2014 年 62 巻 4 号 p. 350-353
2014 年 62 巻 4 号 p. 350-353
For the purpose of obtaining a creatinine-specific antibody, a creatinine derivative with 4-aminobutyl, which was served as a linker for preparing the creatinine–bovine serum albumin (BSA) conjugate, was synthesized from 4-benzylaminobutan-1-ol in 8 steps. Production of anti-creatinine antibodies was observed in two rabbits using the creatinine–BSA conjugate, although their titer was rather low.
Recently, we developed an immunochromatographic system for determination of the N1,N12-diacetyl spermine (DiAcSpm) concentration in human urine1) (Fig. 1). DiAcSpm is a newly developed tumor marker and is unique among existing tumor markers in that it is sensitive in detecting early cancers.2,3) The immunochromatographic testing device can be used for personal health control and would eventually contribute to improvement in the efficiency of early detection of cancer, which is important for reducing the number of fatal cases of cancer. The quantity of daily excretion of urine components is usually estimated from arbitrarily excreted spot urine samples by creatinine normalization and is expressed in nmol substance per g creatinine. The validity of creatinine normalization in estimating daily DiAcSpm excretion in urine was in fact confirmed,4) and creatinine-normalized DiAcSpm in urine has been shown to be a promising tumor marker as described above. To determine the quantity of creatinine-normalized DiAcSpm by immunochromatography, it is necessary to simultaneously determine the quantities of creatinine in urine samples. Yaffe’s reaction, which utilizes the reaction of creatinine with picric acid to form orange-red-colored creatinine picrate in an alkaline solution, can be used for this purpose.5) However, substances in urine such as pyruvate, glucose, ascorbate, and billirubin are also known to form colored complexes and interfere with accurate determination of creatinine. An enzymatic method utilizing creatinine imidohydrolase and glutamate dehydrogenase is more accurate6) but is not readily applicable to creatinine assays on a solid support that are run in parallel with the immunochromatographic determination of DiAcSpm. A creatinine-specific antibody, if available, would be readily applicable to immunochromatographic determination of creatinine. Moreover, DiAcSpm and creatinine could be determined on the same strip by using gold nanoparticles labeled with two antibodies. To obtain a creatinine-specific antibody, creatinine as a hapten must be conjugated to an appropriate carrier macromolecule. With this in mind, we designed a creatinine derivative (10) with 4-aminobutyl.

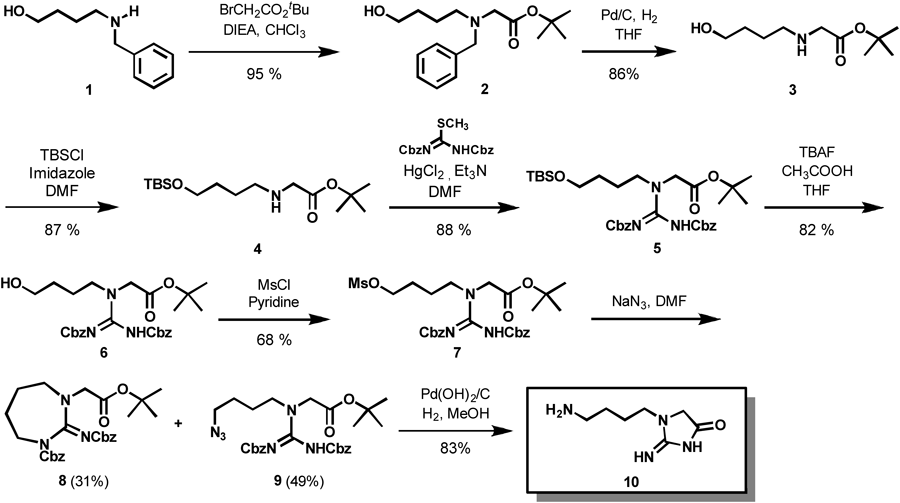
In order to avoid handling highly polar and basic compounds as much as possible, we planned to perform the catalytic hydrogenation of guanidine 9 in the last stage, which was expected to construct iminoimidazolidinone framework,7–11) as shown in Chart 1. Our synthesis started with installation of an acetic acid unit to commercially available 1. Thus, alkylation of 1 with tert-butyl bromoacetate in the presence of diisopropylethylamine proceeded smoothly to afford 2 in high yield. Hydrogenolysis of 2 with palladium on carbon in tetrahydrofuran (THF) under an H2 atmosphere produced secondary amine 312) in 86% yield, which was converted to silyl ether 4 in 87% yield upon treatment with tert-butyldimethylsilyl chloride in the presence of imidazole in N,N-dimethylformamide (DMF). As a key step, guanidinylation of 4 was next carried out upon treatment with S-methylisothiourea in the presence of HgCl2 and Et3N to furnish 5 in 88% yield.13,14) Treatment of 5 with TBAF in the presence of acetic acid generated the alcohol 6 in 82% yield. Mesylation of 6 followed by substitution with sodium azide in DMF gave the desired azide 9 and unexpected cyclic compound 8. Finally, treatment of 9 with Pd(OH)2/C under an H2 atmosphere resulted in reduction of azide, deprotection of two Cbz groups and intramolecular amide formation to give the targeted molecule 10 in 83% yield. The 1H- and 13C-NMR, IR and MS spectra satisfied the shown structure.
Anti-creatinine antibodies were raised in two rabbits (STO150 and STO151) using the 10–bovine serum albumin (BSA) conjugate15) as the antigen. The antibody titer was 1000- to 5000-fold at seven weeks, after the fourth immunization; further increase was not observed at 12 weeks after the sixth immunization. We prepared immunoglobulin fractions from STO150 and STO151 antisera and examined whether the quantity of creatinine can be determined by the anti-creatinine antibodies. To eliminate interference due to the antibodies against the carrier protein portion of the antigen, the 10–OVA conjugate was immobilized on the enzyme-linked immunosorbent assay (ELISA) plate. As shown in Fig. 2, the amount of anti-creatinine antibody bound to the immobilized antigen gradually decreased as the amount of creatinine, the competing antigen, was increased in the range of 0.2–7 mg/mL, which covers the range usually found in human urine. This indicates that creatinine concentrations in this range may be determined with the ELISA system.
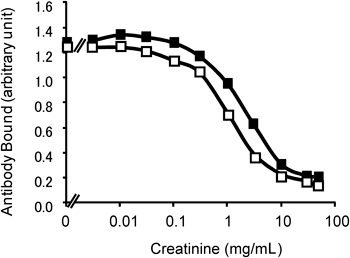
STO150 (□) and STO 151 (■) immunoglobulins, 1 μg and 0.5 μg, respectively, and creatinine at appropriate concentrations were added to ELISA wells in which 10–OVA conjugate was immobilized, and the amount of antibodies bound to immobilized 10 in each well was determined.
In summary, 1-(4-aminobutyl)-2-iminoimidazolidin-4-one (10) was synthesized from commercially available 1 in 8 steps via a guanidinylation of 4 and an intramolecular amide formation of azide 9. Production of anti-creatinine antibodies was observed in two rabbits using the 10–BSA conjugate, although their titer was rather low. The antibodies obtained may not be readily used for further practical application, because the antibody titer was not high enough. However, compound 10 may serve as a useful prototype of a hapten for raising creatinine-specific antibodies.
ChemistryInfrared spectra were recorded on a SHIMADZU IRPrestage-21 spectrophotometer as a thin film using NaCl crystal. 1H- and 13C-NMR spectra were recorded on a JEOL JNM-ECX-400 spectrometer at 400 and 100 MHz, respectively. Chemical shifts were expressed in δ parts per million with tetramethylsilane as an internal standard (δ=0 ppm) for 1H-NMR. Chemical shifts of carbon signals were referenced to CDCl3 (δC=77.0 ppm). The following abbreviations are used: s=singlet, d=doublet, t=triplet, q=quartet, m=multiplet, and br=broad. Electron ionization (EI)-mass spectra were recorded on a JEOL JMS-GCmate II. Electrospray ionization (ESI)-mass spectra were recorded on a Agilent 6530 Accurate-Mass Q-TOF. Column chromatography was carried out on Silica gel 60N (spherical, neutral, 63–210 µm) purchased from KANTO CHEMICAL.
[Benzyl-(4-hydroxy-butyl)-amino]acetic Acid tert-Butyl Ester (2):A mixture of 1 (194 mg, 1.08 mmol), diisopropylethylamine (0.94 mL, 5.40 mmol), tert-butyl bromoacetate (0.50 mL, 3.32 mmol) and CHCl3 (5 mL) was stirred at room temperature for 16 h, then quenched with saturated aqueous NaHCO3 at room temperature and extracted with AcOEt. The extract was dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel eluted with n-hexane–AcOEt (1 : 1) to give 2 (301 mg, 1.03 mmol, 95%) as a colorless oil. 1H-NMR (CDCl3) δ: 7.36–7.24 (5H, m), 3.81 (2H, s), 3.61 (2H, m), 3.18 (2H, s), 2.71 (2H, m), 1.70–1.61 (4H, m), 1.46 (9H, s); 13C-NMR (CDCl3) δ: 170.23 (C), 137.88 (C) 129.46 (CH×2), 128.39 (CH×2), 127.34 (CH), 81.12 (C), 62.65 (CH2), 58.18 (CH2), 54.38 (CH2), 54.05 (CH2), 31.50 (CH2), 28.18 (CH3×3), 24.95 (CH2); IR (neat) cm−1: 3387, 1728; EI-MS m/z 293 [M]+, 192, 179; high resolution (HR)-EI-MS m/z Calcd for C17H27NO3 [M]+ 293.1991 Found 293.1994.
(4-Hydroxy-butylamino)acetic Acid tert-Butyl Ester (3):To a solution of 2 (301 mg, 1.03 mmol) in THF (5 mL) was added 10% Pd–C (30.4 mg). The mixture was stirred under H2 atmosphere at room temperature for 13 h and then filtered with AcOEt. The filtrate was concentrated under reduced pressure. The residue was purified by column chromatography on silica gel eluted with AcOEt to give 3 (180 mg, 0.884 mmol, 86%) as a colorless oil. 1H-NMR (CDCl3) δ: 3.60 (2H, t, J=5.2 Hz), 3.29 (2H, s), 3.16 (1H, br s), 2.66 (2H, t, J=5.6 Hz), 1.72–1.58 (4H, m), 1.47 (9H, s); 13C-NMR (CDCl3) δ: 171.16 (C), 81.59 (C), 62.63 (CH), 51.04 (CH2), 49.28 (CH2), 31.97 (CH2), 28.17 (CH2), 28.11 (CH3×3); IR (neat) cm−1: 3318, 1728; EI-MS m/z 203 [M]+, 174, 158; HR-ESI-MS m/z Calcd for C10H22NO3 [M+H]+ 204.1600 Found 204.1595.
[4-(tert-Butyldimethylsilyloxy)butylamino]acetic Acid tert-Butyl Ester (4):To a mixture of 3 (3.16 g, 15.6 mmol) and tert-butyldimethylsilyl chloride (3.76 g, 25.0 mmol) in DMF (90 mL) was added imidazole (2.20 g, 32.4 mmol) at 0°C. The mixture was stirred at room temperature for 1 h under Ar, then quenched with H2O and extracted with Et2O. The extract was dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel eluted with n-hexane–AcOEt (3 : 1) to give 4 (4.27 g, 13.5 mmol, 87%) as a colorless oil. 1H-NMR (CDCl3) δ: 3.62 (2H, m), 3.29 (2H, s), 2.61 (2H, m), 1.81 (1H, br s), 1.64–1.50 (4H, m), 1.47 (9H, s), 0.89 (9H, s), 0.04 (6H, s); 171.79 (C), 81.12 (C), 63.02 (CH2), 51.73 (CH2), 49.41 (CH2), 30.52 (CH2), 28.13 (CH3×3), 26.54 (CH2), 25.97 (CH3×3), 18.35 (C), −5.29 (CH3×2); IR (neat) cm−1: 1736; EI-MS m/z 260 [M−C4H9]+, 246, 218; HR-ESI-MS m/z Calcd for C16H36NO3Si [M+H]+ 318.2464 Found 318.2465.
N-4-(tert-Butyldimethylsilyloxy)butyl-N-[[[(phenylmethoxy)carbonyl]amino][[(phenylmethoxy)carbonyl]imino]methyl]glycine tert-Butyl Ester (5):A mixture of 4 (144 mg, 0.454 mmol), S-methylisothiourea (191 mg, 0.533 mmol), triethylamine (600 µL, 4.29 mmol), HgCl2 (136 mg, 0.501 mmol) and DMF (6 mL) was stirred at room temperature for 18 h, then concentrated under reduced pressure, diluted with Et2O and filtered. The filtrate was washed with saturated aqueous NH4Cl and brine. The organic layer was dried over MgSO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel eluted with n-hexane–AcOEt (8 : 1) to give 5 (251 mg, 0.400 mmol, 88%) as a colorless oil. 1H-NMR (CDCl3) δ: 10.25 (1H, br s), 7.43–7.24 (10H, m), 5.20–5.09 (4H, m), 4.03 (2H, br s), 3.60 (2H, t, J=6.2 Hz), 3.51 (2H, br s), 1.75–1.64 (2H, m), 1.53–1.41 (2H, m), 1.44 (9H, s), 0.88 (9H, s), 0.03 (6H, s); 13C-NMR (CDCl3) δ: 167.54 (C), 162.70 (C), 156.04 (C), 151.63 (C), 136.64 (C), 135.26 (C), 128.8–127.8 (various types of C×10), 82.23 (C), 68.02 (CH2), 67.39 (CH2), 62.52 (CH2), 50.56 (CH2), 29.82 (CH2), 27.97 (CH3×3), 25.95 (CH3×3), 23.58 (CH2), 18.32 (C), −5.32 (CH3×2); IR (neat) cm−1: 3171, 1751, 1612; EI-MS m/z 570 [M−C4H9]+, 462, 406; HR-ESI-MS m/z Calcd for C33H50N3O7Si [M+H]+ 628.3418 Found 628.3423.
N-4-Hydroxybutyl-N-[[[(phenylmethoxy)carbonyl]amino][[(phenylmethoxy)carbonyl]imino]methyl]glycine tert-Butyl Ester (6):To a solution of 5 (550 mg, 0.877 mmol) in THF (10 mL) were added CH3COOH (250 µL) and TBAF (1.0 M THF sol., 1.9 mL, 1.90 mmol) at room temperature. The reaction mixture was stirred for 16 h at room temperature under Ar and then concentrated under reduced pressure. The residue was purified by column chromatography on silica gel eluted with n-hexane–AcOEt (1 : 1) to give 6 (367 mg, 0.715 mmol, 82%) as a colorless oil. 1H-NMR (CDCl3) δ: 10.32 (1H, br s), 7.43–7.27 (10H, m), 5.17–5.10 (4H, m), 4.02 (2H, s), 3.70–3.40 (4H, m), 1.76 (2H, quintet, J=7.6 Hz), 1.65 (1H, br s), 1.54 (2H, m), 1.44 (9H, s); 13C-NMR (CDCl3) δ: 167.62 (C), 162.57 (C), 155.96 (C), 151.77 (C), 136.61 (C), 135.22 (C), 128.9–127.6 (various types of C×10), 82.30 (C), 68.10 (CH2), 67.37 (CH2), 61.98 (CH2), 50.16 (br, CH2), 29.04 (CH2), 27.97 (CH3×3), 23.24 (br, CH2); IR (neat) cm−1: 3395, 1751, 1605; EI-MS m/z 495 [M−H2O]+, 439, 254; HR-ESI-MS m/z Calcd for C27H36N3O7 [M+H]+ 514.2553 Found 514.2549.
N-4-Mesyloxybutyl-N-[[[(phenylmethoxy)carbonyl]amino][[(phenylmethoxy)carbonyl]imino]methyl]glycine tert-Butyl Ester (7):To a solution of 6 (114 mg, 0.222 mmol) in pyridine (3.5 mL) was added methanesulfonyl chloride (350 µL, 0.446 mmol) at 0°C. The reaction mixture was stirred at room temperature for 3 h, then quenched with H2O, extracted with AcOEt and washed with 50% aqueous CuSO4. The organic layer was dried over Mg2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel eluted with n-hexane–AcOEt (1 : 1) to give 7 (89.8 mg, 0.152 mmol, 68%) as a colorless oil. 1H-NMR (CDCl3) δ: 10.29 (1H, br s), 7.42–7.24 (10H, m), 5.13 (4H, br s), 4.23 (2H, t, J=5.6 Hz), 4.00 (2H, s), 3.56 (2H, br s), 2.95 (3H, s), 1.82–1.68 (4H, m), 1.44 (9H, s); IR (neat) cm−1: 3179, 1744, 1605; EI-MS m/z 495 [M−CH3SO3H]+, 439, 363; HR-ESI-MS m/z Calcd for C28H38N3O9S [M+H]+ 592.2329 Found 592.2327.
N-4-Azidobutyl-N-[[[(phenylmethoxy)carbonyl]amino][[(phenylmethoxy)carbonyl]imino]methyl]glycine tert-Butyl Ester (9):To a solution of 7 (193 mg, 0.327 mmol) in DMF (5.5 mL) was added sodium azide (47.9 mg, 0.722 mmol). The reaction mixture was stirred at room temperature for 24 h, then quenched with H2O and extracted with AcOEt. The organic layer was dried over Mg2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel eluted with n-hexane–AcOEt (4 : 1–2 : 1) to give azide 9 (86.5 mg, 0.161 mmol, 49%) and cyclic guanidine 8 (50.5 mg, 0.102 mmol, 31%) as colorless oils. For 9: 1H-NMR (CDCl3) δ: 10.28 (1H, br s), 7.41–7.26 (10H, m), 5.14 (4H, br s), 4.01 (2H, s), 3.67–3.36 (2H, br), 3.28 (2H, t, J=6.4 Hz), 1.72 (2H, m), 1.56 (2H, m), 1.44 (9H, s); IR (neat) cm−1: 3179, 2099, 1751, 1605; EI-MS m/z 430 [M−C6H5CH2OH]+, 388, 374; HR-ESI-MS m/z Calcd for C27H35N6O6 [M+H]+ 539.2618 found 539.2615. For 8: 1H-NMR (CDCl3) δ: 7.38–7.20 (10H, m), 5.11–4.76 (4H, m), 4.52 (1H, d, J=14.8 Hz), 4.31 (1H, d, J=11.2 Hz), 3.78 (1H, d, J=14.4 Hz), 3.67 (1H, t, J=12.6 Hz), 3.25–3.03 (2H, m), 1.84–1.63 (4H, m), 1.42 (9H, s); 13C-NMR (CDCl3) δ: 167.67 (C), 160.57 (C), 157.43 (C), 152.94 (C), 136.70 (C), 135.72 (C), 128.6–127.7 (various types of C×10), 82.22 (C), 68.01 (CH2), 67.21 (CH2), 53.74 (CH2), 52.95 (CH2), 46.95 (CH2), 27.99 (CH3×3), 26.75 (CH2), 25.34 (CH2); IR (neat) cm−1: 1728, 1690, 1589; ESI-MS m/z 496 [M+H]+, 429, 405; HR-ESI-MS m/z Calcd for C27H34N3O6 [M+H]+ 496.2448 Found 496.2442.
1-(4-Aminobutyl)-2-iminoimidazolidin-4-one (10):To a solution of 9 (101 mg, 0.188 mmol) in MeOH (4 mL) was added Pd(OH)2/C (30 mg) at 0°C. The reaction mixture was stirred for 4 h at room temperature under H2 atmosphere and then filtered. The filtrate was concentrated under reduced pressure to give 10 (26.6 mg, 0.156 mmol, 83%), which was suggested to be a tautomeric mixture by a 1H-NMR spectra. 1H-NMR (CD3OD) δ: 3.85 (2H, s), 3.31 (2H, t, J=6.8 Hz), 2.64 (1.4H, t, J=6.8 Hz), 2.53 (0.6H, t, J=6.8 Hz), 1.55 (2H, m), 1.44 (2H, m); 13C-NMR (CD3OD) δ: 188.37 (C), 171.38 (C), 55.12 (CH2), 44.74 (CH2), 41.45 (CH2), 29.04 (CH2), 26.17 (CH2); IR (neat) cm−1: 3325, 3156, 1636, 1582, 1489; EI-MS m/z 170 [M]+, 154, 141; HR-ESI-MS m/z Calcd for C7H15N4O [M+H]+ 171.1246 Found 171.1237.
Antibody Production and AssayCompound 10 was conjugated to bovine serum albumin (BSA) and ovalbumin (OVA) using glutaraldehyde.15 Briefly, 0.8 µL of 10 (10 mg/mL) and 0.2 µL of 2.5% glutaraldehyde were added to BSA or OVA (30 mg) dissolved in 0.5 mL of 50 mM Na–borate buffer (pH 8.0). The mixture was stirred for 2 h at room temperature, and the reaction was stopped by adding 1 mL of 1 M glycine. 10–BSA or 10–OVA conjugate was then desalted by gel filtration through a PD-10 column (GE Healthcare Japan, Tokyo, Japan). Rabbit anti-creatinine antisera were prepared by Sigma (St. Louis, MO, U.S.A.) using 10–BSA conjugate as the antigen. The use of rabbits for antibody production received prior approval from the review board in the Tokyo Metropolitan Institute of Medical Science. Titer of anti-creatinine antibodies was determined by ELISA as described below. Immunoglobulin fraction was obtained from the antisera by using Protein A column (Amersham, Piscataway, NJ, U.S.A.).
An ELISA was carried out essentially as described previously using the 10–OVA conjugate in place of the AcSpm–GMBS–BSA conjugate as the plate antigen.1) To determine the antibody titer, serial dilutions of rabbit antisera were added to each well of an ELISA plate, incubated for 1 h, and then processed to determine the amount of antibody bound to the immobilized 10 (10–OVA) in each well. To assess the efficiency of creatinine in competing for the binding sites on the antibody with the immobilized 10, serial dilutions of creatinine and the anti-creatinine immunoglobulin were added to each well, incubated at room temperature for 1 h, and then processed to determine the amount of antibody bound to the immobilized 10 in each well.