2014 年 62 巻 4 号 p. 373-378
2014 年 62 巻 4 号 p. 373-378
A series of novel cyano oximino sulfonate derivatives were prepared from the reaction of arylsulfonyl chloride with different cyanoacetamide-based oximes ranging from the simplest unsubstituted amide to analogues containing N-ethyl (mimicking the Oxyma template), N-piperidinyl and N-morpholinyl chains. In addition, the cyano oximes, N-hydroxybenzimidoyl cyanide and N-hydroxypicolinimidoyl cyanide were also used in the synthesis of the novel cyano oximino sulfonate derivatives. The structures of the prepared compounds were confirmed by 1H-NMR, 13C-NMR, and elemental analysis. The preliminary bioassays showed that some of the title compounds, such as 2-oxo-2-(piperidin-1-yl)-N-(tosyloxy)acetimidoyl cyanide (TsPipOx), N-(tosyloxy)benzimidoyl cyanide (TsPhOX), N-(naphthalen-2-ylsulfonyloxy)-2-oxo-2-(piperidin-1-yl)acetimidoyl cyanide (NpsPipOx), 2-amino-N-(naphthalen-2-ylsulfonyloxy)-2-oxoacetimidoyl cyanide (NpsAmOx), N-(naphthalen-2-ylsulfonyloxy)benzimidoyl cyanide (NpsPhCN), and N-(naphthalen-2-ylsulfonyloxy)picolinimidoyl cyanide (NpsPyCN), showed anti-proliferation effect on the mouse fibroblast L929. The calculated IC50-values were ranging between 36.5 µg/mL and 0.235 mg/mL. However the anti-proliferation effects seem to be cytostatic rather than cytotoxic. The compounds only minimize the growth activity without completely killing the cells.
Oximes are one of the most versatile building blocks in organic and organometallic chemistry. The substituent pending at α-position account for the specific properties of each oxime, such as dissociation constant, solubility, chelating ability, etc.1–3) Their multifaceted nature derives in a broad range of applications in chemistry in addition to be biologically relevant moieties.4–7)
Biologically active heterocyclic compounds containing amine moiety, specifically piperidine and morpholine skeletons are attractive targets of organic synthesis owing to their pharmacological activity and wide occurrence in nature.8,9) Piperidines are known to have central nervous system (CNS) depressant activity at low dosage levels but stimulant activity with increased doses. In addition, the piperidine skeleton possesses analgesic, ganglionic blocking and anesthetic properties.10,11) Morpholine derivatives have been reported to possess antiinflammatory12–14) antimicrobial15,16) and central nervous system activities.17,18)
The sulfonate ester is considered an important class in the sulfonylation reaction for the synthesis of sulfonamide which exhibits a wide range of biological activities.19–22)
Sulfonylation is also used as a protection technique for OH and NH functionalities, as it enables crystallization and the protecting group can be selectively cleaved in the presence of acid-labile tert-butyl-based groups by using HCl or HBr in alcohol23) or in aprotic solvents such as ethylacetate or dimethylformamide.24,25)
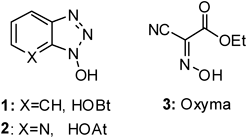
Esters of sulfonic acids and 1-hydroxybenzotriazole (HOBt, 1) have been used for peptide coupling.26–30) The reactivity of these sulfonate esters was shown to be directly related to the presence of electron-withdrawing substituents in both the HOBt 1 and the sulfonic acid moieties.31–36) 1-Hydroxy-7-azabenzotriazole (HOAt, 2) has been reported to be more efficient than HOBt 1 because of the anchimeric assistance effect caused by the pyridine ring.37,38)
The explosive character of HOBt 1 and its derivatives,39) prompted the reevaluation of the strongly acidic oxime ethyl 2-cyano-2-(hydroxyimino) acetate (Oxyma, 3) as a potential replacement (Fig. 1).
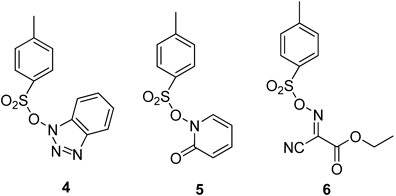
Recently we reported40) the synthesis of sulfonate esters based on ethyl cyanoglyoxylate-2-oxime as well as 1-hydroxy-2-pryridinone as new coupling agents. The biological activity studies of some selected sulfonate ester derivatives (Fig. 2) showed a moderate anti-proliferative effect against human cancer cell line SW756 for compounds 4 and 5, while the sulfonate ester 6 showed a strong growth inhibition for mosquito Culex pipiens 4th instar larvae.41)
We are extending our work by the synthesis of a series of sulfonate ester derivatives of the recently prepared N-alkyl-cyanoacetamido oximes 8–11 (Fig. 3), N-hydroxybenzimidoyl cyanide 12, and N-hydroxypicolinimidoyl cyanide 13 (Fig. 4).

The structure of the target sulfonate ester derivatives were confirmed by their spectral characterization. The biological activity study of the target compounds was further investigated.
We are reporting here the synthesis, spectral characterization and the biological activity study of a novel series of sulfonate ester derivatives.
The cyanoacetamide oximes 8–11 were prepared using the Meyer nitrosation reaction.42) Several more convenient variations of this reaction are known, using alkyl nitrites such as isobutyl nitrite (iBuONO), isopropyl nitrite (iPrONO) and methyl nitrite (MeONO) as source of nitrous acid.1,3,43) Among these modified procedures, in situ generation of gaseous MeONO was selected in view of the efficacy in previous preparation of oximes.3,44) Thus, nitrosation of 3-morpholino-3-oxopropanenitrile, 3-oxo-3-(piperidin-1-yl)propanenitrile, 2-cyanoacetamide, and 2-cyano-N-ethylacetamide took place smoothly by this methodology in presence of sodium hydroxide, followed by acidification to afford the cyanoacetamide oximes 8–11, Chart 1.44–47) Whereas 2-amino-N-hydroxy-2-oxoacetimidoyl cyanide (AmOX, 10) and 2-(ethylamino)-N-hydroxy-2-oxoacetimidoyl cyanide (N-Oxyma, 11) precipitated immediately after the acidic treatment, other oximes, such as N-hydroxy-2-morpholino-2-oxoacetimidoyl cyanide (MorOX, 8) and N-hydroxy-2-oxo-2-(piperidin-1-yl)acetimidoyl cyanide (PipOX, 9), needed addition of cold ether, affording white crystalline, stable solids in all cases.46,47) N-Hydroxybenzimidoyl cyanide (PhOX, 12) and N-hydroxypicolinimidoyl cyanide48) (PyOX, 13) were prepared by the nitrosation of 2-phenylacetonitrile and 2 pyridyl acetonitrile in glacial acetic acid with sodium nitrite.

The 4-tosyl esters 15–20 and 2-naphthalenesulfonyl esters 22–27 were prepared via the reaction of 4-tosyl chloride or 2-naphthalenesulfonyl chloride with MorOX 8, PipOX 9, AmOX 10, N-Oxyma 11, PhOX 12 and PyOX 13 in the presence of triethyl amine (Et3N) in anhydrous dicloromethane (CH2Cl2) under a nitrogen atmosphere at 0°C40) (Chart 2).Recrystallization from CH2Cl2-hexane gave pure reagents as crystals. These reagents are suitable for long-term storage and are stable toward moisture in the air. IR, elemental analysis and NMR spectroscopy confirmed the structure of compounds 15–20, 22–27.

The Infrared spectra of compounds 15–20 and 22–27 showed one sharp peak at the range 2234 and 2291 cm−1 corresponding to the CN group. In addition, compounds 15–18 and 22–25 showed one sharp peak at the range 1650–1710 cm−1 corresponding to the amidic carbonyl group. This is in accordance with the 13C-NMR spectra of compounds 15–20 and 22–27, which showed a peak at the range 105.96–107.77 ppm corresponding to the CN group, whereas compounds 15–18 and 22–25 showed a peak at the range 154.00–157.24 ppm corresponding to the amidic carbonyl group.
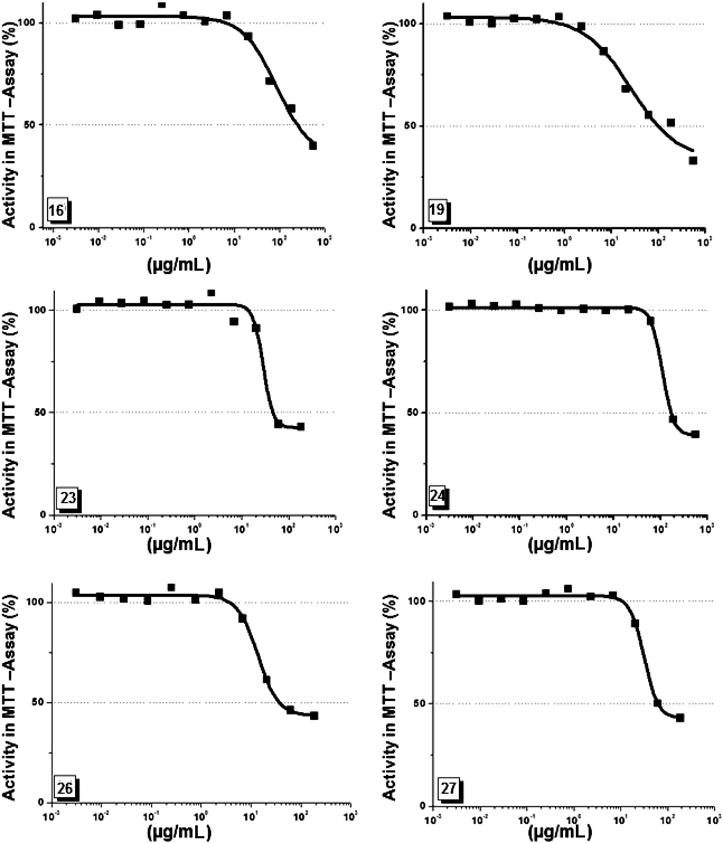
The anti-proliferation activities of the prepared compounds have been measured in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-assay using the mouse fibroblast L929. The cells were incubated with serial dilutions of the compounds for 5 d (0.003–555 µg/mL) in 96-well plates for 5 d then tested for growth inhibitions by use of MTT. The analysis showed that the tested compounds 16, 19, 23, 24, 26 and 27 showed growth inhibition activity on L929 cells (Fig. 5). The measured IC50-values range between (36.5, 233.5 µg/mL). As shown in Table 1, Compounds 26 and 23 showed the strongest growth inhibition effects with IC50 values of 36.5 and 51 µg/mL, respectively. Compounds 19 and 27 showed a moderate growth inhibition effects with IC50 values of 93.8 and 107 µg/mL respectively. The lowest growth inhibition activity of the group was measured for compound 16 (IC50 value=233.5 µg/mL). All other tested compounds (15, 17, 18, 20, 22, 25) showed no effect on the growth of L929 cells under our experimental conditions.
| Compounds | 15 | 16 | 17 | 18 | 19 | 20 | 22 | 23 | 24 | 25 | 26 | 27 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (µg/mL) | NDa) | 233.5 | NDa) | NDa) | 93.8 | NDa) | NDa) | 51.0 | 108.8 | NDa) | 36.5 | 107.0 |
a) Not determined.
In general, the effect of the naphthyl moiety was clear in the oximinopiperidino moiety (NpsPipOx, 23), comparing its growth inhibition effects with its tosyl analogs (TsPipOx, 16). Further investigations are running in our lab to get insights into the mode of action of the active compounds.
The present work led to development of novel sulfonate ester derivatives of the recently prepared N-alkyl-cyanoacetamido oximes, N-hydroxybenzimidoyl cyanide, and N-hydroxypicolinimidoyl cyanide. The mouse fibroblast L929 was used to measure the cytotoxic activities of the derivatives. The test compound showed different activity against the selected cell line. However, some of the tested compounds were inactive. The bioactivities of the active derivatives seem to be rather cytostatic than cytotoxic. The oximinophenyl moiety in the tosyl series (TsPhOX, 19) gave the highest growth inhibition effects with IC50 value=93.8 µg/mL. The same behavior was observed in the naphthyl derivatives, where the oximinophenyl moiety derivative (NpsPhOx, 26) was the most effective one. The combination between the naphthyl and oximinophenyl showed the strongest growth inhibition effects with IC50 value of 36.5 µg/mL. The effect of the naphthyl moiety was also clear in the oximinopiperidino moiety (NpsPipOx, 23), where its growth inhibition effects with IC50 values of 51 µg/mL compared to its tosyl analogs (TsPipOx, 16), which gave the lowest growth inhibition activity (IC50 value=233.5 µg/mL). Further investigations are running in our lab to get insights into the mode of action of the active compounds.
4-Tosyl chloride and 2-naphthalenesulfonyl chloride were obtained from commercial sources (Aldrich). TLC was performed on silica gel-protected aluminum sheets (type 60 GF254, Merck), and the spots were detected by exposure to a UV lamp at λ 254 nm for a few seconds. Melting points were obtained in open capillary tubes using a Mel-Temp apparatus and were uncorrected. Infrared (IR) spectra were recorded on a Perkin-Elmer 1600 series Fourier transform instrument as KBr pellets. Nuclear Magnetic resonance spectra (1H- and 13C-NMR spectra) were recorded on 400 MHz JEOL spectrometer at room temperature. Chemical shifts are reported in parts per million (ppm) and are referenced relative to residual solvent (e.g. CHCl3 at δH 7.26 ppm for CDCl3, DMSO at δH 2.50 ppm for DMSO-d6). Spin multiplicities are represented by the following signals: singlet (s), broad singlet (br s), doublet (d), broad doublet (br d), doublet of doublets (dd), triplet (t), doublet of triplets (dt), quartet (q), sextet (sex) and multiplet (m). Elemental analyses were performed on a Perkin-Elmer 2400 elemental analyzer, and the values found were within ±0.3% of the theoretical values. The compounds were illustrated using Chem Draw Ultra version 11, Cambridge Soft Corporation.
General Procedure for the Preparation of Cyanoacetamide Oximes (8–11)45)Into an ice-cooled solution of 2-acetonitrile derivatives, 3-morpholino-3-oxopropanenitrile, 3-oxo-3-(piperidin-1-yl)propanenitrile, 2-cyano-N-ethylacetamide or 2-amino-N-hydroxy-2-oxoacetimidoyl cyanide (100 mmol) and sodium hydroxide (4 g, 100 mmol) in methanol (30 mL) was introduced gaseous methyl nitrite which was generated from a suspension of sodium nitrite (8.3 g) in a mixture of methanol (10 mL) and water (10 mL) by dropwise addition of a mixture of concentrated sulphuric acid (5 mL) and water (10 mL). The mixture was stirred for 2 h at room temperature and concentrated to dryness. The residue was dissolved in the least amount of water (5 mL), ice cooled, and acidified with concentrated hydrochloric acid to precipitate. In some cases precipitation occurs only by the addition of diethyl ether.
N-Hydroxy-2-morpholino-2-oxoacetimidoyl Cyanide (8): The product was obtained as white crystals, mp 193–194°C (lit. 155–160°C)1) in yield 79% (from ethyl cyanoacetic ester). 1H-NMR (C3D6O) δ: 3.66–3.75 (m, 8H, 4CH2), 13.12 (s, 1H, OH, D2O exchangeable). 13C-NMR (C2D6SO) δ: 43.13, 47.62, 66.22, 66.51, 109.79, 127.31, 158.23.
N-Hydroxy-2-oxo-2-(piperidin-1-yl)acetimidoyl Cyanide (9): The product was obtained as a white powder, mp 159–160°C (lit. 159–162°C)1,49) in yield 87% (from ethyl cyanoacetic ester). 1H-NMR (C3D6O) δ: 1.59–1.71 (m, 6H, 3CH2), 3.60–3.64 (m, 4H, 2CH2). 13C-NMR (C2D6SO) δ: 24.14, 25.59, 26.19, 43.66, 48.03, 109.79, 127.44, 157.73.
2-Amino-N-hydroxy-2-oxoacetimidoyl Cyanide (10): The product was obtained as a white powder, mp 199–200°C in yield 84%. 1H-NMR (CDCl3) δ: 7.07, 7.37 (2 br s, 2H, NH2), 13.38 (s, 1H, OH, D2O exchangeable). 13C-NMR (C2D6SO) δ: 109.45, 128.58, 160.32.
2-(Ethylamino)-N-hydroxy-2-oxoacetimidoyl Cyanide (11): The product was obtained as white crystals, mp 174–175°C in yield 77% (from ethyl cyanoacetic ester). 1H-NMR (C3D6O) δ: 1.14 (t, 3H, CH3, J=7.2 Hz), 3.34 (quart., 2H, CH2, J=7.2 Hz), 7.83 (br s, 1H, NH), 13.59 (s, 1H, OH, D2O exchangeable). 13C-NMR (C3D6O) δ: 14.21, 34.47, 108.39, 157.96, 158.01.
General Method for the Synthesis of Sulfonate Esters (15–20, 22–27)To a suspension of 5 mmol of oxymes 8–13 in 30 mL of anhydrous CH2Cl2 was added 0.71 mL (5 mmol) of triethylamine with magnetic stirring. The resulting clear yellow solution was cooled in an ice bath under an atmosphere of N2 and treated slowly with 5 mmol of 4-tosyl chloride 14 or 2-naphthalenesulfonyl chloride 21. The reaction mixture was stirred at 0°C for 30 min and then at room temperature for 2 h. After dilution with 30 mL of CH2Cl2, the organic phase was washed with water and saturated aqueous NaCl (30 mL) and dried over Na2SO4 anhydrous. After removal of the solvent with a rotary evaporator, the residue was recrystallized from CH2Cl2/hexane to give the sulfonate esters.
2-Morpholino-2-oxo-N-(tosyloxy)acetimidoyl Cyanide (TsMorOx, 15): This compound was obtained as white crystals, 1.54 g (91.3%), mp 118–119°C; IR (KBr) cm−1: 2238 (CN), 1650 (CO, amide), 1387, 1193 (SO2, sulfonate). 1H-NMR (400 MHz, CDCl3) δ: 2.47 (3H, s, CH3), 3.50–3.51 (2H, m, CH2), 3.63–3.73 (6H, m, 3CH2), 7.39 (2H, d, Ar-H, J=8.8 Hz), 7.84 (1H, d, Ar-H, J=8.8 Hz). 13C-NMR (100 MHz, CDCl3) δ: 21.92, 21.99, 43.54, 47.56, 66.38, 66.59, 106.47, 129.37, 130.32, 130.40, 130.44, 147.32, 155.15. Anal. Calcd for C14H15N3O5S: C, 49.84; H, 4.48; N, 12.46. Found: C, 50.07; H, 4.67; N, 12.28.
2-Oxo-2-(piperidin-1-yl)-N-(tosyloxy)acetimidoyl Cyanide (TsPipOx, 16): This compound was obtained as white crystals, 1.49 g (87.8%), mp 98–99°C; IR (KBr) cm−1: 2245 (CN), 1654 (CO, amide), 1396, 1189 (SO2, sulfonate). 1H-NMR (400 MHz, CDCl3) δ: 1.57–1.69 (6H, m, 3CH2), 2.47 (3H, s, CH3), 3.37–3.40 (2H, m, CH2), 3.58–3.61 (2H, m, CH2), 7.39 (2H, d, Ar-H, J=8.8 Hz), 7.85 (1H, d, Ar-H, J=8.8 Hz). 13C-NMR (100 MHz, CDCl3) δ: 21.93, 24.15, 25.33, 26.39, 44.33, 48.38, 106.60, 129.30, 130.27, 130.67, 132.88, 147.04, 154.76. Anal. Calcd for C15H17N3O4S: C, 53.72; H, 5.11; N, 12.53. Found: C, 53.97; H, 5.39; N, 12.29.
2-Amino-2-oxo-N-(tosyloxy)acetimidoyl Cyanide (TsAmOx, 17): This compound was obtained as white crystals, 1.19 g (89.1%), mp 105–106°C; IR (KBr) cm−1: 3425, 3243 (NH2), 2236 (CN), 1710 (CO, amide), 1399, 1200 (SO2, sulfonate). 1H-NMR (400 MHz, CDCl3) δ: 2.48 (3H, s, CH3), 6.31, 6.72 (2H, br s, 2NH), 7.41 (2H, d, Ar-H, J=8.1 Hz), 7.89 (1H, d, Ar-H, J=8.1 Hz). 13C-NMR (100 MHz, CDCl3) δ: 22.00, 105.96, 125.98, 129.07, 129.34, 130.07, 130.58, 133.02, 147.63, 156.92. Anal. Calcd for C10H9N3O4S: C, 44.94; H, 3.39; N, 15.72. Found: C, 45.12; H, 3.55; N, 15.99.
2-(Ethylamino)-2-oxo-N-(tosyloxy)acetimidoyl Cyanide (TsN-Oxyma, 18): This compound was obtained as white crystals, 1.31 g (88.7%), mp 115–116°C; IR (KBr) cm−1: 3359 (NH), 2291 (CN), 1690 (CO, amide), 1396, 1177 (SO2, sulfonate). 1H-NMR (400 MHz, CDCl3) δ: 1.20 (3H, t, CH3, J=7.3 Hz), 2.48 (3H, s, CH3), 3.40 (2H, q, CH2, J=7.3 Hz), 6.58 (H, br s, NH), 7.41 (2H, d, Ar-H, J=8.0 Hz), 7.87 (1H, d, Ar-H, J=8.0 Hz). 13C-NMR (100 MHz, CDCl3) δ: 14.46, 21.97, 35.38, 106.08, 129.22, 130.28, 130.50, 133.76, 147.50, 155.01. Anal. Calcd for C12H13N3O4S: C, 48.81; H, 4.44; N, 14.23. Found: C, 48.62; H, 4.59; N, 13.97.
N-(Tosyloxy)benzimidoyl Cyanide (TsPhCN, 19): This compound was obtained as whiter crystals, 1.28 g (85.3%), mp 110–111°C; IR (KBr) cm−1: 2242 (CN), 1393, 1187 (SO2, sulfonate). 1H-NMR (400 MHz, CDCl3) δ: 2.46 (3H, s, CH3), 7.39 (2H, d, Ar-H, J=8.8 Hz), 7.46 (2H, t, Ar-H), 7.57 (1H, t, Ar-H, J=7.3 Hz), 7.78 (2H, d, Ar-H, J=8.0 Hz), 7.94 (1H, d, Ar-H, J=8.0 Hz). 13C-NMR (100 MHz, CDCl3) δ: 21.92, 107.77, 127.32, 127.54. 129.31, 129.44, 130.15, 131.22, 133.47, 139.35, 146.53. Anal. Calcd for C15H12N2O3S: C, 59.99; H, 4.03; N, 9.33. Found: C, 60.16; H, 4.25; N, 9.05.
N-(Tosyloxy)picolinimidoyl Cyanide (TsPyCN, 20): This compound was obtained as grey crystals, 1.35 g (89.7%), mp 128–129°C; IR (KBr) cm−1: 2247 (CN), 1394, 1173 (SO2, sulfonate). 1H-NMR (400 MHz, CDCl3) δ: 2.46 (3H, s, CH3), 7.39–7.47 (3H, m, Ar-H), 7.78–7.95 (4H, m, Ar-H), 8.72 (1H, d, Ar-H, J=4.4 Hz). 13C-NMR (100 MHz, CDCl3) δ: 21.92, 107.38, 121.74, 126.90. 129.32, 130.24, 130.97, 137.28, 140.06, 146.79, 146.86, 150.43. Anal. Calcd for C14H11N3O3S: C, 55.80; H, 3.68; N, 13.95. Found: C, 55.96; H, 3.46; N, 14.09.
2-Morpholino-N-(naphthalen-2-ylsulfonyloxy)-2-oxoacetimidoyl Cyanide (NpsMorOx, 22): This compound was obtained as beige powder, 1.60 g (85.8%), mp 95–96°C; IR (KBr) cm−1: 2240 (CN), 1661 (CO, amide), 1376, 1184 (SO2, sulfonate). 1H-NMR (400 MHz, CDCl3) δ: 3.38–3.40 (2H, m, CH2), 3.48–3.51 (2H, m, CH2), 3.64–3.69 (4H, m, 2CH2), 7.68–7.78 (2H, m, Ar-H), 7.85–7.91 (1H, m, Ar-H), 7.96–8.07 (3H, m, Ar-H), 8.58 (1H, s, Ar-H). 13C-NMR (100 MHz, CDCl3) δ: 43.49, 47.46, 66.33, 66.44, 106.41, 128.24, 129.64, 130.28, 130.64, 131.90, 131.95, 136.04, 154.00. Anal. Calcd for C17H15N3O5S: C, 54.68; H, 4.05; N, 11.25. Found: C, 54.57; H, 4.34; N, 11.48.
N-(Naphthalen-2-ylsulfonyloxy)-2-oxo-2-(piperidin-1-yl)acetimidoyl Cyanide (NpsPipOx, 23): This compound was obtained as beige powder, 1.59 g (85.7%), mp 75–76°C; IR (KBr) cm−1: 2245 (CN), 1655 (CO, amide), 1392, 1183 (SO2, sulfonate). 1H-NMR (400 MHz, CDCl3) δ: 1.31–1.63 (6H, m, 3CH2), 3.14–3.30 (2H, m, CH2), 3.54–3.57 (2H, m, CH2), 7.65–7.75 (2H, m, Ar-H), 7.79–8.03 (4H, m, Ar-H), 8.58 (1H, s, Ar-H). 13C-NMR (100 MHz, CDCl3) δ: 24.08, 25.28, 26.26, 44.32, 48.32, 106.59, 122.90, 128.22, 128.36, 129.63, 130.07, 130.45, 131.90, 133.08, 136.02, 154.64. Anal. Calcd for C18H17N3O4S: C, 58.21; H, 4.61; N, 11.31. Found: C, 58.47; H, 4.38; N, 11.09.
2-Amino-N-(naphthalen-2-ylsulfonyloxy)-2-oxoacetimidoyl Cyanide (NpsAmOx, 24): This compound was obtained as white crystals, 1.34 g (88.5%), mp 118–120°C; IR (KBr) cm−1: 3439, 3329 (NH2), 2236 (CN), 1696 (CO, amide), 1402, 1192 (SO2, sulfonate). 1H-NMR (400 MHz, DMSO-d6) δ: 7.65 (1H, t, Ar-H, J=7.3 Hz), 7.84 (1H, t, Ar-H, J=7.3 Hz), 8.08–8.30 (6H, m, 2NH, 4Ar-H), 8.94 (1H, s, Ar-H). 13C-NMR (100 MHz, DMSO-d6) δ: 106.68, 128.59, 128.77, 130.08, 130.46, 130.66, 131.10, 132.15, 132.59, 136.16, 136.24, 157.24. Anal. Calcd for C13H9N3O4S: C, 51.48; H, 2.99; N, 13.85. Found: C, 51.69; H, 3.12; N, 13.67.
2-(Ethylamino)-N-(naphthalen-2-ylsulfonyloxy)-2-oxoacetimidoyl Cyanide (NpsN-Oxyma, 25): This compound was obtained as white crystals, 1.39 g (83.7%), mp 114–115°C; IR (KBr) cm−1: 3302 (NH), 2235 (CN), 1676 (CO, amide), 1400, 1185 (SO2, sulfonate). 1H-NMR (400 MHz, CDCl3) δ: 1.17 (3H, t, CH3, J=7.4 Hz), 3.40 (2H, q, CH2, J=7.4 Hz), 6.62 (H, br s, NH), 7.68 (1H, t, Ar-H, J=7.3 Hz), 7.68 (1H, t, Ar-H, J=7.3 Hz), 7.89–8.05 (4H, m, Ar-H), 8.60 (1H, s, Ar-H). 13C-NMR (100 MHz, CDCl3) δ: 14.41, 35.39, 106.11, 122.71, 128.26, 128.48, 129.70, 130.06, 130.30, 130.63, 131.88, 131.96, 133.97, 136.15, 154.94. Anal. Calcd for C15H13N3O4S: C, 54.37; H, 3.95; N, 12.68. Found: C, 54.63; H, 3.79; N, 12.87.
N-(Naphthalen-2-ylsulfonyloxy)benzimidoyl Cyanide (NpsPhCN, 26): This compound was obtained as white crystals, 1.45 g (86.3%), mp 133–134°C; IR (KBr) cm−1: 2234 (CN), 1390, 1186 (SO2, sulfonate). 1H-NMR (400 MHz, CDCl3) δ: 7.44 (2H, t, Ar-H, J=8.1 Hz), 7.54 (1H, t, Ar-H, J=7.3 Hz), 7.65–7.78 (4H, m, Ar-H), 7.93–8.05 (4H, m, Ar-H), 8.67 (1H, s, Ar-H). 13C-NMR (100 MHz, CDCl3) δ: 107.75, 123.23, 127.23, 127.57, 128.14, 129.41, 129.70, 129.83, 130.14, 131.11, 131.68, 132.01, 133.51, 135.91, 139.60. Anal. Calcd for C18H12N2O3S: C, 64.27; H, 3.60; N, 8.33. Found: C, 64.11; H, 3.35; N, 8.05.
N-(Naphthalen-2-ylsulfonyloxy)picolinimidoyl Cyanide (NpsPyCN, 27): This compound was obtained as grey crystals, 1.50 g (89.0%), mp 135–136°C; IR (KBr) cm−1: 2237 (CN), 1392, 1185 (SO2, sulfonate). 1H-NMR (400 MHz, CDCl3) δ: 7.43–7.45 (1H, m, Ar-H), 7.66–8.05 (8H, m, Ar-H), 8.67–8.72 (2H, m, Ar-H). 13C-NMR (100 MHz, CDCl3) δ: 107.34, 121.82, 123.13, 126.91, 128.18, 128.22, 129.70, 129.95, 130.26, 130.87, 131.76, 132.00, 135.97, 137.30, 140.23, 146.72, 150.41. Anal. Calcd for C17H11N3O3S: C, 60.52; H, 3.29; N, 12.46. Found: C, 60.76; H, 3.47; N, 12.19.
L929 (mouse fibroblast) cells was obtained from the American Type Culture Collection, and cultivated under the condition described by their respective depositor. Cell culture reagents were purchased from Life Technologies Inc. (GIBCO BRL). Plastic ware was obtained from Nunc and Saarstedt.
Cell Proliferation AssayGrowth inhibitions were measured in 96-well plates. Aliquots of 120 µL of the suspended cells (50000 mL−1) were given to 60 µL of a serial dilution of the inhibitor(s). After 5 d of incubations, growth were determined the MTT assay.50)
The work in Egypt was supported by the Science and Technology Development Fund (STDF) through the Research Project TC/12/ RSG /2012 (Proposal ID (4769). The authors thank the Deanship of Scientific Research at King Saud University for funding this work through research group “RGP-VPP-234.”