2014 Volume 62 Issue 7 Pages 725-728
2014 Volume 62 Issue 7 Pages 725-728
Transannular cyclizations of germacrone-4,5-epoxide under acidic and thermal conditions have been reported in our previous study. However, this process gave the different and interesting results under basic conditions. (4S,5S)-Germacrone-4,5-epoxide (1) was treated under basic conditions to yield four products (2–5). Compound 2 was an isomer of 1 —(4S,5S,9Z)-4,5-epoxygermacra-7(11),9-dien-8-one— and the remaining three compounds (3–5) were eudesmane-type derivatives. Compounds 4 and 5 are new compounds. The structures of the new compounds were determined using high resolution (HR)-MS, one dimensional (1D)-NMR, 2D-NMR and circular dichroism (CD) spectroscopic data. Products 3–5 had the same carbon skeleton as that of eudesmane-type compounds; however, these compounds showed different arrangement of isoprene units to the natural eudesmane-type sesquiterpenes.
Terpenoids are the major natural products that have various biological activities. They are biosynthesized from variably sized oligo-isoprenyl-diphosphates via cyclization followed by transannular (T-A) cyclization to yield numerous carbon skeletons having complicated stereo-structures. Thus, T-A cyclizations are very important for the production of various carbon skeletons having polycyclic carbon rings to facilitate the biosynthesis of natural products and synthesis of polycyclic terpenes.1–3) Sesquiterpenes biosynthesized from farnesyl-diphosphates belong to relatively small molecule groups having C15 and various carbon skeletons. Germacrane-type compounds having ten-membered rings are important intermediates during the biosynthesis of sesquiterpenes having multi-ring carbon skeletons via T-A cyclization.4–7) Germacrane intermediates convert to polycyclic terpenes such as eudesmane, guaiane, pseudoguaiane, eremophilane, and vetispyrane.
Many guaiane-, secoguaiane- and rearranged guaiane-type sesquiterpenes, as well as several germacrane-type sesquiterpenes, have been obtained from Curcuma sp. plants.8–12) Guaiane and its related compounds are thought to be obtained from (4S,5S)-germacrone-4,5-epoxide (1) by T-A cyclization in Curcuma sp. plants. Antipodal guaiane-type sesquiterpenes like those obtained from Curcuma sp. plants were also isolated along with (4R,5R)-1 from Asarum caulescens.13) This indicated that antipodal sesquiterpenes are biosynthesized via T-A cyclization of (4R,5R)-1 in A. caulescens.
The T-A cyclizations of 1 are important for the biosynthesis of various types of sesquiterpenes in plants and can be performed under acidic or thermal conditions. We previously reported the biotransformation of germacrone into guaiane-type sesquiterpenes by using cultured plant cells14,15) Many studies have reported the T-A cyclization of germacrone-4,5-epoxide to guaiane-type sesquiterpenes under acidic and thermal conditions.13,16,17) However, there have been few reports on T-A cyclization performed under basic conditions.17) We treated 1 under basic conditions to yield four products (2–5). Two (4, 5) of them were new compounds. This study aimed to determine the structure of new compounds derived from 1 after T-A cyclization was performed under basic conditions and to elucidate the mechanism underlying the transformation from 1 to eudesmane skeleton.
(4S,5S)-1 was obtained as one of the main constituents along with many guaiane, secoguaiane, and germacrane compounds from fresh rhizomes of Curcuma aromatica cultivated at the Medicinal Botanical Garden of Kagawa School of Pharmaceutical Sciences, Tokushima Bunri University. Compound 1 was treated under basic conditions with methanol solution, and the resultant reaction products were purified using preparative TLC and HPLC using an octadecyl silica (ODS) column to yield four products, 2–5.
Product 2 showed a pseudo-molecular ion peak [M+H]+ at m/z 235.1702 for C15H23O2, which was consistent with the molecular formula C15H22O2. The UV spectrum showed absorption at 246 nm (ε 8454), which indicated the presence of a conjugated ketone. 1H-NMR of 2 showed the presence of three olefinic methyl groups [δH 1.89 (3H, s), 1.72 (3H, s), and 1.70 (3H, s)] and a singlet methyl group [δH 1.22 (3H, s)]. The 13C-NMR of 2 showed four olefinic carbons (δC 152.7, 133.3, 130.6, and 130.5), a conjugated carbonyl carbon (δC 202.8), and two epoxide carbons (δC 64.2, 60.1). These 1H- and 13C-NMR data of 2 were similar to those of 1. The heteronuclear multiple bond connectivity (HMBC) spectral data of 2 showed that 2 was the 9-ene isomer of 1 (Fig. 1). The rotating frame nuclear Overhauser enhancement spectroscopy (ROESY) data showed a correlation between Me-14 and H-9. Thus the structure of 2 was determined to be (4S,5S,9Z)-4,5-epoxygermacra-7(11),9-dien-8-one. Racemic 2 was reported as a derivative of isogermacrone-epoxide.18)
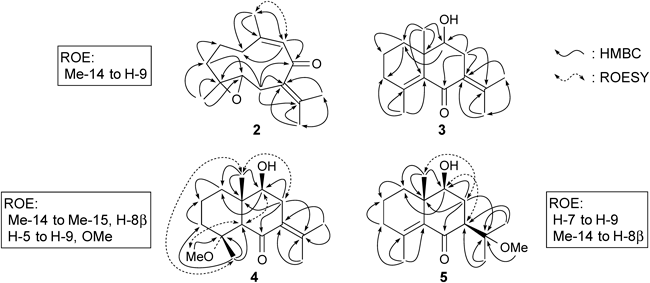
Product 3 showed a pseudo-molecular ion peak [M+H]+ at m/z 235.1707, which was consistent with molecular formula C15H22O2. The 1H- and 13C-NMR spectral data of 3 showed the presence of three olefinic methyl groups, four quaternary olefin carbons, a secondary hydroxy group, and a quaternary aliphatic methyl group. The HMBC data indicated that the structure of 3 was (9S,10S)-9-hydroxyeudesma-4,7(11)-dien-6-one. This compound was identified as a product obtained from 1 under basic conditions.17)
New product 4 showed the pseudo-molecular ion peak [M+Na]+ at m/z 289.1768 to C16H26O3Na and [M+H]+ at m/z 267.1966 to C16H27O3, which was consistent with the molecular formula C15H26O3. The UV spectrum showed the absorption at 250 nm (ε 5832), which indicated the presence of a conjugated ketone. The 1H-NMR spectrum of 4 showed the presence of two olefinic methyl groups [δH 1.74 (3H, s), 1.87 (3H, s)], two quaternary methyl groups [δH 1.58 (3H, s), 0.94 (3H, s)], a methoxy group [δH 3.19 (3H, s)], and an oxygen-bearing methine group [δH 3.73 (1H, dd, J=11.5, 5.4 Hz)], among others. The 13C-NMR spectrum of 4 showed the presence of two olefinic carbons (δC 139.1, 130.8), two oxygen-bearing carbons (δC 78.3, 75.3), a carbonyl group (δC 202.8), four methyl groups (δC 22.5, 21.5, 18.6, 13.2), four methylene groups (δC 37.1, 35.1, 38.1, 19.2), a methine group (δC 61.6) and a quaternary carbon (δC 43.0). These data indicated that 4 should be a derivative of 3 and contain a methoxy group. The HMBC spectrum of 4 showed the following correlations (Fig. 1); Me-14 (δH 0.94, 3H, s) showed correlation with C-1, C-5, and C-9 (δC 37.1, 61.6, 78.3); Me-15 (δH 1.58, 3H, s) with C-3, C-4, and C-5 (δC 38.1, 75.3, 61.6); MeO (δH 3.19, 3H, s) with C-4 (δC 75.3); H-1 (δH 1.17, br t, J=13.2 Hz) with C-2, C-3, C-9, and C-10 (δC 19.2, 38.1, 78.3, 43.0); H-5 (δH 2.37, s) with C-1, C-3, C-6, C-7, and C-9 (δC 37.1, 38.1, 205.4, 130.8, 78.3); H-8 (δH 2.93, dd, J=14.6, 5.4 Hz, 2.23, br t, J=13.2 Hz) with C-6, C-7, C-9, C-10, and C-11 (δC 202.8, 130.8, 78.3, 43.0, 139.1); H-9 (δH 3.73 (dd, J=11.5, 5.4 Hz) with C-1, C-5, and C-7 (δC 37.1, 61.6, 130.8). These data showed that the planar structure of 4 was 9-hydroxy-4-methoxyeudesm-7(11)-en-6-one. The ROESY spectrum of 4 showed the following ROE correlations: Me-14 showed correlation with Me-15 and H-8β, and H-5 showed correlation with H-9 and OMe. These ROE data indicated the relative configuration of 4, which is shown in Fig. 1. The circular dichroism (CD) spectrum of 4 showed a positive Cotton effect ([θ]325 +6198) for the n→π* transition and a negative Cotton effect ([θ]249 −27535) for the π→π* transition on the basis of the α,β-unsaturated ketone.19) These results and T-A cyclization mechanism indicated that the structure of 4 was (4R,5R,9S,10S)-9-hydroxy-4-methoxyeudesm-7(11)-en-6-one.
New product 5 showed the pseudo-molecular ion peaks [M+Na]+ at m/z 289.1770 for C16H26O3Na and [M+H]+ at m/z 267.1970 for C16H27O3, which were consisted with the molecular formula C16H26O3. The UV spectrum showed absorption at 249 nm (ε 4075), which indicated the presence of a conjugated ketone. The 1H-NMR spectrum of 5 showed the presence of an olefinic methyl group [δH 1.66 (3H, s)], three aliphatic singlet methyl groups [δH 1.39 (3H, s), 1.25 (3H, s), 0.92 (3H, s)], a methoxy group [δH 3.16 (3H, s)] and a secondary hydroxy group [δH 3.78 (1H, dd, J=11.2, 5.4 Hz)]. The 13C-NMR spectrum of 5 showed the presence of two olefin carbons (δC 137.9, 138.7), two oxygen-bearing carbons (δC 77.0, 75.0), a carbonyl carbon (δC 205.43), four methyl groups (δC 23.7×2, 20.9, 17.9), four methylene carbons (δC 34.9, 32.3, 30.4, 19.2), a methine carbon (δC 25.8), and a quaternary carbon (δC 42.4). These data indicated that 5 should have a structure similar to that of 4. The HMBC spectrum showed the following H–C correlations: Me-14 (δH 0.92, 3H, s) with C-1, C-5, C-9, and C-10 (δC 34.9, 137.9, 77.0, 42.4); Me-15 (δH 1.66, 3H, s) with C-3, C-4, and C-5 (δC 32.3, 138.7, 137.9); Me-12 and Me-13 (δH 1.25, 3H, s, 1.39, 3H, s) with C-7 and C-11 (δC 55.7, 75.0); MeO (δH 3.16, 3H, s) with C-11 (δC 75.0); H-8 (δH 2.29, 1H, dt J=12.9, 7.0 Hz, 1.74, overlap) with C-6, C-10, and C-11 (δC 205.4, 75.0, 42.4), etc. (Fig. 1). These data showed that the planer structure of 5 was 9-hydroxy-11-methoxyeudesm-4-en-6-one. The ROESY spectrum of 5 showed the correlations between H-7 and H-9, and Me-14 and H-8β. The CD spectrum of 5 showed a positive Cotton effect ([θ]250 +23519) for the π→π* transition and a negative Cotton effet ([θ]323 −4698) for the n→π* transition on the basis of the α,β-conjugated ketone.19) These results and T-A cyclization mechanism indicated that the structure of 5 was (7S,9S,10S)-9-hydroxy-11-methoxyeudesm-4-en-6-one.
T-A cyclization mechanism of 1 converted to 3–5 after treatment under basic conditions were speculated to be as follows. First, substrate 1 was transformed to dienol anion intermediate (1a) under the basic condition (Fig. 2). Intermediate 1a changed to the (Z)-9-ene isomer (2). Alternatively, 1a was transformed to 3 through T-A cyclization with an epoxide ring opening followed by bond formation between C-4 and C-9 (Fig. 2). Conversion of 2 to 3 under basic conditions has been reported18) and supported the cyclization mechanism described above. Product 3 was further transformed to 4 and 5 via the Michael addition of MeOH under the basic condition. In the T-A-cyclization, new bond formation between C-4 and C-9 of intermediate 2 yielded 3, and then 4 and 5. Products 3–5 have a eudesmane skeleton, but were generated from different arrangement of three prenyl units with that of natural eudesmane skeleton (Fig. 3). In the biosynthesis, the germacrane intermediate transformed to eudesmane skeleton by the formation of a new bond between C-5 and C-10 via T-A-cyclization. Thus, the mechanisms of incorporation of C5-units between the T-A cyclization by biosynthesis and the T-A cyclization under the basic condition were different (Fig. 3).

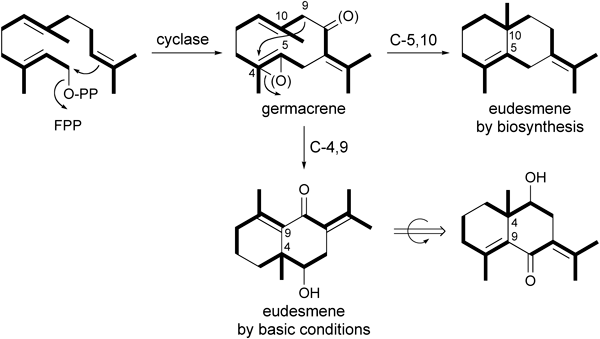
In the case of biosynthesis T-A cyclization was done by new bond formation between C-5 and C-10; however, in the case of basic conditions, the new bond formation was done between C-4 and C-9.
UV spectral data were recorded using a Hitachi U-2001 spectrophotometer. CD data were recorded at 22°C in MeOH by using a JASCO J-820 spectrometer. High resolution (HR)-electrospray ionization (ESI)-MS data were obtained using Waters/Micromass Q-Tof micro mass spectrometer. 1H-NMR (700 MHz) and 13C-NMR (175 MHz) data were measured using a Bruker Avance 700 NMR spectrometer in CDCl3 by TMS as an internal standard. Preparative and analytical HPLC were carried out on a reversed-phase ODS column (Mightysil C-18; Kanto Chemical Co., Ltd.) by using a CH3CN–H2O solvent system. Analytical and preparative TLC were carried out using precoated silica gel TLC plates (0.25-mm thick for analytical TLC and 0.5-mm thick for preparative TLC; Merck).
Isolation of 1 from the Rhizomes of C. aromaticaFive kilograms of fresh rhizome of C. aromatica cultivated at the Medicinal Botanical Garden of Kagawa School of Pharmaceutical Sciences, Tokushima Bunri University, in October 2010, were crushed and extracted with MeOH twice at room temperature. The MeOH extract was evaporated and partitioned between ethyl acetate (EtOAc) and water. The EtOAc-soluble fraction was chromatographed on a silica gel column by using a gradient chloroform–MeOH solvent system to yield crude 1 along with curdione (5.5 g), germacrone (500 mg), etc. Crude 1 was recrystallized from n-hexane to yield pure 1 (2.0 g).
Alkaline Treatment of 1 in MeOHFor this treatment, 110 mg of 1 was dissolved in 30 mL 0.5% NaOH in MeOH and was stirred for 5 d at room temperature. The reaction solution was poured into water and extracted with CHCl3 to yield a CHCl3 solution, which was washed with water and evaporated under reduced pressure to obtain viscous pale brown oil. The reaction mixture was purified using preparative HPLC by using an ODS column and 40–60% CH3CN gradient solvent to yield products 2 (9 mg), 3 (60 mg), 4 (5 mg), and 5 (7 mg).
(4S,5S,9Z)-4,5-Epoxygermacra-7(11),9-dien-8-one (2)HR-ESI-MS m/z: 235.1702 [M+H]+ (Calcd 235.1698 for C15H23O2). UV λmax nm (ε): 246 (8454) (MeOH). CD: [θ]244 +27014 (c=0.003, MeOH). 1H-NMR (700 MHz, CDCl3) δH: 6.14 (1H, s), 2.96 (1H, m, H-1), 2.86 (1H, d, J=14.9 Hz, H-6), 2.81 (1H, d, J=11.2 Hz, H-5), 2.32 (1H, m, H-1), 2.17 (1H, dd, J=14.9, 11.2 Hz, H-6), 2.05 (1H, br dd, J=11.7, 2.4 Hz, H-3), 1.90 (1H, overlap, H-2), 1.89 (3H, s, H-14), 1.72 (3H, s, H-12), 1.70 (3H, s, H-13), 1.67 (1H, overlap, H-2), 1.22 (3H, s, H-15), 1.02 (1H, dt, J=2.4, 13.4 Hz, H-3). 13C-NMR (175 MHz, in CDCl3) δC: 202.8 (C-8), 152.7 (C-10), 133.3 (C-11), 130.6 (C-7), 130.5 (C-9), 64.2 (C-5), 60.1 (C-4), 36.8 (C-3), 28.9 (C-1), 28.3 (C-6), 23.5 (C-14), 21.9 (C-2), 21.5 (C-12), 19.8 (C-13), 15.9 (C-15).
9-Hydroxyeudesma-4,7(11)-dien-6-one (3)HR-ESI-MS m/z: 235.1707 [M+H]+ (Calcd 235.1698 for C15H23O2). UV λmax nm (ε): 281 (10434) (MeOH). CD: [θ]329 −723, [θ]274 −5324 (c=0.003, MeOH). 1H-NMR (700 MHz, CDCl3) δH: 3.72 (1H, dd, J=11.2, 5.6 Hz, H-9), 2.90 (1H, dd, J=14.9, 5.6 Hz, H-8), 2.37 (1H, dd, J=14.9, 11.2 Hz, H-8), 2.1 (2H, overlap, H-3), 2.06 (3H, s, H-13), 1.89 (3H, s, H-15), 1.82 (3H, s, H-12), 1.79 (1H, br d, J=13.4 Hz, H-1), 1.67 (1H, overlap, H-2), 1.58 (1H, overlap, H-2), 1.38 (1H, br t, J=12.7 Hz, H-1), 1.00 (3H, s, H-14). 13C-NMR (175 MHz, in CDCl3) δC: 196.9 (C-6), 143.9 (C-11), 143.4 (C-4), 137.6 (C-5), 130.1 (C-7), 75.8 (C-9), 40.6 (C-10), 34.6 (C-1), 34.1 (C-8), 33.9 (C-3), 23.3 (C-13), 22.7 (C-12), 22.1 (C-15), 18.1 (C-2), 17.9 (C-14).
(4R,5R,9S,10S)-9-Hydroxy-4-methoxyeudesm-7(11)-en-6-one (4)HR-ESI-MS m/z: 289.1768 [M+H]+ (Calcd 289.1780 for C16H26O3Na), m/z 267.1966 [M+H]+ (Calcd 267.1960 for C16H27O3). UV λmax nm (ε): 250 (5832) (MeOH). CD: [θ]325 +6198, [θ]249 −27535 (c=0.003, MeOH). 1H-NMR (700 MHz, CDCl3) δH: 3.73 (1H, dd, J=11.5, 5.4 Hz, H-9), 3.19 (3H, s, OMe), 2.93 (1H, dd, J=14.6, 5.6 Hz, H-8), 2.37 (1H, s, H-5), 2.23 (1H, br t, J=13.2 Hz, H-8), 1.87 (3H, s, H-13), 1.80 (2H, overlap, H-1, 3), 1.74 (3H, s, H-12), 1.68 (1H, overlap, H-2), 1.58 (3H, s, H-15), 1.48 (1H, br q, J=13.7 Hz, H-2), 1.29 (1H, br t, J=13.2 Hz, H-3), 1.17 (1H, br t, J=13.2 Hz, H-1), 0.94 (3H, s, H-14). 13C-NMR (175 MHz, in CDCl3) δC: 202.8 (C-6), 139.1 (C-11), 130.8 (C-7), 78.3 (C-9), 75.3 (C-4), 61.6 (C-5), 48.4 (OMe), 43.0 (C-10), 38.1 (C-3), 37.1 (C-1), 35.1 (C-8), 22.5 (C-13), 21.5 (C-12), 19.2 (C-2), 18.6 (C-15), 13.2 (C-14).
(7S,9S,10S)-9-Hydroxy-11-methoxyeudesm-4-en-6-one (5)HR-ESI-MS m/z: 289.1770 [M+H]+ (Calcd 289.1780 for C16H26O3Na), m/z 267.1970 [M+H]+ (Calcd 267.1960 for C16H27O3). UV λmax nm (ε): 249 (4075) (MeOH). CD: [θ]323 −4698, [θ]250 +23519 (c=0.003, MeOH). 1H-NMR (700 MHz, CDCl3) δH: 3.78 (1H,dd, J=11.2, 5.2 Hz, H-9), 3.16 (3H, s, OMe), 2.58 (1H, dd, J=13.4, 5.4 Hz, H-7), 2.29 (1H, dt, J=12.9, 7.0 Hz, H-8), 2.02 (2H, overlap, H-3), 1.79 (1H, overlap, H-1), 1.74 (1H, overlap, H-8), 1.66 (1H, overlap, H-2), 1.66 (3H, s, H-15), 1.54 (1H, m, H-2), 1.42 (1H, br t, J=12.7, H-1), 1.39 (3H, s, H-12), 1.25 (3H, s, H-13), 0.92 (3H, s, H-14). 13C-NMR (175 MHz, in CDCl3) δC: 205.4 (C-8), 138.7 (C-4), 137.9 (C-5), 77.0 (C-9), 75.0 (C-11), 55.7 (C-7), 48.3 (OMe), 42.4 (C-10), 34.9 (C-1), 32.3 (C-3), 30.4 (C-8), 23.7 (6H, C-12, 15), 20.9 (C-13), 18.2 (C-2), 17.9 (C-14).