2, 51.0, 47.8, 45.5, 28.8, 27.3; MS (ESI) m/z=489 [M+H]+; Anal. Calcd for C28H32N4O4: C, 68.83; H, 6.60; N, 11.47; Found: C, 68.76; H, 6.73; N, 11.59.
(S,E)-2-(3-(4-Hydroxyphenyl)acryloyl)-9-(3-(4-methylpiperazin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic Acid (9e): The title compound was obtained starting from 8e. Pale yellow solid, mp 157–160°C; yield: 83%. Analytical data for 9e: IR (KBr, cm−1): 3443, 2940, 1726, 1618, 1423, 1170, 1025; 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.69 (m, 2H, Ar-H), 7.57 (d, 1H, J=16.2 Hz, CH), 7.17–7.30 (m, 4H, Ar-H), 6.98 (d, J=7.5 Hz, 1H, Ar-H), 6.85 (m, 1H, Ar-H), 6.33 (d, 1H, J=16.2 Hz, CH), 4.41–4.55 (m, 2H, NCH, NCH2Ar), 4.22 (m, 1H, NCH2Ar), 4.01 (t, J=6.9 Hz, 2H, NCH2), 2.99–3.08 (m, 4H, 2×NCH2), 2.92 (m, 1H, ArCH2), 2.59 (m, 1H, ArCH2), 2.55 (s, 3H, NCH3), 2.27–2.50 (m, 6H, 3×NCH2), 1.80–1.91 (m, 2H, NCH2CH2); 13C-NMR (DMSO-d6, 75 MHz, δ ppm): 174.2, 163.5, 153.6, 138.6, 136.8, 131.1, 128.7, 127.7, 125.3, 121.5, 118.8, 117.9, 116.0, 112.1, 106.0, 62.4, 57.7, 56.1, 54.9, 49.6, 47.1, 29.5, 26.5; MS (ESI) m/z=503 [M+H]+; Anal. Calcd for C29H34N4O4: C, 69.30; H, 6.82; N, 11.15; Found: C, 69.44; H, 6.71; N, 11.32.
(S,E)-2-(3-(4-Hydroxy-3-methoxyphenyl)acryloyl)-9-(3-(pyrrolidin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic Acid (9f): The title compound was obtained starting from 8f. Pale yellow solid, mp 130–132°C; yield: 87%. Analytical data for 9f: IR (KBr, cm−1): 3416, 2933, 1709, 1611, 1405, 1131, 1017; 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.69 (m, 1H, Ar-H), 7.61 (d, 1H, J=16.2 Hz, CH), 7.27 (m, 1H, Ar-H), 7.18 (m, 1H, Ar-H), 6.99 (m, 1H, Ar-H), 6.71–6.88 (m, 3H, Ar-H), 6.36 (d, 1H, J=16.2 Hz, CH), 4.44–4.58 (m, 2H), 4.25 (m, 1H), 4.05 (t, J=6.9 Hz, 2H), 2.94 (m, 1H), 2.59 (d, J=7.5 Hz, 1H), 2.20–2.33 (m, 6H, 3×NCH2), 1.85–1.94 (m, 2H, NCH2CH2), 1.57–1.66 (m, 4H, CH2CH2); 13C-NMR (DMSO-d6, 75 MHz, δ ppm): 173.7, 164.5, 153.1, 149.2, 137.4, 135.6, 130.2, 128.4, 127.1, 125.6, 121.3, 118.4, 117.3, 116.3, 112.3, 105.8, 60.8, 56.3, 54.8, 51.6, 46.5, 28.7, 26.5, 23.2; MS (ESI) m/z=504 [M+H]+; Anal. Calcd for C29H33N3O5: C, 69.17; H, 6.60; N, 8.34; Found: C, 69.06; H, 6.75; N, 8.23.
(S,E)-2-(3-(4-Hydroxy-3-methoxyphenyl)acryloyl)-9-(3-(piperidin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic Acid (9g): The title compound was obtained starting from 8g. Pale yellow solid, mp 123–125°C; yield: 90%. Analytical data for 9g: IR (KBr, cm−1): 3445, 2952, 1731, 1617, 1422, 1231, 1024; 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.67–7.71 (m, 1H, Ar-H), 7.58 (d, 1H, J=16.2 Hz, CH), 7.30 (m, 1H, Ar-H), 7.19 (m, 1H, Ar-H), 7.02 (d, J=7.5 Hz, 1H, Ar-H), 6.66–6.87 (m, 3H, Ar-H), 6.35 (d, 1H, J=16.2 Hz, CH), 4.59 (m, 1H, NCH), 4.47 (m, 1H, NCH2Ar), 4.29 (m, 1H, NCH2Ar), 4.07 (t, J=6.9 Hz, 2H, NCH2), 2.97 (m, 1H, ArCH2), 2.60 (m, 1H, ArCH2), 2.21–2.38 (m, 6H, 3×NCH2), 1.87–1.95 (m, 2H, NCH2CH2), 1.59–1.65 (m, 4H, 2×NCH2CH2), 1.41–1.49 (m, 2H, CH2); 13C-NMR (DMSO-d6, 75 MHz, δ ppm): 174.3, 163.5, 152.6, 149.1, 138.1, 136.3, 130.7, 128.4, 127.1, 124.2, 120.9, 118.3, 116.9, 112.7, 106.1, 61.5, 57.0, 54.3, 50.8, 45.9, 27.8, 26.6, 22.7; MS (ESI) m/z=518 [M+H]+; Anal. Calcd for C30H35N3O5: C, 69.61; H, 6.82; N, 8.12; Found: C, 69.74; H, 6.69; N, 8.18.
(S,E)-2-(3-(4-Hydroxy-3-methoxyphenyl)acryloyl)-9-(3-morpholinopropyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic Acid (9h): The title compound was obtained starting from 8h. White solid, mp 139–141°C; yield: 85%. Analytical data for 9h: IR (KBr, cm−1): 3432, 2943, 1728, 1610, 1413, 1146, 1009; 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.70 (m, 1H, Ar-H), 7.61 (d, 1H, J=16.2 Hz, CH), 7.19–7.27 (m, 2H, Ar-H), 7.01 (m, 1H, Ar-H), 6.69–6.90 (m, 3H, Ar-H), 6.35 (d, 1H, J=16.2 Hz, CH), 4.42–4.55 (m, 2H, NCH, NCH2Ar), 4.23 (m, 1H, NCH2Ar), 4.02 (m, 2H, NCH2), 3.75 (t, J=4.2 Hz, 4H, 2×OCH2), 2.89 (m, 1H, ArCH2), 2.52 (m, 1H, ArCH2), 2.25–2.42 (m, 6H, 3×NCH2), 1.83–1.95 (m, 2H, NCH2CH2); 13C-NMR (DMSO-d6, 75 MHz, δ ppm): 172.8, 162.5, 151.8, 137.5, 135.2, 130.1, 128.8, 127.5, 125.3, 120.5, 118.1, 117.4, 115.8, 111.8, 106.7, 66.5, 61.7, 58.8, 56.9, 55.5, 52.0, 47.2, 29.9, 27.5; MS (ESI) m/z=520 [M+H]+; Anal. Calcd for C29H33N3O6: C, 67.04; H, 6.40; N, 8.09; Found: C, 67.11; H, 6.53; N, 7.96.
(S,E)-2-(3-(4-Hydroxy-3-methoxyphenyl)acryloyl)-9-(3-(piperazin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic Acid (9i): The title compound was obtained starting from 8i. Pale yellow solid, mp 161–163°C; yield: 82%. Analytical data for 9i: IR (KBr, cm−1): 3467, 2953, 1742, 1630, 1425, 1217, 1129, 1021; 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.66 (m, 1H, Ar-H), 7.55 (d, 1H, J=16.2 Hz, CH), 7.32 (m, 1H, Ar-H), 7.20 (m, 1H, Ar-H), 7.03 (m, 1H, Ar-H), 6.72–6.91 (m, 3H, Ar-H), 6.41 (d, 1H, J=16.2 Hz, CH), 4.47–4.61 (m, 2H, NCH, NCH2Ar), 4.28 (m, 1H, NCH2Ar), 4.08 (t, J=6.9 Hz, 2H, NCH2), 2.97–3.09 (m, 5H, 2×NCH2, ArCH2), 2.63 (m, 1H, ArCH2), 2.30–2.45 (m, 6H, 3×NCH2), 1.85–1.92 (m, 2H, NCH2CH2); 13C-NMR (DMSO-d6, 75 MHz, δ ppm): 174.1, 164.0, 153.9, 139.1, 136.3, 130.7, 128.5, 127.6, 125.8, 121.1, 118.7, 117.8, 115.6, 111.9, 106.6, 62.1, 57.6, 54.7, 51.8, 48.3, 45.7, 29.4, 27.6; MS (ESI) m/z=519 [M+H]+; Anal. Calcd for C29H34N4O5: C, 67.16; H, 6.61; N, 10.80; Found: C, 66.98; H, 6.74; N, 10.68.
(S,E)-2-(3-(4-Hydroxy-3-methoxyphenyl)acryloyl)-9-(3-(4-methylpiperazin-1-yl)propyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic Acid (9j): The title compound was obtained starting from 8j. Pale yellow solid, mp 156–158°C; yield: 83%. Analytical data for 9j: IR (KBr, cm−1): 3451, 2940, 1727, 1614, 1410, 1136, 1012; 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.73 (m, 2H, Ar-H), 7.62 (d, 1H, J=16.2 Hz, CH), 7.23–7.30 (m, 2H, Ar-H), 7.05 (d, J=7.5 Hz, 1H, Ar-H), 6.76–6.93 (m, 3H, Ar-H), 6.40 (d, 1H, J=16.2 Hz, CH), 4.43–4.65 (m, 2H, NCH, NCH2Ar), 4.27 (m, 1H, NCH2Ar), 4.11 (t, J=6.9 Hz, 2H, NCH2), 3.11 (m, 4H, 2×NCH2), 2.96 (m, 1H, ArCH2), 2.63 (m, 1H, ArCH2), 2.59 (s, 3H, NCH3), 2.31–2.54 (m, 6H, 3×NCH2), 1.84–1.97 (m, 2H, NCH2CH2); 13C-NMR (DMSO-d6, 75 MHz, δ ppm): 173.6, 162.7, 151.8, 138.0, 137.2, 130.3, 127.9, 127.0, 125.1, 120.2, 118.4, 117.3, 115.3, 110.8, 105.2, 62.8, 56.8, 55.9, 54.1, 49.0, 46.6, 28.5, 26.1; MS (ESI) m/z=533 [M+H]+; Anal. Calcd for C30H36N4O5: C, 67.65; H, 6.81; N, 10.52; Found: C, 67.82; H, 6.93; N, 10.41.
Cell CultureSMMC-7721, HepG2, SGC7901, HCT-116, MCF-7, SKOV-3, or human normal liver cell line LO2 cells were maintained in 10% fetal bovine serum (FBS) Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Invitrogen), which were supplemented with 10% fetal calf serum (PAA, Austria) and antibiotics [100 IU/mL penicillin and 100 IU/mL streptomycin (Amresco)]. All of the cell lines were purchased from the Shanghai Institute of Cell Biology (Shanghai, China) and were grown at 37°C in a 5% CO2 atmosphere.
MTT AssayThe inhibitory effects on cell proliferation of test compounds were investigated by the MTT method. SMMC-7721, HepG2, SGC7901, HCT-116, MCF-7, SKOV-3, or LO2 cells at a final density of 1.0×104 cells/well were placed in 96-well cell plates overnight and treated with or without different concentrations of test compounds for various periods of time. During the last 4 h culture, the cells were exposed to MTT (5 mg/mL), and the resulting formazan crystals were dissolved in 150 µL of dimethyl sulfoxide (DMSO) and measured using a spectrophotometer (Tecan) at a test wavelength of 570 nm. Experiments were conducted in triplicate. Inhibition rate (%)=[(Acontrol−Atreated)/Acontrol]×100%.
Apoptotic Cell AnalysisHepG2 cells were cultured overnight and incubated in triplicate with or without various concentraions of 9j (3.0, 6.0, or 12 µM) for 48 h. To quantify apoptosis, prepared cells were washed twice with cold PBS and then resuspended in 500 µL of binding buffer at a concentration of 1×106 cells/mL. Five microliters of annexin-V-FITC and 5 µL of PI were then added to these cells, which were kept in the dark at 25°C for 10 min. Data acquisition and analysis were performed in a FACS calibur flow cytometer (Becton Dickinson) and values calculated with Cell Quest (BD Biosciences, Franklin Lakes, NJ, U.S.A.).