2014 Volume 62 Issue 7 Pages 627-635
2014 Volume 62 Issue 7 Pages 627-635
A novel γ-cyclodextrin (γ-CD) based carrier for molecular encapsulation of cancer chemotherapeutic agent doxorubicin (DOX) was synthesized and fully characterized by various analytical approaches. The γ-CD derivative, with a β-naphthyl alanine residue attached in its primary face, exhibits potent binding capacity with DOX. The encapsulation efficiency was assessed under various temperatures and pHs and it was demonstrated that the carrier-DOX inclusion complex is highly stable under a wide range of acidic conditions (pH 1.0–7.0); however, the encapsulated drug is slowly released under hyperthermic conditions (up to 50°C). Cell culture studies showed that the complexation of DOX with the carrier protected the drug from being uptaken by the cells and also greatly reduced its toxicity. Thermo-triggered DOX release was validated and the increase in cellular uptake was observed in in-vitro experiments. We concluded that this novel γ-CD derivative is able to effectively encapsulate DOX and the inclusion is responsive to temperature change, hence renders it a potential encapsulating agent for DOX delivery in combination with hyperthermia treatments.
Cyclodextrins (CDs) are natural cyclic torus-shaped oligosaccharides mostly composed of 6, 7, or 8 D(+)-glucose units, and named α-, β-, or γ-CD, respectively.1,2) Because of the unique capability of forming inclusion complexes in the inner cavities and many other favourable physicochemical and biological properties, natural CDs and their derivatives have been applied in drug delivery systems to improve the solubility, stability, bioavailability, therapeutic index, efficacy, pharmacokinetics and biosafety of many hydrophobic drugs.3,4)
In aqueous solutions, CDs form inclusion complexes with anthracyclines5) which are potent antitumour antibiotics.6) In present, cancer chemotherapeutic agent doxorubicin (DOX, 4), a DNA-intercalating antitumour agent, is the most frequently used member of the anthracycline group7) in spite of being chemically unstable8) in aqueous media with major problems of acute and chronic toxicity9) and appearance of cellular multidrug-resistance. The best way to overcome serious side effects associated with DOX administration is to limit the amount of DOX intake or alternatively, to encapsulate the drug in various drug-delivery systems. An ideal targeted drug delivery vehicle should be able to solubilise, protect and transport the drug specifically to cancer cells and slowly release the drug molecules inside the cells to the site of their pharmacological activities. This can be achieved by attaching biological signals10) (hormones, vitamins or antibodies), which can recognize cancer cells, to the surface of the vehicles.11,12) Moreover, drug targeting can also be achieved by using drug vehicles sensitive to the surrounding enzymes, temperature or pH.13–15)
Recently, several studies reported16–23) that introduction of a suitable substituent (e.g., glucose, galactose, arbutin, hydroquinone α-glycoside, porphyrin, or β-carboline) to the rim of α- and β-CDs, can enhance the binding affinity towards DOX through cooperative interactions between the CD’s cavity and guest molecules. Although, α- and β-CD are the most exploited CDs,24) little has been published on γ-CD (1) as targeted drug delivery system. Having 8 glucopyranoside units thus the largest cavity, the γ-CD possesses higher water solubility, better inclusion ability of large-size drugs, and is orally and parenterally administered non-toxic.25)
In this report we synthesized and performed detailed characterization of a novel chemically modified γ-CD derivative, mono-6′-deoxy-6-[3-(naphthalen-2-yl)-2-(amino)propionylamino]-γ-cyclodextrin (3b), in which a β-naphthyl alanine residue is attached in the primary face of γ-CD. This study was carried out in parallel to our recently reported work26) which focused on the application of ultrasound-mediated drug release by employing this novel γ-CD derivative. We further evaluated the host (3b)-guest (DOX) interaction and the encapsulation efficiency of DOX by this carrier under various temperatures and pHs. In addition, the drug inclusion was evaluated in in-vitro studies by cellular drug uptake and cytotoxicity. The potential of the novel γ-CD derivative as a thermo-sensitive carrier for DOX was discussed.
DOX and γ-CD were obtained from Mesochem Technology Co., Ltd., Beijing, China. P2O5, N,N-dimethyl-4-aminopyridine (DMAP), N-hydroxybenzotriazole (HOBt), N-boc-3-(2-naphthyl)-D-alanine, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), N,N-dimethylformamide (DMF), dimethyl sulphoxide (DMSO), trifluoroacetic acid (TFA), dichloromethane (CH2Cl2), deuterated water (D2O), acetonitrile (ACN), acetone, methanol, sulfuric acid, 1-butanol, ethanol, and NH3(aq) were all bought from Sigma-Aldrich, Israel. LiChroprep RP-18 reversed-phase column (40–63 µm) was purchased from Merck, Israel.
Cell CultureHuman nasopharyngeal epidermal carcinoma (KB) was obtained from American Type Culture Collection (ATCC CCL-17, ATCC, U.S.A.). KB cells were cultured in complete medium (CM) under standard conditions (37°C, 5% CO2). The CM consisted of Dulbecco’s modified Eagle’s medium (DMEM) culture medium supplemented with 10% fetal bovine serum, 1% L-glutamine and 1% penicillin–streptomycin which all were obtained from Invitrogen, Paisley, U.K.
Thiazolyl blue tetrazolium bromide (MTT) reagent for cell viability test and bicinchoninic acid (BCA) kit for cell protein quantitation were obtained from Sigma-Aldrich, U.K. Sodium dodecyl sulphate (SDS) for preparation cell lysis buffer (0.5%, w/w) was obtained from Sigma-Aldrich, U.K. PBS solution was prepared by dissolving 1 tablet (Oxoid, U.K.) in 100 mL distilled water (Millipore, U.K.).
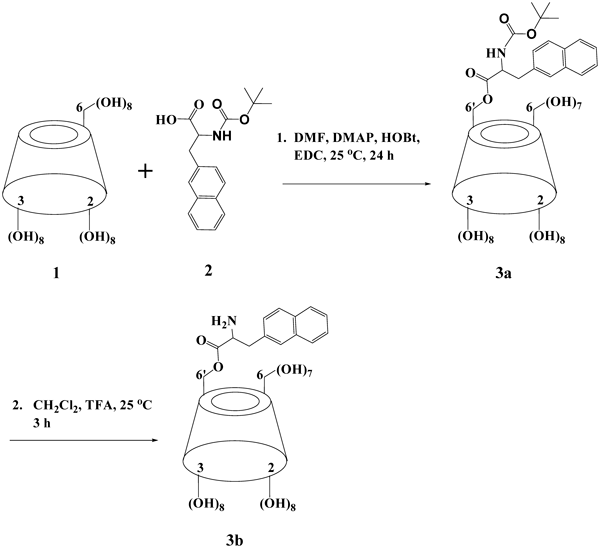
Synthesis of the γ-CD DerivativeThe synthesis of the γ-CD derivative was performed as essentially described in our previously reported study26) with minor modifications. In brief, mono-6′-deoxy-6-[3-(naphthalen-2-yl)-2-(tert-butyloxycarbonylamino)propionylamino]-γ-cyclodextrin (3a) was prepared (Fig. 1, Step 1) by direct coupling of dry γ-CD (1) with 2-((tert-butoxy carbonyl)amino)-3-(naphthalen-2-yl)propanoic acid (2) by using HOBt, DMAP and EDC27) instead of using N,N-dicyclohexylcarbodiimide (DCC). The resultant compound (3a) was then converted to mono-6′-deoxy-6-[3-(naphthalen-2-yl)-2-(amino)propionylamino]-γ-cyclodextrin (3b) by removal of the tert-butoxycarbonyl protecting group (Fig. 1, Step 2).26) The final product 3b was then fully characterized.
TLC analysis was performed on silica gel 60 F254 PLC plates (Yavin-Yeda, Israel). TLC eluent solution was prepared by mixed solvent of 1-butanol–ethanol–NH3(aq) at 4 : 5 : 6 (v/v/v). The spot detection was then carried out by spraying with 5% (v/v) concentrated sulfuric acid in ethanol and heated to 150°C on a hot plate for 5 min.
HPLC analysis was performed on a Thermo Electron instrument equipped with UV/Vis & ELSD (Sedex 75) detectors (Sedere, France). The column used was a Gemini 5 µm octadecyl carbon chain bonded silica (C18, 110 Å, 250–4.6 mm, Sedere, France) mobile phase: ACN/H2O, with a gradient elution from 98% H2O/2% ACN to 10% H2O/90% ACN, within 30 min at a constant flow rate of 1.2 mL/min.
Characterization of 3bThe ultraviolet (UV) spectroscopy signal of 3b was measured at λ=254 nm. UV analysis was performed on a UV spectrophotometer (Thermo Electron Corporation Instrument, U.S.A.). Mass spectroscopy (MS) was performed using Micromass ZQ 2000 detector (Waters, Micromass ZQ, U.S.A.), nitrogen flow 500 L/h, nitrogen cone flow 70 L/h, source temperature 110°C, desolvation temperature 350°C, and cone voltage 30 V. Melting point (mp) was performed by using Electrothermal IA9100 Digital Melting Point Apparatus (Thermo Scientific, Israel). Nuclear magnetic resonance (1H-NMR, 13C-NMR, correlation spectroscopy (COSY) and nuclear Overhauser effect spectroscopy (NOESY)) spectra were recorded on Bruker 400 MHz Advance III spectrometer (Bruker, U.S.A.) with D2O as a solvent. All the chemical shifts are expressed as δ units (ppm).
Fluorescence SpectrometryInclusion Association Analysis of 3b with DOXThe inclusion association of 3b with DOX was quantitatively evaluated by titration method, i.e., measuring the fluorescence intensity of DOX at a constant concentration (5 µM) in the presence of different concentrations (0.67 to 5000 µM) of 3b. The 3b : DOX molar ratios in the tested solutions varied from 0.13 : 1 to 1000 : 1. Fluorescence spectra were measured by fluorescence spectrophotometer of Model Varian Cary Eclipse (Agilent Technologies, U.S.A.). Experiments were performed in 0.1 M PBS buffer (pH=7.4) at 37°C. A solution of 5 µM DOX was prepared by dilution of DOX 100 mM stock solution (in DMSO) by PBS. The final concentration of DMSO in the diluted solution was 0.005%. To 10 mL of the 5 µM DOX solution, 7.47 mg/mL of carrier 3b was added to obtain a carrier concentration of 5000 µM (1000 : 1 M ratio of 3b : DOX). Double dilutions from the 1000 : 1 (3b : DOX) solution by using 5 µM DOX solution was performed to obtain the various ratios of 3b : DOX in the final test solutions. Each sample was transferred into a 4 mL cuvette (Yavin-Yeda, Israel) and the fluorescence intensity was measured at λexcitation=480 nm, λemission=500–800 nm (λmax=592 nm),28,29) with excitation and emission slits of 10 nm.
Thermal/pH Responsivity of the Inclusion Complex of 3b : DOXSamples were prepared in the same way as described above “Inclusion Association Analysis of 3b with DOX.” Fluorescence intensity was measured by a multi-mode plate reader (Tecan infinite M200, Austria) under optimal gain mode. Thermal responsivity of 3b : DOX was evaluated in 0.1 M PBS. INV064 oven (−10°C to 50°C) (Scientific Laboratory Supplies, U.K.) was used for achieving the desired temperatures. The pH dependence of 3b : DOX was assessed by dissolving the complex in different buffer solutions. The buffer solutions were prepared by using 0.2 M/0.1 M dibasic sodium phosphate, 0.1 M sodium phosphate monobasic, 0.1 M citrate acid monohydrate, 0.1 M potassium chloride and 0.1 M hydrochloric acid.30) All chemicals for preparation of buffers were purchased from Sigma-Aldrich, U.K.
In-Vitro Cellular AssayCytotoxicity AssayCultured KB cells were harvested and seeded at the density of 4000 per well in a 96-well micro-plate one day prior to the assay.
To test 3b’s cytotoxicity, carrier solution at 1250 µM was prepared in CM and was filtered by a 0.45 µm filter (Fisher Scientific, U.K.). It was then double diluted by CM to obtain gradient concentrations of 3b solutions until 4.88 µM. For the cytotoxicity assay of 3b : DOX complex, 100 µM DOX was prepared by dilution of the DOX stock solution (100 mM in DMSO) by CM. The final concentration of DMSO in the diluted solution was 0.1%. 3b was added at 1.5 mg/mL to the 100 µM DOX solution to obtain a concentration of 1000 µM (10 : 1 of 3b : DOX molar ratio). This solution was then filtered by a 0.45 µm filter and double diluted by CM to obtain DOX at 50 µM, 25 µM, 12.5 µM, 6.25 µM, and 3.125 µM; the ratio of 3b : DOX was kept constant at 10 : 1.
Cells were exposed to the pre-heated (37°C) test solutions by incubation with 100 µL of each solution/well for 30 min in triplicates. At the end of incubation, test solutions and CM in control wells were removed and cells were washed by pre-heated CM twice and replaced with 100 µL/well fresh CM. Cells were then observed under the microscope for cell loss. The cells were further incubated for 48 h before the assay.
Cells were then evaluated with MTT colorimetric assay that uses the enzymatic activities of cells as a measure for their viability. For the MTT assay, 20 µL/well of 5 mg/mL MTT dissolved in PBS was added directly into cultured cells (100 µL CM) and incubated for 3 h in a standard incubator. After incubation, the solution was gently aspirated from the wells without touching the bottom of wells. 100 µL/well of DMSO was then added to dissolve the formazan, a chromogenic product created by the reduction of the tetrazolium salt in the metabolically active cells. The absorbance for each well, which is proportional to the total number of live cells, was read using the same multi-mode Infinite M200 plate reader at a wavelength 550 nm. Values of viability of treated cells were expressed as a percentage of that from corresponding control cells. All experiments were repeated at least three times.
Cellular Uptake AssayKB cells were harvested and seeded at the density of 1.3×105 per well in a 24-well plate and allowed to grow over three days prior to the assay. DOX solutions at 12.5 µM were prepared by dilution of the DOX stock solution (100 mM in DMSO) by CM. For the 3b : DOX complex, appropriate amount of 3b was added to the corresponding DOX solutions to obtain the concentration of 125 µM so that the 3b : DOX ratio was kept at 10 : 1. Cells were then exposed to the test solutions that were pre-heated to 37°C, 42°C and 50°C by incubation with 300 µL of each solution/well for 5 min in triplicates. At the end of the incubation, test solutions were removed and the wells were washed twice with PBS followed by the addition of 250 µL of 0.5% SDS. Upon observation under the microscope to ensure complete cell disruption by SDS, 200 µL total cell lysates from each well were transferred into wells of a black 96-well plate with µ-clear bottom (Greiner, Austria) for the quantification of intracellular DOX by fluorescence intensity. Meanwhile, 25 µL of cell lysates from the same samples were transferred to wells of a transparent 96-well plate (TPP, Switzerland) for total cellular protein determination by the BCA assay.31) Standard calibration curves of DOX and BSA with known concentrations were obtained and used to calculate the concentrations of the intracellular DOX and total cellular protein, respectively. The total cellular protein is proportional to the number of cells present in the well at the time of assay and was used to normalize the measured intracellular DOX. The normalized amount of intracellular DOX was expressed as µmol/µg cellular protein. In addition, the cellular uptake of encapsulated DOX under standard culture conditions (37°C) was compared with and expressed as a percentage of that from cells incubated with free DOX.
EquationsCalculation of Encapsulation Efficiency of 3b to DOXThe encapsulation efficiency of 3b to DOX was calculated by Eq. 1:
 | (1) |
Where, I0 and Ix are the fluorescence intensity of DOX in the absence and presence of different concentrations (x) of 3b, respectively. The release rate of DOX (ESM Table 1) from the encapsulation by the temperature increase was calculated by the subtraction between encapsulation efficiencies (%) that were obtained at varying temperatures.
| pH | 3b : DOX | ||||
|---|---|---|---|---|---|
| 1000 : 1 | 300 : 1 | 40 : 1 | 10 : 1 | 5 : 1 | |
| 7.4 | 92.57±0.16 | 91.82±0.37 | 83.51±2.03 | 66.19±4.69 | 46.30±5.19 |
| 7.0 | 91.00±0.35 | 89.83±0.19 | 82.50±0.67 | 68.19±2.21 | 53.17±2.72 |
| 6.5 | 90.99±0.44 | 89.92±0.51 | 81.43±1.79 | 65.73±3.32 | 51.69±5.41 |
| 5.5 | 90.55±0.27 | 89.88±0.38 | 80.81±1.72 | 65.13±3.87 | 48.28±5.07 |
| 5.0 | 91.04±0.23 | 90.30±0.15 | 82.90±0.10 | 67.55±0.35 | 50.40±1.26 |
| 4.5 | 91.27±0.05 | 89.85±0.09 | 81.49±0.43 | 65.30±0.40 | 50.20±0.35 |
| 4.0 | 90.81±0.43 | 90.16±0.22 | 80.12±1.12 | 62.21±1.47 | 46.92±2.16 |
| 3.0 | 91.82±0.08 | 90.27±0.17 | 80.81±0.62 | 63.36±1.83 | 46.67±1.01 |
| 2.0 | 92.44±0.19 | 90.62±0.20 | 82.58±0.56 | 62.74±1.21 | 44.52±1.26 |
| 1.0 | 91.72±0.11 | 90.97±0.22 | 82.43±0.88 | 62.57±1.58 | 42.61±0.56 |
a) The concentration of DOX is 5 µM. b) The concentrations of 3b are between 25 µM and 5000 µM to keep appropriate ratios between 3b and DOX as indicated.
It has been well reported in the literature that, addition of native γ-CD added to DOX at a ratio of up to 5000 : 1 could significantly stabilize DOX in aqueous solution via complex formation.32) Other reports,33) in which DOX encapsulation within CDs could significantly lower the fluorescence and absorbance signals of the guest molecule, demonstrated that CDs can be considered as a fluorophore quencher for DOX. Therefore, the binding constant can be calculated according to modified Stern–Volmer24) Eq. 2:
 | (2) |
Where, [H]x is the concentration of the host compound 3b and 1/f is equal to 1 since only one fluorescence quencher was employed in our study.34) The corresponding K binding was calculated from the linear plot of I0/(I0−Ix) vs. 1/[H]x where 1/fK is the slope.
To analyze the conjugated product of the carrier synthesis, TLC and HPLC were conducted. TLC analysis shows Rf (γ-CD)=0.18, Rf (3a)=0.48, and Rf (3b)=0.25, respectively. The chromatogram obtained by HPLC (Fig. 2) of crude 3b contains the following peaks: 12.06 min (3b), 12.9, 13.5, 14.1 min (unknown impurities), 17.1, 17.8, 18.8 min (di-substituted γ-CD), 36.6, 37.5, 46.8 min (unknown impurities). The yield of the monosubstituted product 3b was 83% (of the total products) with retention time occurred at 12.06 min. The unwanted di-substituted and tri-substituted (14.1% in total) were manily in three parts with retention times being at 12.9/13.5/14.1 min, 17.1/17.8/18.8 min, and 36.6/37.7/46.8 min, respectively, depending on the relative position of the substituents to each other.
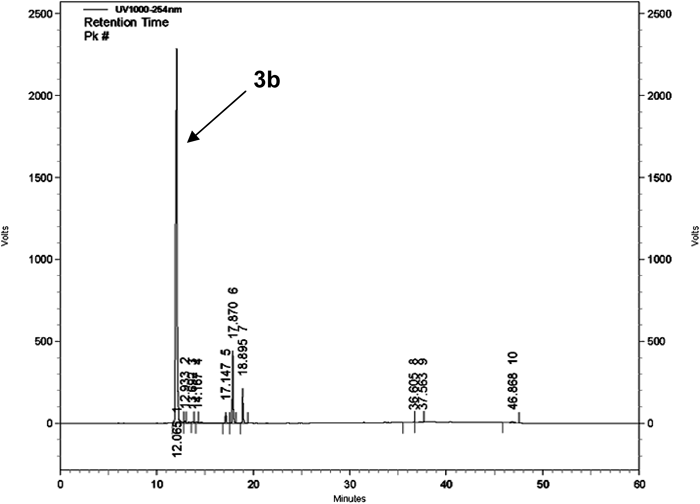
It contains the following peaks: 12.06 min (3b), 12.9, 13.5, 14.1 min (unknown impurities), 17.1, 17.8, 18.8 min (di-substituted γ-CD), 36.6, 37.5, 46.8 min (unknown impurities).
Eight peaks were found in the UV spectra above −3.00A between wavelengths 190.00 nm and 1100.00 nm with the highest peak at 278 nm (0.895) (ESM Fig. 1). MS result of ESI-MS m/z as Calcd for C61H91NO41 (3b) was 1494.51, and was measured 1494.77 (ESM Fig. 2). mp (3b, H2O)=220–222°C.
NMR Characterization1H-NMR and 13C-NMR mesurements were performed to validate the structure of desired 3b. The 1H-NMR spectra (Fig. 3a) and especially 13C-NMR spectra (Fig. 3b) suggested that the resultant chemical structure of the carrier is in aggreement with the desired yield being the monosubstituted γ-CD with a β-naphthyl alanine residue on one of the hydroxyl groups on the primary face.
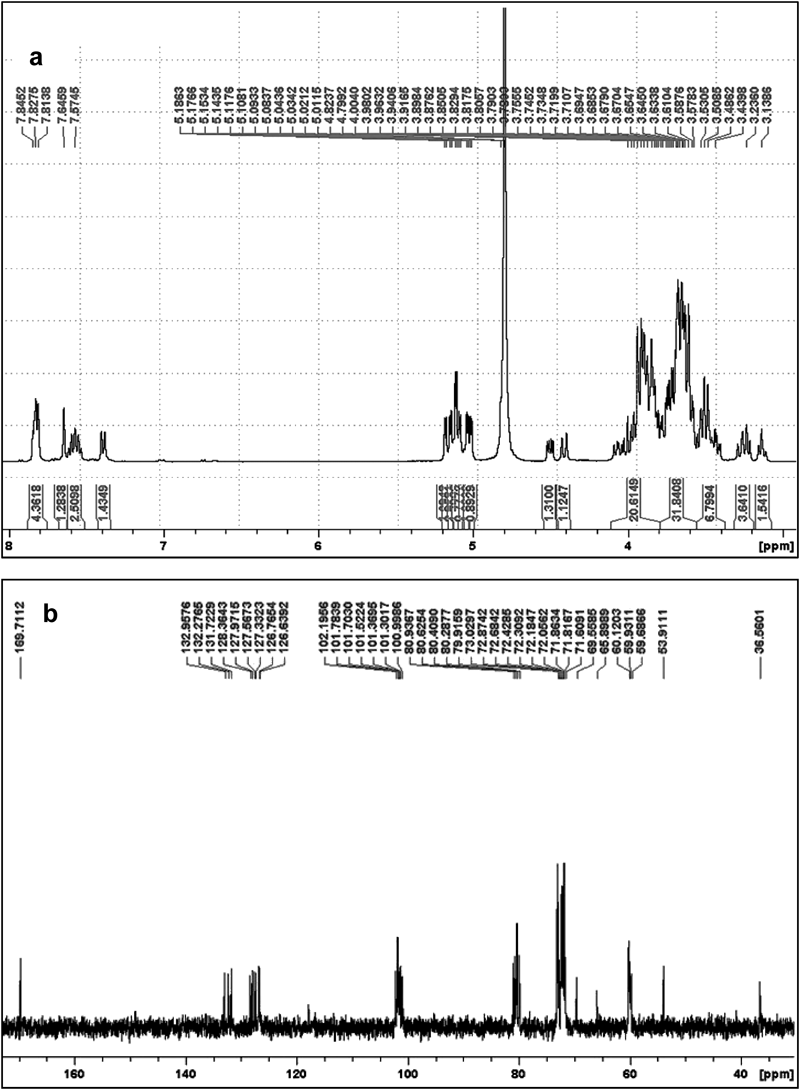
1H-NMR spectrum (a); 13C-NMR spectrum (b).
1H-NMR (D2O) of 3b: 3.20 (4H, d), 3.52 (2H, d), 3.86 (6H, s), 3.79 (5H, t), 3.97 (3H, d), 4.41 (9H, d), 4.50 (8H, s), 5.10 (1H, d), 7.39 (19H, s), 7.57 (15H, m), 7.57 (14H, m), 7.65 (11H, m), 7.81 (18H, m), 7.83 (16H, m), 7.85 (13H, m).
13C-NMR (D2O) of 3b: 36.53 (9C), 53.87 (8C), 59.63–60.15 (6C), 65.84 (6C*), 69.52 (5C*), 71.56–71.82 (5C), 72.01–72.38 (2C), 72.64–73.06 (3C), 79.83–80.90 (4C), 100.97–102.17 (1C), 126.59 (15C), 126.73 (14C), 126.81 (18C), 127.31 (13C), 127.53 (16C), 127.94 (19C), 128.35 (11C), 131.69 (17C), 132.23 (12C), 132.92 (10C), 169.68 (7C). (* Carbon of the substituted ring.)
It is well documented in the literature35) that 1H, 2H, 4H and 6H protons in γ-CD are located on the exterior surface of the molecule and exposed to bulk environments, while 3H (δ=3.95 ppm) and 5H (δ=3.88 ppm) protons are located in the interior cavity of γ-CD. 1H-NMR analysis of 3b (Fig. 3a), shows that 3H (δ=3.97 ppm) and 5H (δ=3.79 ppm) protons may experience magnetic perturbation, in particular the 5H, which has an upfield shift of 0.09 ppm. This observation suggests the possibility of certain cooperative hydrophobic interactions between the CD’s interior cavity and the flexible cap β-naphthalene moiety, achieved likely by partial or fully self-assembly inclusion. Further evidence regarding the interaction and relevant discussion can be found in “NMR Analysis of 3b to DOX Complexation” where the existence of DOX was included in the complexation.
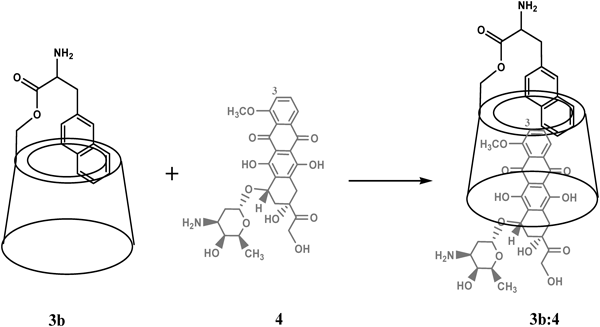
Inclusion Association of 3b to DOXNMR Analysis of 3b to DOX ComplexationIn order to investigate the inclusion behavior of the host molecule for DOX, further NMR measurements were performed in D2O. The results showed significant chemical shifts (black circled positions in Fig. 4) in the majority of the protons from both 3b and DOX molecules, where the complex spectrum of 3b : DOX mixture in D2O is not a simple superposition of the carrier and DOX spectra36) (ESM Fig. 3). As mentioned above in “NMR Characterization,” 3H and 5H of 3b both face the inner space of the cone thus could be affected by the removal of D2O and the entrance of DOX.37) Therefore, at least one explanation for the cavity inclusion of DOX might be found from the upfield chemical shifts of these protons within the γ-CD ring as shown in Fig. 4 in which interaction between 3H and 5H of CD’s ring with 4-OCH3 of DOX and protons on β-naphthalene was observed. This piece of data also provides further information in supporting that the β-naphthalene moeity is partially or fully assembled within the CD’s interior cavity as predicted in “NMR Characterization.” Additional information obtained from the 1H-NMR spectrum of the complex can indicate the orientation of the DOX within the carrier. As can be seen from Fig. 4, the methyl moiety on the 5th position of the amino sugar ring with the peak position of δ=1.33 ppm (5′-CH3, arrow 1 and 1′ in Fig. 4) and the proton on the 8th position of the aromatic ring with peak position of δ=2.30 ppm (8-H2, arrow 2 and 2′ in Fig. 4) of the DOX molecule are not influenced by the addition of the 3b carrier, suggesting that this part of DOX is located outside the γ-CD cavity.

5′-CH3 (arrow 1 and 1′) δ=1.33 ppm and 8-H2 (arrow 2 and 2′) δ=2.30 ppm of DOX are as indicated. Black circles indicate interations of (from left to right): 3H of DOX with protons on β-naphthalene; 1′H of DOX with 1H of CD’s ring; 3H ad 5H interior protons of CD’s ring with 4-OCH3 of DOX and protons on β-naphthalene; 10H2 of DOX with protons on CD’s ring.
Additional insights in the 3D structure of the complex were achieved by NOESY and COSY measurements. Subtraction of the COSY and NOESY full spectrums (ESM Figs. 4–6) provided the information on the non-covalently bonded protons present in proximity to each other in the 3D structure of the complex. In the measurement of the 1H-NOESY spectra of the 3b : DOX38) complex in D2O, the NOE interaction between the proton 3H of DOX (6.9 ppm), which is located on the first phenyl ring next to the methyl group, and the β-naphthalene protons (7.6 ppm) of 3b was observed (ESM Fig. 5c). The COSY/NOESY subtraction also revealed again the interaction between 1′H of DOX (5.75 ppm) and 1H of CD’s ring on 3b’s exterior surface (5.17 ppm) (ESM Fig. 6c). Taken together, data from the NMR analysis indicate multi-interactions between the DOX molecule and the interior protons of the CD’s ring or those on β-naphthalene, and a strong probability of the formation of π–π stacking between the naphthyl group and the aromatic ring of the included DOX within the 3b’s cavity. The predicted model of inclusion is illustrated in Fig. 5, in which the orientation of the DOX is also considered.
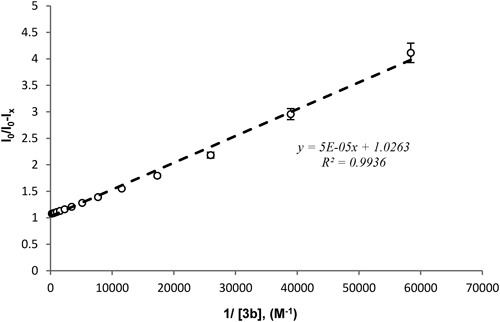
Fluorescence intensity of DOX (5 µM) that was mixed with 3b (0.67 to 5000 µM) was measured and K binding was calculated as described in Materials and Methods (K=20526 M−1).
As shown in Fig. 6, a good liner relationship between the fluorescence intensity of DOX in the presence of 3b and 1/[3b] was observed. The corresponding K binding value was then calculated by the obtained liner plot of y=5×105x+1.0263 based on the Eq. 2 where y=I0/(I0−Ix) and x=1/[H]x (1/[3b]); the K binding value was 20,526 M−1 at physiological temperature. This value is 102 fold higher than that reported by Anand et al.39) for the native γ-CD with DOX in 1 : 1 complex formation. The high value of K binding of our modified 3b can be attributed to the modification by the naphthalene group, which potentially exhibits π–π interaction between the aromatic rings in 3b and DOX molecules as suggested by the NMR studies.
Drug Loading Efficiency of the CarrierThe fluorescence intensity of DOX was gradually reduced by the presence of increasing concentration of the carrier (ESM Fig. 7). This signal decay suggests good coupling between the DOX and 3b due to encapsulation that allows energy transfer between the guest and host molecules to occur. The result also validated that the carrier 3b is a fluorophore quencher for DOX, in agreement with previous reports in which similar quenching effect was observed by this type of molecules.40,41)
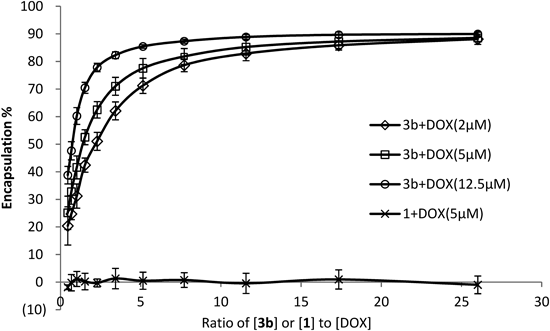
The DOX concentrations were as indicated (2 µM, 5 µM, and 12.5 µM) and 3b (or γ-CD) was applied at ratios to each concentration of DOX from 26 : 1 down to 0.5 : 1.
As shown in Fig. 7, the encapsulation efficiency for DOX, as calculated by the Eq. 1, was overall gradually elevated with increasing concentration of carrier 3b, i.e., increase in the 3b : DOX ratio could enhance the encapsulation, although significant encapsulation was already seen at ratios as low as 1 : 1. The data also suggest that increasing the concentration of DOX led to increased drug loading. No inclusion was observed by the non-modified carrier 1 under same conditions.
The 1H-NMR spectra of 3b : DOX complexes in Fig. 8, and in the Supporting Information (Fig. 8), may provide more evidence in supporting the above observation. Higher 3b : DOX ratio (10 : 1) exhibited broader, more widely distributed peaks than lower ratio (1 : 1) complex. This NMR spectral variation suggests that in the 1 : 1 solution (Fig. 8, bottom spectrum) the complexation has a more rigid structure than that in 10 : 1 complex in which excessive amount of the carrier 3b is present. In the latter case (10 : 1, Fig. 8, upper spectrum), several 3b molecules could interact one DOX encouraging it to exhibit a faster dynamic equilibrium between the free and encapsulated forms thus facilitating the encapsulation. Additionally, the comparison of the two spectra can aid in the attribution of the peaks to the appropriate molecules. A good example is the peak at 6.9 ppm which appears only in the 1 : 1 spectrum and corresponds to 3H of DOX (black arrow in Fig. 8, bottom spectrum).

Twenty millimolar 3b and 2 mM DOX were applied for the 10 : 1 complex; and 10 mM 3b and 10 mM DOX were applied for the 1 : 1 complex. 3H (black arrow) δ=6.9 ppm of DOX is as indicated.
The pH responsiveness of 3b : DOX complex was investigated by fluorescence spectroscopy. The fluorescence of free DOX was found to be unaffected in different pH media (ESM Fig. 9). Therefore, any change in the fluorescence intensity of DOX should be a result of the encapsulation. The encapsulation of 3b to the drug was estimated under a wide range of pH values (1.0–7.4) at 37°C (Table 1). Although clearly affected by the 3b : DOX ratio as already demonstrated in Figs. 7 and 8, the encapsulation efficiencies are all in the same order of magnitude. The results thus indicate a similar chemical stability of the complex8,32) and comparable encapsulation mode in a wide range of acidic environments and at a wide range of 3b : DOX ratios.
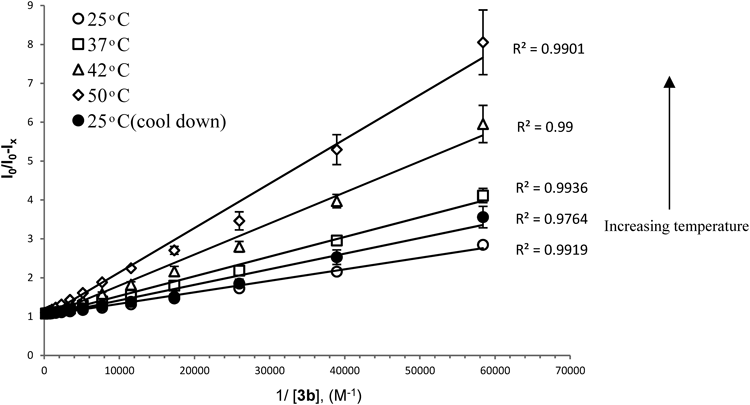
K25°C=34433, K37°C=20526, K42°C=12475, K50°C=9960, K25°C (cool down)=25377 M−1.
The same method was utilized to evaluate the thermal response42) of the inclusion complex. The samples were heated up from 25°C, to 37, 42, and 50°C and then cooled down to 25°C (ESM Fig. 10). The modified Stern–Volmer plots show a clear correlation between the encapsulation efficiency of 3b with DOX and the temperature of the surrounding environment (Fig. 9). Increasing the temperature from 25 to 50°C led to a decrease of the K binding by ca. 3.5 fold and the release of the DOX from the inclusion complex. In addition, after cooling down to 25°C, a re-encapsulation process of DOX by 3b was observed. This phenomenon demonstrates the reversible equilibrium process of 3b+DOX⇆3b : DOX and further confirms the dynamic complexation interaction between 3b and DOX. It is known in the literature that the complexation process of CDs43) is driven by enthalpy and entropy, typically exhibiting large negative enthalpy changes as well as small negative entropy changes. The negative enthalpy changes results from van der Waals interaction and hydrogen bonding between guests and the cavities of CDs, while the negative entropic change is due to the steric barrier caused by the molecule’s geometrical shape and the limit of CD cavity to the freedom of shift and rotation of guest molecules.44) Optimal (highest) DOX release from the inclusion by temperature changes occurred when the 3b : DOX ratio was around 10 : 1 as indicated by the release rate (ESM Table 1). We applied this ratio formation thereafter in our in-vitro cell culture studies “In-Vitro Cell Culture Study.”
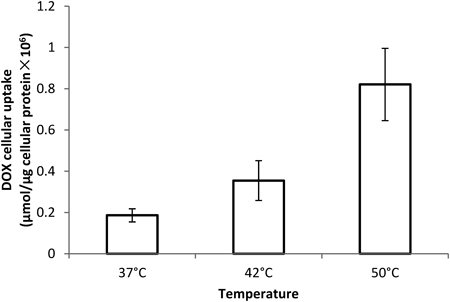
KB cells were incubated with the encapsulated DOX at concentration of 12.5 µM for 5 min at 37, 42, and 50°C, respectively, and intracellular DOX was measured as described in Materials and Methods.
To validate the encapsulation capability of the carrier in physiological environment, in-vitro cell culture experiments were conducted. The results showed that this modified γ-CD derivative itself had no obvious cytotoxic effect to KB cells (ESM Table 2); the average cell viability was all over 92.92±6.10 (%). Whilst the free DOX dose-dependently killed the cells, inclusion by the carrier dramatically reduced its cytotoxicity: over 74.73±3.42 (%) of the cells were viable at the drug dose up to 100 µM (Table 2). The significant protecting effects of the carrier when the DOX concentration was above 6.25 µM, which remained at a constant level, demonstrate the encapsulating capacity of this compound and its potential as a viable carrier for DOX delivery.
| DOX concentration (µM) | KB cell viability (% of control) | |
|---|---|---|
| Free DOX | 3b : DOX (10 : 1) | |
| 0.00 | 100.00±4.11 | 100.00±4.98 |
| 1.56 | 75.41±4.05 | 80.50±3.88 |
| 3.13 | 75.34±3.53 | 74.73±3.42 |
| 6.25 | 69.85±5.40 | 77.23±3.78a) |
| 12.50 | 56.81±3.56 | 81.32±4.97a) |
| 25.00 | 36.00±0.72 | 77.51±1.40a) |
| 50.00 | 23.44±1.53 | 75.37±2.04a) |
| 100.00 | 12.99±2.42 | 77.32±4.46a) |
a) Donates a significant increase from the cells treated with free DOX (p<0.05).
The thermo-response of 3b : DOX inclusion was tested by cellular uptake of the drug. Carrier 3b was found to be very efficient in reducing the DOX uptake into KB cells: the uptake was found to be only 12.15% of free drug-treated cells under standard culture conditions. The data suggests that the encapsulation of DOX by 3b inhibits its cellular internalization therefore reduces its cytotoxicity as shown in Table 2. Increase in the culture temperature significantly facilitated the drug uptake from the inclusion complex (Fig. 10), suggesting the enhanced drug release from the encapsulation upon hyperthermia which is in agreement with the data presented in “Thermal Responsivity of the Complex.” These biological observations confirm the encapsulation ability of 3b and emphasize the potential thermo-triggered application of this vehicle in targeted drug delivery for DOX.
In conclusion, we have successfully designed and constructed a novel γ-CD derivative 3b that showed significantly improved encapsulation ability for DOX due to the possible multi-interactions between the DOX molecule and the interior protons of the CD’s ring or those on the β-naphthalene moiety. Our data from the encapsulation analyses under various conditions showed that the carrier-drug complexation is thermo-responsive and highly stable in a wide range of pH environment. Encapsulation of DOX by the carrier protected the drug from being uptaken by the cultured cells and also greatly reduced its toxicity. Further in-vitro studies in cells upon temperature rise validated the thermo-triggered drug release and increased cellular uptake under physiologically relevant conditions. This study demonstrates the potential of this novel γ-CD derivative as an effective encapsulating vehicle for DOX which can be further exploited for cancer chemotherapy in combination with heat treatments, such as radiofrequency thermal ablation (RFA), microwave hyperthermia, and high intensity focused ultrasound (HIFU).45)
The NANOPORATION project has received funding from the European Community’s Seventh Framework Programme (FP7/2007–2013) under Grant Agreement No. 230674.