2014 Volume 62 Issue 7 Pages 675-694
2014 Volume 62 Issue 7 Pages 675-694
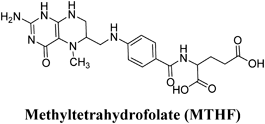
Cobalamin-dependant cytosolic enzyme methionine synthase (MetS) catalyses the transfer of a methyl group from the methyltetrahydrofolate (MTHF) to homocysteine (Hcy) to produce methionine and tetrahydrofolate (THF). MetS is over-expressed in the cytosol of certain breast and prostate tumour cells. Methionine used as a source of one carbon atom for the building of the DNA of the tumour cells, structural protein and enzymes. In this study, we designed, synthesized and evaluated the cytotoxic activity of a series of substituted methyl 2-(2-(4-oxo-3-aryl-3,4-dihydroquinazolin-2-ylthio)acetamido)acetate and dipeptide that mimic the substructure of MTHF. These inhibitors were docked in to the MTHF binding domain in such the same way as MTHF in its binding domain. The free energies of the binding were calculated and compared to the IC50 values. This series has been developed by dicyclohexylcarbodiimide (DCC) and azide coupling methods of amino acid esters with carboxylic acid derivatives, respectively. Compound methyl 3-hydroxy-2-(2-(3-(4-methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-ylthio)acetamido)propanoate exhibited the highest IC50 value 20 µg/mL against PC-3 cell line and scored the lowest free energy of the binding (−207.19 kJ/mol).
Methionine synthase (MetS) is one of the folic acid cycle enzymes that catalyses the transfer of a methyl group from 5-methyltetrahydrofolate to homocysteine to produce methionine and tetrahydrofolate. The catalysis occurred via the coenzyme cobalamin cofactor (CH3-Cb).1) This enzyme has been linked to the pathogenesis of anemias, neurodegenerative disorders and cancer.2) Using mammalian methionine synthase, X-ray studies show that it is constructed of 1227 amino acid residues and has a molecular mass of 136 kDa.3) Cobalamin-dependent methionine synthase is made up of four modules 5-methyltetrahydrofolate and homocysteine binding regions from the C-terminal of the protein and cobalamin and the S-adenosylmethionine binding regions from N-terminal.3) Methionine is an essential amino acid necessary for normal growth. Methionine is used as a methyl group donor, providing one carbon units for DNA synthesis.4) Methionine dependence is defined as the inability of cancer cells to grow in a methionine depleted medium even when replaced with the immediate precursor, homocysteine.5) Normal cells remain unaffected by methionine restrictions if supplemented with homocysteine, as they are able to synthesize methionine via methylation of homocysteine in sufficient quantities. Most metastatic tumours, such as those originating in prostate, lung and gastrointestinal tract, respond poorly to conventional chemotherapy. Novel treatment strategies for advanced cancer are therefore needed. Dietary restriction of the essential amino acid methionine offers promise as such a strategy, either alone or in combination with chemotherapy or other treatments.6) Although there are many approaches for developing inhibitors for folic acid enzymes system collectively named antifolates. There are a limited number of reports concerning the inhibition of MetS. A series of inhibitors of substituted 2,4-diaminopyrimidine derivatives that could bind the substrate-binding pocket of dihydrofolate reductase was synthesized.7) 6-Substituted pyrrolo- and thieno[2,3-d]pyrimidine derivatives were identified as antifolates with selective membrane transport by proton-coupled folate transporter (PCFT) and folate receptors (FRs) over reduced folate carrier (RFC).8) A series of pyrido[2,3-d]pyrimidines was successfully synthesized and used by Gangjee et al. as selective and potent DHFR inhibitors.9) In a strategy for discovering specific inhibitors of MetS, some benzo-fused heterocycles have been designed and synthesized that mimic the substructures of 5-methyltetrahydrofolate of MetS.10) Taking in consideration the structural requirements for the design of novel inhibitors that have high affinity to methyltetrahydrofolate (MTHF) receptor, a series of dihydroquinazolinone derivatives have been synthesized. The structure requirement for the pharmacophoric portion was maintained with the presence of quinazoline nucleus which mimics the pteridine ring of MTHF with varying the side chain at positions 2 and 3 of quinazoline nucleus. Meanwhile the phenyl ring moiety was dislocated from its familiar position at the side chain of the tested inhibitors in previous work11,12) and in MTHF and relocated at N-3 of quinazoline nucleus to attempt the compensation of London interaction contacts, including complimentary π–π stacking with the phenyl ring of phenylalanine 511.
The chemoselective reactions of heterocyclic thioamides always attracted our research group. We earlier reported the chemoselective S- and N-alkylation of the model compound 4-methyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one with different electrophiles. These results were supported by theoretical density functional theory (DFT) computational calculations.13–15) We also invested these findings in the syntheses of interesting heterocycles via domino,15–17) rearrangement reactions18) and the preparation of heterocyclic coupled amino acid esters.19,20) These results motivated the development of a series of S-substituted amino acid esters of biologically promising quinazoline ring system.
Molecular modeling was carried out on Schrodinger computational software workstation using Maestro 9.3 graphic user interface (GUI) and Red Hat Linux nash Enterprise version V 4.1.18 on Batchmin V 9.3 modeling engine. The atomic coordinates for the polypeptide segments of MTHF and Hcy binding domains of MetS enzyme extracted from human liver were obtained from the protein bank database PDB (Brookhaven protein database (PDB: 1Q8J)). Explicit calcium ion counter ions were included instead of cadmium and positioned at a 4 Å distance from the homocysteine binding site. The basic and acidic amino acids were neutralized at pH 7±2 by protonation of the terminal amino groups and basic amino acids such as histidine, lysine and arginine. Moreover, the terminal carboxylic acid groups of amino acids and acidic amino acids such as glutamic acid, aspartic acid, asparagine and glutamine were deprotonated. Prime 3.1 was used to check the energy content of the protein segments and loops. The docking process involved the standard precision docking (SP) in which ligand poses that were expected to have unfavourable energies would be rejected from the conditioned set up of the hierarchal filter. The presumption is that only active compounds will have available poses that avoid these penalties. SP docking is appropriate for screening ligands of unknown quality in large numbers. SP is a soft docking programme that was adept at identifying ligands that have a reasonable propensity to bind and 20% of the final poses produced from the SP docking were subjected to the Extra Precision mode of Glide docking (XP) to perform the more expensive docking simulation on worthwhile poses. XP docking mode is harder than SP docking mode in that it penalizes the poses that violate established charges. Flexible docking was selected to generate conformations of all possible ligand poses, which is more realistic as this occurs in reality because the protein undergoes side chain and back bone movement or both, upon ligand binding. Five and six-membered rings were allowed to flip and amide bonds which were not cis or trans configuration were penalised. Five thousand poses per ligand for the initial phase of docking with a scoring window for keeping poses of 100 kJ/mol were set up. The best poses which fulfil those conditions were subjected to energy minimization on the OPLS-AA nonbonded-interaction grid with a distance dielectric constant of 2 and maximum number of conjugate gradient steps of 10000 iterations. The ligands of the poses selected by the initial screening were subsequently minimized in the field of the receptor using a standard molecular mechanics energy function (OPLS-AA force field) in conjunction with a distance-dependant dielectric model. Finally, the lowest energy poses obtained in this fashion were subjected to a Monte Carlo procedure that examines nearby torsion minima. The complex was minimized using the conjugate gradients algorithm until an energy convergence criterion of 0.1 kJ/mol was reached with iteration cycle of 10000. Molecular dynamics (MD) at 300 K were then performed on the solvated system for a 50 ps equilibration and 300 ps of production employing a 1 fs time step using OPLS 2005 force field, from which 100 structures were sampled at 1 ps intervals and averaged. The final averaged structure was then finally minimized.21)
Experimental Procedure. General ProceduresSolvents were purified and dried in the usual way. The boiling range of the petroleum ether used was 40–60°C. Thin layer chromatography (TLC): silica gel 60 F254 plastic plates (E. Merck, layer thickness 0.2 mm) detected by UV absorption. Melting points were determined on a Buchi 510 melting-point apparatus and the values are uncorrected. NMR spectra measured with Bruker AC 250 (250 MHz). Tetramethylsilane (TMS) (0.00 ppm) as internal standard. Mass spectra were measured on a GC-MSQP 1000EX Shimadzu. Elemental analyses were performed on a Flash EA-1112 instrument at the Microanalytical laboratory, Faculty of Science, Suez Canal University, Ismailia, Egypt. Quinazoline derivatives 1, 5, 6 were prepared according to the method described in literature.17,22,23)
General Preparation of 2-(3-Aryl-4-oxo-3,4-dihydroquinazolin-2-ylthio)acetohydrazide (6)To a solution of ester 5a (0.33 g, 1.0 mmol) in methyl alcohol (30 mL), hydrazine hydrate (2.4 mL, 5 mmol) was added. The reaction mixture was refluxed for 4 h, cooled and the resultant precipitate was filtered off, washed with ethanol and ether then crystallized from aqueous ethanol to yield the hydrazide 6. White crystals (0.29 g, 89%); mp 195–196°C. 1H-NMR (300 MHz, dimethylsulfoxide (DMSO)) δ: 9.14 (1H, s, NH), 8.72 (2H, d, J=8.0 Hz, ArH), 8.55 (1H, d, J=8.0 Hz, ArH), 8.15–8.11 (1H, m, ArH), 7.79–7.66 (2H, m, ArH), 7.68–7.61 (3H, m, ArH), 4.21 (2H, s, SCH2CO), 1.92 (2H, m, NH2). Anal. Calcd for C16H14N4O2S (326.4): C, 58.88; H, 4.32; N, 17.17 Found: C, 58.73; H, 4.28; N, 17.03.
General Preparation of 3-Phenylaryl Quinazoline-2,4(1H,3H)-dione (3)To a cold solution (−5°C) of hydrazide 6 (0.33g, 1.0 mmol) in AcOH (6 mL), 1 N HCl (3 mL), and water (25 mL) was added a solution of NaNO2 (0.34 g, 5.0 mmol) in cold water (3 mL). After stirring at −5°C for 1 h. a thick precipitate was formed. The reaction mixture was extracted in cold ethyl acetate (30 mL), washed with cold 3% NaHCO3, H2O and finally dried (Na2SO4). A solution of amino acid esters hydrochloride (1.0 mmol) in ethyl acetate (20 mL) containing 0.2 mL of triethyl amine was added to the azide solution. The mixture was kept at −5°C for 24 h, then at 25°C for another 24 h, followed by washing with 0.5 N HCl, water, 3% solution of NaHCO3 and finally dried (Na2SO4). The solution was evaporated to dryness, and the residue was recrystallized from petroleum ether–ethyl acetate to give the corresponding quinazoline dione 3a. White crystals (0.31 g, 88%); mp 235–236°C. 1H-NMR (300 MHz, DMSO) δ: 11.31 (1H, s, NH), 7.93 (1 H, d, J=7.8 Hz, ArH), 7.65–7.51 (1H, d, J=8.0 Hz, ArH), 7.45–7.41 (3H, m, ArH), 7.27–7.15 (4H, m, ArH). Anal. Calcd for C14H10N2O2 (238.2): C, 70.58; H, 4.23; N, 11.76. Found: C, 70.45; H, 4.11; N, 11.53.
General Preparation of 2-(3-Aryl-4-oxo-3,4-dihydroquinazolin-2-ylthio)acetic Acid (2)To a solution ester 5a–c (1.0 mmol) in methyl alcohol (30 mL), was added sodium hydroxide (0.112 g, 2.0 mmol) solution in 10 mL H2O. The reaction mixture was stirred for 3 h till complete consumption of the ester (monitored by TLC). The reaction mixture was evaporated under reduced pressure, diluted with water and acidified by 1 N HCl to pH 3. The separated whitish ppt was filtered off and washed several times with water, dried and crystallized by DMF–H2O.
2-(4-Oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetic Acid (2a): White crystals (0.31 g, 88%); mp 245–246°C. 1H-NMR (300 MHz, DMSO) δ: 8.01 (1H, d, J=8.1 Hz, ArH), 7.78 (1H, t, J=8.3 Hz, ArH), 7.61–7.55 (4H, m, ArH), 7.48–7.42 (3H, m, ArH), 4.18 (2H, s, SCH2CO). Anal. Calcd for C16H12N2O3S (312.3): C, 61.53; H, 3.87; N, 8.97; S, 10.27. Found: C, 61.48; H, 3.65; N, 8.81.
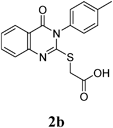
2-(4-Oxo-3-p-tolyl-3,4-dihydroquinazolin-2-ylthio)acetic Acid (2b): White crystals (0.31 g, 88%); mp 235–236°C. 1H-NMR (300 MHz, DMSO) δ: 8.03 (1H, d, J=8.1 Hz, ArH), 7.91 (1H, t, J=8.1 Hz, ArH), 7.49 (2H, d, J=8.0 Hz, ArH), 7.47–7.30 (4H, m, ArH), 4.16 (2H, s, SCH2CO), 2.41 (3H, s, CH3). Anal. Calcd for C17H14N2O3S (326.4): C, 62.56; H, 4.32; N, 8.58; S, 9.82. Found: C, 62.43; H, 4.15; N, 8.38.
2-(3-(4-Methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-ylthio)acetic Acid (2c): White crystals (0.31 g, 88%); mp 235–236°C. 1H-NMR (300 MHz, DMSO) δ: 8.03 (1H, d, J=8.0 Hz, ArH), 7.78 (1H, t, J=7.8 Hz, ArH), 7.59 (1H, d, J=7.8 Hz, ArH), 7.45 (1H, t, J=7.9 Hz, ArH), 7.33 (2H, d, J=8.7 Hz, ArH), 7.19 (2H, d, J=8.7 Hz, ArH), 4.21 (2H, s, SCH2CO), 3.85 (2H, s, OCH3). Anal. Calcd for C17H14N2O4S (342.4): C, 59.64; H, 4.12; N, 8.18; S, 9.37. Found: C, 59.58; H, 4.01; N, 8.13.
General Preparation of Methyl 2-(2-(4-Oxo-3-aryl-3,4-dihydroquinazolin-2-ylthio)acetamido)alkanoates (8–13)To a cold solution (−5°C) of the amino acid methyl ester hydrochloride (1.0 mmol) in acetonitrile (6 mL) containing triethyl amine (0.14 mL, 1.0 mmol), 2-(3-aryl-4-oxo-3,4-dihydroquinazolin-2-ylthio)acetic acid 2a–c (1.0 mmol), dicyclohexyl-carbodiimide (DCC) (0.207 g, 1.0 mmol) and hydroxybenzotriazole (HOBT) (0.135 g, 1.0 mmol) were added successively. The reaction mixture was stirred at 0°C for one hour, at 5°C for one hour, then at room temperature for 8 h. The reaction mixture was set aside overnight. The precipitated dicyclohexylurea was filtered off and the filtrate was evaporated under reduced pressure. The residue was dissolved in ethyl acetate, filtered and the filtrate was washed with 5% NaHCO3, 1 N HCl and saturated solution of sodium chloride then dried over anhydrous sodium sulphate. After evaporation of the solvent, the remaining oily residue was triturated with petroleum ether (bp 40–60°C) at 0°C with scratching, afterwards the formed solid was filtered off and crystallized from petroleum ether–ethyl acetate.
Methyl 2-(2-(4-Oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetamido)acetate (8a): From GlyOCH3·HCl (0.126 g). White crystals (0.29 g, 77%); mp 234–235°C. 1H-NMR (300 MHz, DMSO) δ: 8.61 (1H, t, J=5.7 Hz, NH), 8.04 (1H, d, J=7.8 Hz, ArH), 7.82 (1H, t, J=8.1 Hz, ArH), 7.62–7.55 (4H, m, ArH), 7.48–7.43 (3H, m, ArH), 3.92 (2H, s, SCH2), 3.84 (2H, d, J=5.7 Hz, NCH2), 3.57 (3H, s, OCH3). 13C-NMR (CDCl3) δ: 170.52 (C=O), 167.79 (C=O), 161.16 (Cq), 157.17 (Cq), 135.77 (Cq), 135.36 (CHAr), 130.46 (CHAr), 130.01 (CHAr), 129.91 (CHAr), 127.00 (CHAr), 126.62 (CHAr), 120.00 (Cq), 52.21 (OCH3), 41.44 (CH2), 36.23 (SCH2). Anal. Calcd for C19H17N3O4S (383.4): C, 59.52%; H, 4.47%; N, 10.96%; S, 8.36%. Found: C, 59.44%; H, 4.39%; N, 10.86%. Mass spectrum, m/z (Ir/%): 383.1.
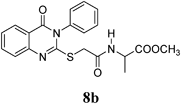
Methyl 2-(2-(4-Oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetamido)propanoate (8b): From L-AlaOCH3·HCl (0.14 g). White crystals (0.21 g, 54%); mp 120–121°C. 1H-NMR (300 MHz, CDCl3) δ: 8.68 (1H, d, J=7.2 Hz, NH), 8.09 (1H, d, J=8.1 Hz, ArH), 7.85 (1H, t, J=8.1 Hz, ArH), 7.62–7.48 (4H, m, ArH), 7.46–7.44 (3H, m, ArH), 4.31–4.27 (1H, m, CH), 3.92 (2H, s, SCH2), 3.59 (3H, s, OCH3), 1.33 (3H, d, J=7.2 Hz, CH3). Anal. Calcd for C20H19N3O4S (397.4): C, 60.44%; H, 4.82%; N, 10.57%; S, 8.07%. Found: C, 60.38%; H, 4.73%; N, 10.45%.
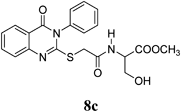
Methyl 3-Hydroxy-2-(2-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetamido)propanoate (8c): From L-SerOCH3·HCl (0.156 g). White crystals (0.28 g, 68%); mp 200–201°C. 1H-NMR (300 MHz, DMSO) δ: 8.59 (1H, d, J=7.8 Hz, NH), 8.05 (1H, d, J=8.1 Hz, ArH), 7.82 (1H, t, J=8.3 Hz, ArH), 7.61–7.55 (4H, m, ArH), 7.48–7.42 (3H, m, ArH), 5.07 (1H, t, J=5.7 Hz, OH, D2O exchangeable), 4.37–4.31 (1H, m, CH), 3.93 (2H, s, SCH2), 3.69–3.55 (5H, m, OCH2, OCH3). 13C-NMR (CDCl3) δ: 170.81 (C=O), 166.95 (C=O), 160.63 (Cq), 156.85 (Cq), 147.14 (Cq), 135.77 (Cq), 134.85 (CHAr), 129.95 (CHAr), 129.38 (CHAr), 129.55 (CHAr), 126.50 (CHAr), 125.99 (CHAr), 119.49 (Cq), 61.25 (OCH2), 53.83 (OCH3), 51.47 (CH), 36.48 (SCH2), Anal. Calcd for C20H19N3O5S (413.4): C, 58.10%; H, 4.63%; N, 10.16%; S, 7.76%. Found: C, C, 57.94% ; H, 4.56%; N, 9.96%;. Mass spectrum, m/z (Ir/%): 413.2.
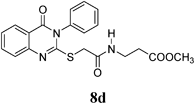
Methyl 3-(2-(4-Oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetamido)propanoate (8d): From β-AlaOCH3·HCl (0.14 g). White crystals (0.29 g, 73%); mp 110–111°C. 1H-NMR (300 MHz, CDCl3) δ: 8.25 (1H, t, J=7.2 Hz, NH), 8.05 (1H, d, J=8.1 Hz, ArH), 7.79 (1H, t, J=8.1 Hz, ArH), 7.67–7.47 (4H, m, ArH), 7.46–7.34 (3H, m, ArH), 3.93 (2H, s, SCH2), 3.52 (3H, m, OCH2, OCH3), 3.30 (2H, q, J=6.4 Hz, NCH2), 2.46 (2H, t, J=6.6 Hz, CH2), Anal. Calcd for C20H19N3O4S (397.4): C, 60.44%; H %, 4.82%; N, 10.57%; S, 8.07%. Found: C, 60.36%; H %, 4.70%; N, 10.49%.
Methyl 4-Methyl-2-(2-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetamido)pentanoate (8e): From L-LeuOCH3·HCl 0.182 g) and 8. White crystals (0.23 g, 54%); mp 215–216°C. 1H-NMR (300 MHz, CDCl3) δ: 8.57 (1H, d, J=7.8 Hz, NH), 8.04 (1H, d, J=7.8 Hz, ArH), 7.91 (1H, t, J=8.0 Hz, ArH), 7.83–7.66 (4H, m, ArH), 7.53–7.30 (3H, m, ArH), 4.30–4.25 (1H, m, CH), 3.93 (1H, d, J=15.0 Hz, SCH2), 3.87 (1H, d, J=15.0 Hz, SCH2), 3.55 (3H, s, OCH3), 1.68–1.45 (2H, m, CH2), 1.22–1.13 (1H, m, CH), 0.97 (6H, d, J=6.0 Hz, 2CH3), 13C-NMR (CDCl3) δ: 173.17 (C=O), 167.29 (C=O), 161.08 (Cq), 157.29 (Cq), 147.61 (Cq), 136.23 (Cq), 135.26 (CHAr), 130.41 (CHAr), 129.89 (CHAr), 129.52 (CHAr), 127.01 (CHAr), 126.46 (CHAr), 122.93 (CHAr), 119.96 (Cq), 52.31 (CH), 51.02 (OCH3), 41.78 (CH2), 36.37 (SCH2), 24.63 (CH), 23.15 (CH3), 21.69 (CH3). Anal. Calcd for C23H25N3O4S (439.5): C, 62.85%; H, 5.73%; N, 9.56%; S, 6.92. Found: C, 62.81%; H, 5.67%; N, 9.54%.
Methyl 3-Hydroxy-2-(2-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetamido)butanoate (8f): From L-ThrOCH3·HCl (0.156 g). White crystals (0.31 g, 74%); mp 155–156°C. 1H-NMR (300 MHz, CDCl3) δ: 8.37 (1H, d, J=8.4 Hz, NH), 8.09 (1H, d, J=7.8 Hz, ArH), 7.83 (1H, t, J=8.0 Hz, ArH), 7.65–7.58 (4H, m, ArH), 7.51–7.44 (3H, m, ArH), 5.07 (1H, d, J=5.1 Hz, OH, D2O exchangeable), 4.31–4.27 (1H, m, CH), 4.07–4.02 (3H, m, SCH2, CH), 3.59 (3H, s, OCH3), 1.04 (3H, d, J=6.3 Hz, CH3), Anal. Calcd for C21H21N3O5S, (427.5): C, 59.00%; H, 4.95%; N, 9.83%; S, 7.50%. Found: C, 58.87%; H, 4.91%; N, 9.69%. Mass spectrum, m/z (Ir/%): 428.1.
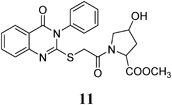
Methyl 4-Hydroxy-1-(2-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetyl)pyrrolidine-2-carboxylate (11): From L-HOPrlOCH3·HCl (0.156 g). White crystals (0.16 g, 38%); mp 103–104°C. 1H-NMR (300 MHz, DMSO) δ: 8.05 (1H, d, J=7.8 Hz, NH), 7.81 (1H, t, J=7.8 Hz, ArH), 7.65 (1H, d, J=8.0 Hz, ArH), 7.62–7.56 (4H, m, ArH), 7.47–7.42 (3H, m, ArH), 5.30 (1H, d, J=6.0 Hz, CH), 4.42 (1H, br s, OH, D2O exchangeable), 4.31 (1H, t, J=7.8 Hz, CH), 4.11 (1H, d, J=15.6 Hz, SCH2), 3.98 (1H, d, J=15.6 Hz, SCH2), 3.75–3.66 (2H, m, CH2), 3.52 (3H, s, OCH3), 2.19–1.96 (2H, m, CH2). 13C-NMR (CDCl3) δ: 172.58 (C=O), 166.25 (C=O), 161.13 (Cq), 157.21 (Cq), 147.67 (Cq), 136.27 (Cq), 135.32 (CHAr), 130.48 (CHAr), 130.04 (CHAr), 129.87 (CHAr), 126.97 (CHAr), 126.65 (CHAr), 126.49 (CHAr), 119.93 (Cq), 69.42 (CH), 58.33 (OCH3), 55.66 (SCH2), 52.25 (CH), 37.84 (CH2), 36.54 (CH2). Anal. Calcd for C22H21N3O5S (439.5): C, 60.12%; H, 4.82%; N, 9.56%; S, 7.30%. Found: C, 60.03%; H, 4.78%; N, 9.45%.
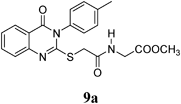
Methyl 2-(2-(4-oxo-3-p-tolyl-3,4-dihydroquinazolin-2-ylthio)acetamido)acetate (9a): From GlyOCH3·HCl (0.126 g). White crystals (0.23 g, 58%); mp 165–166°C. 1H-NMR (300 MHz, DMSO) δ: 8.60 (1H, t, J=7.2 Hz, NH), 8.04 (1H, d, J=8.1 Hz, ArH), 7.84 (1H, t, J=8.1 Hz, ArH), 7.63 (2H, d, J=8.0 Hz, ArH), 7.50 (1H, t, J=8.1 Hz, ArH), 7.47–7.30 (3H, m, ArH), 3.93 (2H, s, SCH2), 3.86 (2H, d, J=5.7 Hz, NCH2), 3.60 (3H, s, OCH3), 2.42 (3H, s, CH3). Anal. Calcd for C20H19N3O4S (397.4): C, 60.44%; H, 4.82%; N, 10.57%; S, 8.07%. Found: C, 60.15%; H, 4.63%; N, 10.43%. Mass spectrum, m/z (Ir/%): 397.2.
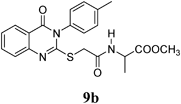
Methyl 2-(2-(4-Oxo-3-p-tolyl-3,4-dihydroquinazolin-2-ylthio)acetamido)propanoate (9b): From L-AlaOCH3·HCl (0.14 g). White crystals (0.19 g, 48%); mp 165–166°C. 1H-NMR (300 MHz, CDCl3) δ: 8.68 (1H, d, J=7.2 Hz, NH), 8.06 (1H, d, J=8.1 Hz, ArH), 7.84 (1H, t, J=8.1 Hz, ArH), 7.60 (1H, d, J=8.1 Hz, ArH), 7.47 (1H, t, J=8.1 Hz, ArH), 7.37 (2H, d, J=8.7 Hz, ArH), 7.31 (2H, d, J=8.7 Hz, ArH), 4.37–4.25 (1H, m, CH), 3.90 (1H, s, SCH2), 3.59 (3H, s, OCH3), 2.41 (3H, s, CH3), 1.28 (3H, d, J=7.2 Hz, CH3). Anal. Calcd for C21H21N3O4S (411.5): C, 61.30%; H, 5.14%; N, 10.26%; S, 7.79%. Found: C, 61.21%; H, 5.08%; N, 10.18%.
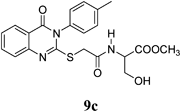
Methyl 3-Hydroxy-2-(2-(4-oxo-3-p-tolyl-3,4-dihydroquinazolin-2-ylthio)acetamido)propanoate (9c): From L-SerOCH3·HCl (0.156 g). White crystals (0.27 g, 65%); mp 152–153°C. 1H-NMR (300 MHz, CDCl3) δ: 8.59 (1H, d, J=7.2 Hz, NH), 8.09 (1H, d, J=7.8 Hz, ArH), 7.86 (1H, t, J=8.1 Hz, ArH), 7.61 (1H, d, J=7.8 Hz, ArH), 7.47 (1H, t, J=8.1 Hz, ArH), 7.38 (2H, d, J=8.1 Hz, ArH), 7.31 (2H, d, J=8.1 Hz, ArH), 5.08 (1H, br s, OH, D2O exchangeable), 4.37–4.33 (1H, m, CH), 3.94 (1H, s, SCH2), 3.70–3.57 (5H, m, CH2, OCH3), 2.41 (3H, s, CH3). Anal. Calcd for C21H21N3O5S (427.5): C, 59.00% ; H, 4.95%; N, 9.83%; S, 7.50%. Found: C, 58.73%; H, 4.85%; N, 9.69%. Mass spectrum, m/z (Ir/%): 428.1.
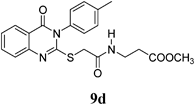
Methyl 3-(2-(4-Oxo-3-p-tolyl-3,4-dihydroquinazolin-2-ylthio)acetamido)propanoate (9d): From β-AlaOCH3·HCl (0.14 g). White crystals (0.29 g, 71%); mp 160–161°C. 1H-NMR (300 MHz, DMSO) δ: 8.23 (1H, t, J=7.2 Hz, NH), 8.07 (1H, d, J=7.8 Hz, ArH), 7.83 (1H, t, J=7.8 Hz, ArH), 7.55 (2H, d, J=8.0 Hz, ArH), 7.49 (1H, t, J=7.8 Hz, ArH), 7.46 (1H, d, J=8.0 Hz, ArH), 7.30 (2H, d, J=8.0 Hz, ArH), 3.81 (2H, s, SCH2), 3.55 (3H, s, OCH3), 3.42–3.32 (2H, m, NCH2), 2.52 (2H, t, J=7.1 Hz, CH2), 2.42 (3H, s, CH3). Anal. Calcd for C21H21N3O4S (411.5): C, 61.30%; H %, 5.14%; N, 10.21%; S, 7.79%. Found: C, 61.28%; H %, 5.06%; N, 10.17%.
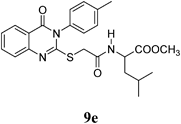
Methyl 4-Methyl-2-(2-(4-oxo-3-p-tolyl-3,4-dihydroquinazolin-2-ylthio)acetamido)pentanoate (9e): From L-LeuOCH3·HCl 0.182 g). White crystals (0.25 g, 56%); mp 159–160°C. 1H-NMR (300 MHz, CDCl3) δ: 8.65 (1H, d, J=7.2 Hz, NH), 8.03 (1H, d, J=8.1 Hz, ArH), 7.86 (1H, t, J=7.8 Hz, ArH), 7.60 (1H, d, J=7.8 Hz, ArH), 7.49 (1H, t, J=8.1 Hz, ArH), 7.44 (2H, d, J=8.1 Hz, ArH), 7.32 (2H, d, J=8.1 Hz, ArH), 4.36–4.21 (1H, m, CH), 3.92 (1H, d, J=14.4 Hz, SCH2), 3.59 (3H, s, OCH3), 3.83 (1H, d, J=14.4 Hz, SCH2), 2.41 (3H, s, CH3), 1.63–1.45 (2H, m, CH2), 0.97–0.95 (1H, m, CH), 0.84 (6H, d, J=7.2 Hz, 2CH3). Anal. Calcd for C24H27N3O4S (453.6): C, 63.56%; H, 6.00%; N, 9.26%; S, 7.07. Found: C, 63.49%; H, 5.93%; N, 9.14%.
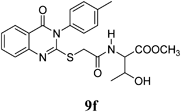
Methyl 3-Hydroxy-2-(2-(4-oxo-3-p-tolyl-3,4-dihydroquinazolin-2-ylthio)acetamido)butanoate (9f): From L-ThrOCH3·HCl (0.156 g). White crystals (0.17 g, 41%); mp 174–175°C. 1H-NMR (300 MHz, CDCl3) δ: 8.74 (2H, d, J=8.0 Hz, ArH), 8.58 (1H, d, J=8.0 Hz, ArH), 8.18 (1H, d, J=8.0 Hz, ArH), 7.94 (1H, d, J=8.0 Hz, NH), 7.69–7.59 (2H, m, ArH), 7.58–7.55 (3H, m, ArH), 5.12 (1H, br s, OH, D2O exchangeable), 4.65 (1H, d, J=6.0 Hz, CH), 4.34–4.13 (3H, m, SCH2, CH), 3.71 (3H, s, OCH3), 1.42 (3H, d, J=7.2 Hz, CH3), Anal. Calcd for C22H23N3O5S, (441.5): C, 59.85%; H, 5.25%; N, 9.52%; S, 7.26%. Found: C, 59.77%; H, 5.18%; N, 9.46%. Mass spectrum, m/z (Ir/%): 442.1.
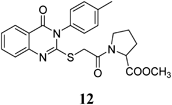
Methyl 4-Hydroxy-1-(2-(4-oxo-3-p-tolyl-3,4-dihydroquinazolin-2-ylthio)acetyl)pyrrolidine-2-carboxylate (12): From L-HOPrlOCH3·HCl (0.156 g). White crystals (0.30 g, 67%); mp 201–202°C. 1H-NMR (300 MHz, CDCl3) δ: 8.08 (1H, d, J=7.8 Hz, ArH), 7.83 (1H, t, J=7.8 Hz, ArH), 7.67 (1H, d, J=7.8 Hz, ArH), 7.49 (1H, t, J=7.8 Hz, ArH), 7.47 (2H, d, J=8.1 Hz, ArH), 7.31 (2H, d, J=8.1 Hz, ArH), 5.31 (1H, d, J=5.3 Hz, CH), 4.43 (1H, br s, OH, D2O exchangeable), 4.30 (1H, d, J=6.0 Hz, CH), 3.81–3.72 (2H, m, SCH2, CH2), 3.58 (3H, s, OCH3), 2.41 (3H, s, CH3), 2.06–1.69 (2H, m, CH2). Anal. Calcd for C23H23N3O5S (453.5): C, 60.91%; H, 5.11%; N, 9.27%; S, 7.07%. Found: C, 60.76%; H, 5.04%; N, 9.16%.
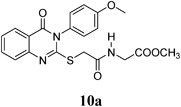
Methyl 2-(2-(3-(4-Methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-ylthio)acetamido)acetate (10a): From GlyOCH3·HCl (0.126 g). White crystals (0.23 g, 57%); mp 188–189°C. 1H-NMR (300 MHz, DMSO) δ: 8.60 (1H, t, J=7.2 Hz, NH), 8.08 (1H, d, J=8.1 Hz, ArH), 7.82 (1H, t, J=8.1 Hz, ArH), 7.61 (1H, d, J=8.1 Hz, ArH), 7.50 (1H, t, J=8.1 Hz, ArH), 7.36 (2H, d, J=9.0 Hz, ArH), 7.11 (2H, d, J=9.0 Hz, ArH), 3.93 (2H, s, SCH2), 3.87–3.84 (5H, m, NCH2, OCH3), 3.60 (3H, s, OCH3). Anal. Calcd for C20H19N3O5S (413.4): C, 58.10%; H, 4.63%; N, 10.16%; S, 7.76%. Found: C, 57.85%; H, 4.51%; N, 10.01%. Mass spectrum, m/z (Ir/%): 413.2.
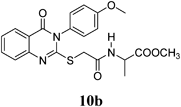
Methyl 2-(2-(3-(4-Methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-ylthio)acetamido)propanoate (10b): From L-AlaOCH3·HCl (0.14 g). White crystals (0.14 g, 34%); mp 130–131°C. 1H-NMR (300 MHz, CDCl3) δ: 8.65 (1H, d, J=6.9 Hz, NH), 8.07 (1H, d, J=8.1 Hz, ArH), 7.84 (1H, t, J=8.1 Hz, ArH), 7.59 (1H, d, J=8.1 Hz, ArH), 7.48 (1H, t, J=8.1 Hz, ArH), 7.36 (2H, d, J=8.7 Hz, ArH), 7.11 (2H, d, J=8.7 Hz, ArH), 4.31–4.26 (1H, m, CH), 3.91 (2H, s, SCH2), 3.85 (2H, s, OCH3), 3.59 (3H, s, OCH3), 1.28 (3H, d, J=7.2 Hz, CH3). Anal. Calcd for C21H21N3O5S (427.5): C, 59.00%; H, 4.95%; N, 9.83%; S, 7.50%. Found: C, 58.91%; H, 4.73%; N, 9.65%.
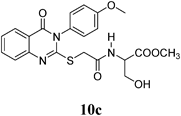
Methyl 3-Hydroxy-2-(2-(3-(4-methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-ylthio)acetamido)propanoate (10c): From L-SerOCH3·HCl (0.156 g). White crystals (0.29 g, 66%); mp 192–193°C. 1H-NMR (300 MHz, DMSO) δ: 8.59 (1H, d, J=6.9 Hz, NH), 8.07 (1H, d, J=8.0 Hz, ArH), 7.83 (1H, t, J=8.1 Hz, ArH), 7.62 (1H, d, J=8.0 Hz, ArH), 7.46 (1H, t, J=8.0 Hz, ArH), 7.37 (2H, d, J=8.9 Hz, ArH), 7.12 (2H, d, J=8.9 Hz, ArH), 5.06 (1H, t, J=5.7 Hz, OH, D2O exchangeable), 4.34–4.26 (1H, m, CH), 3.94 (2H, s, SCH2), 3.85 (2H, s, OCH3), 3.68–3.60 (5H, m, OCH2, OCH3). Anal. Calcd for C21H21N3O6S (443.5): C, 56.87% ; H, 4.77%; N, 9.48%; S, 7.23%. Found: C, 56.85%; H, 4.76%; N, 9.45%. Mass spectrum, m/z (Ir/%): 443.2.
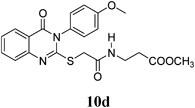
Methyl 3-(2-(3-(4-Methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-ylthio)acetamido)propanoate (10d): From β-AlaOCH3·HCl (0.14 g). White crystals (0.32 g, 76%); mp 212–213°C. 1H-NMR (300 MHz, CDCl3) δ: 8.78 (1H, dt, J=7.2 Hz, NH), 8.02 (1H, d, J=8.0 Hz, ArH), 7.84 (1H, t, J=8.1 Hz, ArH), 7.58 (1H, d, J=8.0 Hz, ArH), 7.51 (1H, t, J=8.0 Hz, ArH), 7.38 (2H, d, J=8.9 Hz, ArH), 7.14 (2H, d, J=8.9 Hz, ArH), 4.22 (2H, s, SCH2), 3.59 (3H, s, OCH3), 3.54 (2H, q, J=6.6 Hz, NCH2), 2.54 (2H, t, J=6.4 Hz, CH2), 13C-NMR (CDCl3) δ: 172.19 (C=O), 167.29 (C=O), 161.14 (Cq), 157.35 (Cq), 147.56 (Cq), 136.26 (Cq), 135.37 (CHAr), 130.44 (CHAr), 129.89 (CHAr), 129.55 (CHAr), 127.04 (CHAr), 126.48 (CHAr), 119.99 (Cq), 51.82 (OCH3), 36.54 (SCH2), 35.34 (CH2), 33.91 (CH2), Anal. Calcd for C21H21N3O5S (427.5): C, 59.00%; H, 4.95%; N, 9.83%; S, 7.50%. Found: C, 58.81%; H %, 4.83%; N, 9.76%.
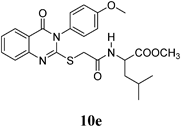
Methyl 2-(2-(3-(4-Methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-ylthio)acetamido)-4-methylpentanoate (10e): From L-LeuOCH3·HCl 0.182 g. White crystals (0.23 g, 51%); mp 184–185°C. 1H-NMR (300 MHz, CDCl3) δ: 8.65 (1H, d, J=6.9 Hz, NH), 8.05 (1H, d, J=8.1 Hz, ArH), 7.82 (1H, t, J=8.1 Hz, ArH), 7.51 (1H, d, J=8.1 Hz, ArH), 7.45 (1H, t, J=8.1 Hz, ArH), 7.34 (2H, d, J=9.0 Hz, ArH), 7.12 (2H, d, J=9.0 Hz, ArH), 4.51–4.32 (1H, m, CH), 3.85 (2H, d, J=6.7 Hz, SCH2), 3.81 (3H, s, OCH3), 3.59 (3H, s, OCH3), 1.63–1.45 (2H, m, CH2), 1.21–1.13 (1H, m, CH), 0.83 (6H, d, J=6.0 Hz, 2CH3). Anal. Calcd for C24H27N3O5S (469.6): C, 61.39%; H, 5.80%; N, 8.95%; S, 6.83. Found: C, 61.43%; H, 5.64%; N, 8.81%.
Methyl 3-Hydroxy-2-(2-(3-(4-methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-ylthio)acetamido)butanoate (10f): From L-ThrOCH3·HCl (0.156 g). White crystals (0.28 g, 62%); mp 180–181°C. 1H-NMR (300 MHz, CDCl3) δ: 8.36 (1H, d, J=8.7 Hz, NH), 8.08 (1H, d, J=8.1 Hz, ArH), 7.83 (1H, t, J=8.1 Hz, ArH), 7.60 (1H, d, J=8.1 Hz, ArH), 7.47 (1H, t, J=8.1 Hz, ArH), 7.34 (2H, d, J=9.0 Hz, ArH), 7.11 (2H, d, J=9.0 Hz, ArH), 5.01 (1H, d, J=5.4 Hz, OH, D2O exchangeable), 4.36 (1H, d, J=6.5 Hz, CH), 4.07–4.02 (3H, m, SCH2, CH), 3.85 (2H, s, OCH3), 3.60 (3H, s, OCH3), 1.03 (3H, d, J=6.3 Hz, CH3). Anal. Calcd for C22H23N3O6S (457.5): C, 57.76%; H, 5.07%; N, 9.18%; S, 7.01%. Found: C, 57.64%; H, 4.83%; N, 9.03%. Mass spectrum, m/z (Ir/%): 457.2.
Methyl 1-(2-(3-(4-Methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-ylthio)acetyl)pyrrolidine-2-carboxylate (13): From L-PrlOCH3·HCl (0.156 g). White crystals (0.27 g, 61%); mp 220–221°C. 1H-NMR (300 MHz, DMSO) δ: 8.08 (1H, d, J=7.8 Hz, NH), 7.81 (1H, t, J=7.8 Hz, ArH), 7.68 (1H, d, J=8.0 Hz, ArH), 7.48 (1H, d, J=8.0 Hz, ArH), 7.33 (2H, d, J=9.0 Hz, ArH), 7.11 (2H, d, J=9.0 Hz, ArH), 5.17 (1H, d, J=6.0 Hz, CH), 4.46 (1H, br s, OH, D2O exchangeable), 4.30 (1H, t, J=7.8 Hz, CH), 4.11 (1H, d, J=15.6 Hz, SCH2), 3.99 (1H, d, J=15.6 Hz, SCH2), 3.85–3.73 (2H, s, CH2, OCH3), 3.54 (1H, t, J=7.8 Hz, CH), 3.54 (3H, s, OCH3), 2.18–2.08 (2H, m, CH2), 2.01–1.91 (2H, m, CH2). Anal. Calcd for C23H23N3O5S (453.5): C, 60.91%; H, 5.11%; N, 9.27%; S, 7.07%. Found: C, 60.77%; H, 5.07%; N, 9.13%.
General Procedure for the Preparation of Thioacetyl Amino Acid Hydrazide (14a, 14d, 14e)To a solution of amino acide ester derivative 8a, 8d, 8e (1.0 mmol) in methyl alcohol (30 mL), hydrazine hydrate (2.4 mL, 5 mmol) was added. The reaction mixture was refluxed for 4 h, cooled and the resultant precipitate was filtered off, washed with ethanol and ether then crystallized from aqueous ethanol to yield the hydrazide 14a, 14d and 14e.
2-(2-(4-Oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetamido)acetohydrazide (14a): White crystals (0.32 g, 83%); mp 185–186°C. 1H-NMR (300 MHz, DMSO) δ: 8.11 (1H, d, J=7.9 Hz, ArH), 8.03 (1H, d, J=8.0 Hz, ArH), 7.82 (1H, t, J=8.0 Hz, ArH), 7.61–7.52 (4H, m, ArH), 7.50–7.43 (2H, m, ArH), 5.13 (1H, t, J=6.1 Hz, NH), 4.25–4.18 (3H, m, NH, NH2), 3.93 (2H, s, SCH2), 3.87 (2H, s, NCH2). Anal. Calcd for C18H17N5O3S (383.4): C, 56.38%; H, 4.47%; N, 18.27%; S, 8.36%. Found: C, 56.17%; H, 4.38%; N, 18.12%.
3-(2-(4-Oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetamido)propanehydrazide (14d): White crystals (0.28 g, 73%); mp 196–197°C. 1H-NMR (300 MHz, DMSO) δ: 8.05 (1H, d, J=8.0 Hz, ArH), 7.86 (1H, d, J=8.0 Hz, ArH), 7.83 (1H, t, J=8.0 Hz, ArH), 7.60–7.58 (4H, m, ArH), 7.47–7.45 (2H, m, ArH), 4.09 (2H, s, SCH2), 3.91 (1H, t, J=7.2 Hz, NH), 3.45–3.33 (5H, m, NHCH2, NH, NH2), 1.89 (2H, t, J=6.6 Hz, CH2). Anal. Calcd for C19H19N5O3S (397.5): C, 57.42%; H, 4.82%; N, 17.62%; S, 8.07%. Found: C, 57.32%; H, 4.76%; N, 17.55%.
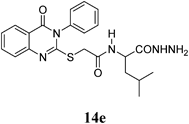
4-Methyl-2-(2-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetamido)pentanehydrazide (14e): White crystals (0.40 g, 92%); mp 165–166°C. 1H-NMR (300 MHz, DMSO) δ: 9.15 (1H, br s, NH), 8.31 (1H, d, J=7.9 Hz, ArH), 8.05 (1H, d, J=8.1 Hz, ArH), 7.81 (1H, t, J=8.1 Hz, ArH), 7.58–7.55 (4H, m, ArH), 7.47–7.43 (2H, m, ArH), 4.25–4.18 (3H, m, CH, NH2), 3.96 (1H, d, J=15.0 Hz, SCH2), 3.87 (1H, d, J=15.0 Hz, SCH2), 1.54–1.39 (3H, m, CH, CH2), 0.80 (6H, d, J=6.0 Hz, 2CH3). 13C-NMR (CDCl3) δ: 171.29 (C=O), 166.87 (C=O), 161.12 (Cq), 157.47 (Cq), 147.62 (Cq), 136.28 (Cq), 135.34 (CHAr), 130.44 (CHAr), 130.02 (CHAr), 129.87 (CHAr), 127.02 (CHAr), 126.50 (CHAr), 119.96 (Cq), 50.47 (CH), 41.78 (SCH2), 36.63 (CH2), 24.59 (CH), 23.33 (CH3), 22.14 (CH3). Anal. Calcd for C22H25N5O3S (439.5): C, 60.12%; H, 5.73%; N, 15.93%; S, 7.30%. Found: C, 60.01%; H, 5.68%; N, 15.81%.
General Procedure of Quinazoline Thioacetyl Dipeptide (16a, 16d, 16e)To a cold solution (−5°C) of hydrazide 14a–c (1.0 mmol) in AcOH (6 mL), 1 N HCl (3 mL), and water (25 mL) was added a solution of NaNO2 (0.34 g, 5.0 mmol) in cold water (3 mL). After stirring at −5°C for 1 h. A thick precipitate was formed. The reaction mixture was extracted in cold ethyl acetate (30 mL), washed with cold 3% NaHCO3, H2O and finally dried (Na2SO4). A solution of amino acid esters hydrochloride (1.0 mmol) in ethyl acetate (20 mL) containing 0.2 mL of triethyl amine was added to the ethyl acetate azide solution 15a–c. The mixture was kept at −5°C for 24 h, then at 25°C for another 24 h, followed by washing with 0.5 N HCl, water, 3% solution of NaHCO3 and finally dried (Na2SO4). The solution was evaporated to dryness, and the residue was recrystallized from petroleum ether–ethyl acetate to give the corresponding quinazoline dipeptide 16a–c.
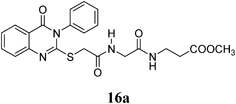
Methyl 3-(2-(2-(4-Oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetamido)acetamido)propanoate (16a): White crystals (0.20 g, 46%); mp 130–131°C. 1H-NMR (300 MHz, CDCl3) δ: 8.43 (1H, t, J=6.9 Hz, NH), 8.09 (1H, d, J=8.0 Hz, ArH), 8.07–7.81 (2H, m, ArH), 7.64–7.57 (4H, m, ArH), 7.50–7.44 (2H, m, ArH), 3.92 (2H, s, SCH2), 3.66 (2H, d, J=5.9 Hz, NHCH2), 3.58 (3H, s, OCH3), 3.24 (2H, q, J=6.5 Hz, NCH2), 2.43 (2H, t, J=6.5 Hz, CH2). Anal. Calcd for C22H22N4O5S (454.5): C, 58.14%; H, 4.88%; N, 12.33%; S, 7.06%. Found: C, 57.89%; H, 4.72%; N, 12.22%.
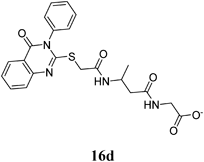
Methyl 2-(3-(2-(4-Oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetamido)propanamido)acetate (16d): White crystals (0.19 g, 42%); mp 145–146°C. 1H-NMR (300 MHz, DMSO) δ: 8.05 (1H, d, J=8.0 Hz, ArH), 7.86 (1H, d, J=8.0 Hz, ArH), 7.83 (1H, t, J=8.0 Hz, ArH), 7.60–7.58 (4H, m, ArH), 7.47–7.45 (2H, m, ArH), 4.22 (2H, s, SCH2), 3.75–3.64 (5H, m, CH2, OCH3), 3.54 (2H, q, J=6.6 Hz, NHCH2), 2.54 (2H, t, J=6.4 Hz, CH2). Anal. Calcd for C22H22N4O5S (454.5): C, 58.14%; H, 4.88%; N, 12.33%; S, 7.06%. Found: C, 57.83%; H, 4.62%; N, 12.14%.
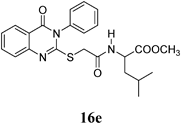
Methyl 2-(4-Methyl-2-(2-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio)acetamido)pentanamido)acetate (16e): White crystals (0.27 g, 56%); mp 180–181°C. 1H-NMR (300 MHz, DMSO) δ: 8.36 (1H, t, J=8.1 Hz, ArH), 8.24 (1H, d, J=7.8 Hz, NH), 8.08 (1H, d, J=8.1 Hz, ArH), 7.84 (1H, t, J=8.1 Hz, ArH), 7.61–7.57 (4H, m, ArH), 7.51–7.43 (2H, m, ArH), 4.28–4.18 (1H, m, CH), 4.01–3.67 (4H, m, SCH2, NHCH2), 3.60 (3H, s, OCH3), 1.69–1.43 (3H, m, CH, CH2), 0.82 (6H, d, J=6.0 Hz, 2CH3). Anal. Calcd for C25H28N4O5S (496.6): C, 60.47%; H, 5.68%; N, 11.28%; S, 6.46%. Found: C, 60.35%; H, 5.54%; N, 11.16%.
The design of the molecules synthesized in this article based on certain character of the structural similarity between the synthesized compounds and the natural substrate.11,12,21) The synthesized compounds compete with the natural substrate to its binding domain and this resulted in the inhibition of the enzyme reaction. The calculations based on this theory relayed initially on calculating the free energy of binding of designed and docked inhibitors in to the binding domain of the methionine synthase and the free energy of the receptor and the ligand individually (Eq. 1).

Comparison was established between the results obtained from the free energy of binding occurred in the docking stage and the IC50 values and hence there is inverse proportional relationship between the IC50 values and the free binding energy.21)
This method was carried out according to Skehan et al.24)
The sensitivity of the human tumor cell lines to the synthesized compounds was determined by the SRB assay. SRB is a bright pink aminoxanthene dye with two sulphonic groups that bind to the amino groups of intracellular proteins under mild acidic conditions; and dissociate under basic conditions. As the binding of SRB is stoichiometric the amount of dye extracted from stained cells is directly proportional to the cell mass.
ProcedureCells were used when 90% confluence was reached in T25 flasks. Adherent cell lines were harvested with 0.025% trypsin. Viability was determined by trypan blue exclusion using the inverted microscope. Cells were seeded in 96-well microliter plates at a concentration of 5×104 cells/well in a fresh medium and left to attach to the plates for 24 h. After 24 h, cells were incubated with the appropriate concentration of the synthesized compounds (100 µg/well), when completed to total of 200 µL volume/well using fresh medium and incubation was continued for 24, 48 and 72 h. Control cells were treated with vehicle alone. For each drug concentration, 4 wells were used. The cells were fixed with 50 µL cold 50% trichloroacetic acid for 1 h at 4°C. Wells were washed 5 times with distilled water and stained for 30 min at room temperature with 50 µL 0.4% SRB dissolved in 1% acetic acid. The cells were then washed 4 times with 1% acetic acid. The plates were air-dried and the dye was solubilized with 100 µL/well of 10 mM Tris base (pH 10.5) for 5 min on a shaker (Orbital shaker OS 20, Boeco, Germany) at 1600 rpm. The optical density (OD) of each well was measured spectrophotometrically at 564 nm with an ELISA microplate reader (Meter tech. Σ 960, U.S.A.). The mean background absorbance was automatically subtracted and mean values of each drug concentration was calculated.
CalculationThe percentage of cell survival was calculated as follows:
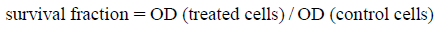 |
The IC50 values: (a parameter reflects potency).
The concentration of tested compound required to produce 50% inhibition of cell growth was calculated using sigmoidal dose response curve-fitting model.
IC50Human tumor cell lines (PC-3) used in this study were obtained from the American Type Culture Collection (ATCC, Minisota, U.S.A.). The tumor cell lines were maintained at the National Cancer Institute, Cairo, Egypt, by serial sub-culturing. Cells were maintained in vitro at 37°C in a 5% carbon dioxide, 95% air humidified incubator. Cells were grown in RPMI media containing 100 µM of l-methionine (abbreviated M+H) or methionine-free RPMI media containing 100 µM of l-homocysteine thiolactone (abbreviated M−H+). Media was supplemented with 10% 1 kDa cut-off dialyzed fetal calf serum (HL60, 20%), sodium pyruvate (1 mM), L-glutamine (2 µM), hepes (24 µM) and sodium bicarbonate (2 µM) Cells in exponential growth were plated (500–1000×10−3 cells per well) in complete M+H−, medium, containing l-methionine (100 µM). After an initial incubation of 24 h at 37°C the medium was changed to M−H− and cells were treated with compound concentrations between 100 and 0.1 µM. The tested compounds were dissolved in DMSO. After 24 h, the cells were washed twice with Hanks solution and transferred to fresh M+H− medium (recovery). Cells were then incubated for a further 5 d at 37°C before performing the MTT assay. Cell culture media was collected at appropriate times during recovery and stored at −80°C. Appropriate control experiments were performed without the inhibitors.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was used to assess the cytotoxicity of the tested compounds in cell culture. Following the above described treatments, media were recovered and the cells were treated with fresh medium containing MTT (0.6 mg/mL). The cells were incubated for 4 h at 37°C, the medium removed and replaced with DMSO (150 µL) to dissolve the resulting crystals. The absorbance of this solution was recorded at 550 nm in a plate reader. The final DMSO concentration was 0.1% and each plate contained the blank group (complete medium 200 µL/well and no cells), the control group (complete medium 200 µL/well and untreated cells). After 96 h of exposure time, 20 µL of MTT solution (5 mg/mL) was added and incubated in the dark at 37°C for 4 h. The supernatant was removed and formazan crystals were dissolved in DMSO (150 µL).
The results of the IC50 values were listed down in Table 1.
| Compound | x-Intercept | y-Intercept | Type of inhibition | Ki (µM) | % Inhibition/[I] (µM) |
|---|---|---|---|---|---|
| 10a | — | 0.19 | Non-competitive | 25.2 | 60±2/100 |
| 10e | — | 0.08 | Competitive | — | 11 STMa)±6 |
a) STM: stimulant.
Adapted methodology from our recent publication was used for the isolation, purification and the assay of methionine synthase from rat liver.12) Frozen rat livers (200 g) (−80°C) were thawed on ice and rinsed in ice cold potassium phosphate buffer (50 mM, pH 7.2). The livers were then chopped into ice-cold homogenising buffer that was made using potassium phosphate buffer (500 mL, 50 mM, pH 7.2) supplemented with protease inhibitors, (phenylmethylsulfonyl fluoride (PMSF) (26 mg) dissolved in 1 mL ethanol), Na-p-tosyl-L-lysine chloromethyl ketone hydrochloride (35 mg), trypsin (12.5 mg) and apotinin (2.6 mg) each dissolved in potassium phosphate buffer (1 mL, 50 mM, pH 7.2) prior to mixing into the homogenising buffer. The livers were then homogenised for six short pulses using a blender taking care to ensure the homogenate remained ice cold throughout. The resulting mixture was centrifuged for 15 min, at 5000 rpm in a 4°C pre-cooled Beckman centrifuge using a JA-20 rotor. The resulting supernatant liquid was centrifuged for a further 30 min at 15000 rpm at 4°C. The supernatant liquid was further centrifuged for 1 h, at 4°C at 28000 rpm using a Beckman ultra centrifuge equipped with a 28-swing rotor. The resulting cytosolic fraction was collected and stored in ice for up to 24 h awaiting purification.
Batch Chromatography on DEAE Cellulose. Preparation of the ResinDiethylaminoethyl (DEAE) cellulose (205 g) was stirred in sodium phosphate buffer (400 mL, 0.2 M, pH 7.2) for 3 min. The slurry was allowed to settle and the supernatant containing any fine partials was decanted. The resin was re-dispersed in sodium phosphate buffer (400 mL, 0.2 M, pH 7.2) the slurry again allowed to settle before decanting the supernatant.
The conditioned DEAE cellulose was then equilibrated with sodium phosphate buffer (400 mL, 20 mM, pH 7.2). In detail, the resin was suspended in sodium phosphate buffer, stirred for 20 min, allowed to settle, and the supernatant decanted. This process was repeated three times.
Batch Chromatography of Cytosolic FractionThe rat liver cytosol (approximately 200 mL) was taken and stirred with the previously equilibrated DEAE cellulose in sodium phosphate buffer (100 mL, 20 mM, pH 7.2) for one hour in a blacked out, cold cabinet. The supernatant containing any unbound proteins was removed by vacuum filtration. The DEAE cellulose was then washed twice with ice cold sodium phosphate buffer (100 mL, 20 mM, pH 7.2) dispersing the red colour from the resin. The bound proteins, including the desired enzyme were then eluted from the resin by stirring the DEAE cellulose in sodium phosphate buffer (200 mL, 20 mM, pH 7.2) containing sodium chloride (0.5 M) for 15 min in a dark, cold cabinet. The mixture was filtered under vacuum and the resin washed twice with the sodium chloride containing buffer (0.5 M, 2×50 mL). The filtrates containing the enzyme were pooled and kept on ice in the dark awaiting further analysis. This solution was kept for up to 24 h.
DEAE Regeneration and Storage ConditionsThe used DEAE cellulose was washed in sodium phosphate buffer (500 mL, 20 mM, pH 7.2) containing sodium chloride (0.5 mM) for one hour. The resin was then allowed to settle and the buffer decanted. The process was repeated twice using sodium phosphate buffer (250 mL, 20 mM, pH 7.2) containing 1 M sodium chloride. Finally the buffer was replaced with a 20% ethanol/ water mixture and stirring continued for 15 min. The ethanol/ water wash was repeated three times in succession before storing the DEAE cellulose at 4°C in 20% ethanol/ water (v/v).
Q-Sepharose FPLC ChromatographyThe Q-Sepharose resin was conditioned prior to use with the separation buffers. In detail, sodium phosphate buffer (20 mM, pH 7.2), (low salt buffer), was run through the column at a rate of 4 mL/min for one hour followed by sodium phosphate buffer (20 mM, pH 7.2) containing sodium chloride (1 M), (high salt buffer), for 1 h. Finally a stable baseline was recorded using the low salt buffer.
The enzyme preparation obtained from the DEAE purification was diluted three fold with milli-Q water to reduce the concentration of sodium chloride. The Q-sepharose packed XK16 column (15×2.5 cm) was loaded with 300 mL of dilute filtrate using a superloop (10 mL) and flow rate of 4 mL/min. The column was then eluted at a flow rate of 2 mL/min using the following gradient: Sodium phosphate buffer (20 mM, pH 7.2) containing sodium chloride (1 M) (high salt buffer) was increased to 0.5 M NaCl over 50 min. This was then increased to 1 M NaCl over 5 min. This condition was held constant for 20 min before washing back with sodium phosphate buffer (20 mM, pH 7.2) (low salt) over 5 min and holding constant for a further 20 min to condition the resin for subsequent separations. Fractions were collected at 12 min intervals of approximately 50 mL throughout the program.
Precautions were undertaken to ensure the enzyme solutions remained cool and dark at all times. To this effect, fractions were collected in pre-cooled tubes kept on ice. The column and super loop were wrapped in foil throughout experiments and the buffers were kept on ice during the separation.
The active fractions, identified using a radiometric methionine synthase assay, were pooled together and 50 mL batches concentrated to one-fifth of the original volume using an Amicon ultra concentrator (30 kDa cut-off size). The combined concentrates were pooled and kept on ice. Glycerol was then added to a final concentration of 20% v/v.
Protein Content DeterminationThe protein content of all the collected samples was determined using a Bio-Rad protein assay kit. The Method used bovine serum albumin (BSA) as the standard. The standard curve ranging from BSA 0 to 5.4 µg. Assays were incubated for 15 min to allow for the formation of the chromophore. The absorptions of the samples were determined using an automatic plate reader at 600 nm on a 96 well plate.
A stock solution of BSA (0.135 µg/mL) was diluted with water to make a working solution of BSA (0.0135 µg/mL). The Bradford reagent (200 µL) was added to each concentration making a final volume of 1 mL. After incubation (15 min at room temperature) aliquots of 200 µL were transfer into a 96 well plate for reading. Protein samples were run along side the known concentrations of BSA. Serial dilutions of the unknown concentrations of protein were made to ensure their absorbance readings fell within the range of the calibration line.
Preparation of ReagentsHomocysteine solution (3.77 mM) was prepared from D,L-homocysteine (5.1 mg) in water (10 mL). Homocysteine solution was prepared daily.
Ascorbic acid solution (10 mM) was prepared form ascorbic acid (0.18 g) in water (100 mL).
5-Methyltetrahydrofolate (MTHF) solution (3.96 mM) was prepared from 5-methyltetrahydrofolic acid (25 mg) in ascorbic acid solution (12.5 mL, 10 mM). 5-Methyltetrahydrofolate solution was stored in 1 mL aliquots at −20°C.
5-14C-Methyltetrahydrofolate (14C-MTHF) solution was prepared from 5-14C-methyltetrahydrofolic acid barium salt (50 µCi) dissolved in ascorbic acid solution (1 mL, 10 mM). 5-14C-Methyltetrahydrofolate solution was stored in 20 µL aliquots at −20°C.
Dithiothreitol (DTT) solution (0.7 µM) was prepared from DTT (13 mg) dissolved in water (120 µL). Dithiothreitol solution was prepared daily.
Hydroxycobalamin (HO-Cbl) solution (1.8 mM) was prepared from hydroxycobalamin (2.5 mg) dissolved in water (1 mL). Hydroxycobalamin solution was stored in 100 µL aliquots at −20°C.
S-Adenosylmethionine (S-AdoMet) solution (1.2 mM) was prepared from S-adenosylmethionine iodine salt (5 mg) dissolved in water (7.9 mL). S-Adenosylmethionine solution was stored in 500 µL aliquots at −20°C.
General AssayInto clean, dry, labelled serum vials prepared in triplicate, MTHF (67.9 nmol) made up of MTHF (20 µL, 3.96 mM) and 14C MTHF (10 µL, 2.47×10−3 MBq), were added. S-AdoMet (10 µL, 1.2 mM), DTT (10 µL, 0.70 µM), HO-Cbl (10 µL, 1.8 mM), and potassium phosphate buffer (50 mM, pH 7.2) (to give a total assay volume of 300 µL) were added. The vials were excluded from light by wrapping in aluminium foil. Enzyme (50 µL) was added to each vial in turn followed by sealing and flushing with nitrogen for 30 s to create anaerobic conditions. The vials were pre-incubated for a period of 5 min at 37°C. The reaction was initiated with the addition of homocysteine (40 µL, 3.77 mM). The total assay volume was 300 µL. Assay vials were then incubated for 45 min at 37°C unless otherwise stated. The reactions were terminated with ice cold milli-Q water (400 µL) and the assay vials placed on ice. The reaction mixture was then passed through AG1-X8 resin columns (0.5×5 cm) pre-conditioned with water (3 mL) followed by water (2 mL) to elute [14C]-methionine. The 2 mL elute was collected and scintillation cocktail added (12 mL) before being quantified using a liquid scintillation counter.
When the assay was used to screen the activity of the inhibitors an aliquot of DMSO (5 µL) vehicle was included and the buffer volume adjusted accordingly. Blank assays were prepared in the same way excluding the enzyme source and substituting for an equivalent volume of assay buffer.
Enzyme Dose-Dependence of the AssayThe enzyme dose-dependence of the assays were performed using a fixed incubation time of 30 min while varying the amount of enzyme added. Aliquots of 10, 25, 75 and 100 µL of purified enzyme were used.
Time-Dependence of the AssaysDetermination of time-dependence was investigated using a fixed amount of enzyme (50 µL) determined from the dose study at varying incubation time. Assays were carried out at 0, 5, 10, 15, 30 and 45 min.
Solvent Effect of the AassaySolvent effects were determined using the optimised conditions determined from the dose and time studies. The effect of 0, 2, 5 and 10 µL of DMSO on enzyme activity was determined. The optimum volume was found to be 5 µL and was subsequently used for all of the inhibition studies.
Preparation of Inhibitor SolutionsStock solutions (30 mM) of all the compounds to be tested were prepared in DMSO. Working solutions of the inhibitors were prepared by further dilution of the stock with DMSO. The concentrations of these working solutions were calculated to give the desired concentration of inhibitor in the assay when 5 µL of working solution was used per assay.
Time Inhibition StudyTime-dependent assays of the inhibitors were performed at 5, 10, 15, 30, 45, 60 and 90 min keeping all other parameters fixed.
Enzyme Kinetics. Kinetic ParametersKinetic parameters (Vmax, Km and Ki) were calculated for both uninhibited and inhibited reactions using the following parameters: Inhibitor concentrations of 500, 250 and 100 µM. 5-Methyltetrahydrofolate concentrations: 200, 100, 80, 40, 20 and 10 µM. The concentrations of all other substrates remained constant. A 5 min pre-incubation and 20 min incubation time was used for all assays ensuring that substrate turnover was ≤20%.
Kinetic Parameters of Methionine SynthasePrior to any inhibition studies the kinetic parameters of the enzyme reaction had to be established. This involved a series of experiments to establish that all assays were performed within the linear region of the enzyme activity, and that the maximum turnover of the enzyme did not exceed 20% to avoid product inhibition and ensure that the concentration of the substrate was much greater than the enzyme. These experiments consisted of time and dose-dependent assays.
Compounds 8a, 9a, 10a and 10f showed the lowest values of free energy of binding (−105.51 kJ/mol, −190.09 kJ/mol, −207.19 kJ/mol and −124.33 kJ/mol, respectively and the lowest IC50 values 22.6 µg/mL, 20.6 µg/mL, 20 µg/mL and 20.8 µg/mL against PC-3 cells, respectively. Compounds 8c and 9c exhibited moderate values of free energy of binding (150.19 kJ/mol and −183.38 kJ/mol) and moderate IC50 values 34 µg/mL and 30.7 µg/mL (Table 1).
The structure assignment of both the S-substituted amino acid derivatives and dipeptide is based on 1H-, 13C-NMR and mass spectroscopy as well as physicochemical analysis. Both the 1H- and 13C-NMR clearly confirm the alkylation site for all the isolated S-substituted derivatives. Thus the 1H-NMR spectrum of 8a exhibits a singlet signal at δ 3.92 ppm typically associated with SCH2 group. The 1H-NMR spectrum also shows an interesting triplet and doublet signals at δ 8.61 and 3.84 ppm associated with the NH and CH2 groups, respectively. This confirming the generation of amino acid coupling that is common for all S-substituted α-amino acid esters 8–13.The 13C-NMR spectrum of 8a shows signals at δ 52.21, 41.44, 36.23 ppm attributed to OCH3, NHCH2 and SCH2, respectively.
We now report the preparation of methyl 2-(2-(4-oxo-3-aryl-3,4-dihydroquinazolin-2-ylthio)acetamido)alkanoates and dipeptide derivative. Quinazoline 1 was prepared either by the reaction of aryl isothiocyanate with anthranilic acid in glacial acetic acid23) or could be obtained by the sequential reaction of aniline derivatives with carbon disulfide followed by alkylation with dimethyl sulfate and finally refluxed with methyl anthranilate in ethyl alcohol for 18h to afford the desired quinazoline 1.22) Structure modification of the model compound 1 could be achieved by the simple chemoselective alkylation reactions with electrophiles. However, the reaction of quinazoline 1 with chloroacetic acid in the presence of NaOH gave a mixture of S-substituted carboxylic acid 2 contaminated with quinazoline dione 3 and benzanilde 4. On the other hand, the reaction of 1 with methyl chloroacetate in the presence of a NEt3 afforded the S-substituted ester 5 in an excellent yield. Saponification of ester 5 with KOH in methanol water mixture at room temperature for 4 h gave S-substituted carboxylic acid 2. The ester 5 was reacted with hydrazine hydrate in ethyl alcohol for 4 h and gave hydrazide 6 (Chart 1). Compounds 2 and 6 are excellent precursors for quinazoline structure modification at sulfur atom via amino acid coupling methods. Azide coupling method was considered one of the important methods to introduce amino acid derivatives from hydrazides. It was also reported that this method decreases the degree of racemization in the amino acid coupling.25,26)
The reaction pathway shows our results obtained by the chemoselective S-alkylation of quinazoline 1 with chloroacetic acid and methyl chloroacetate and the subsequent hydrolysis and hydrazinolysis of the ester 5.
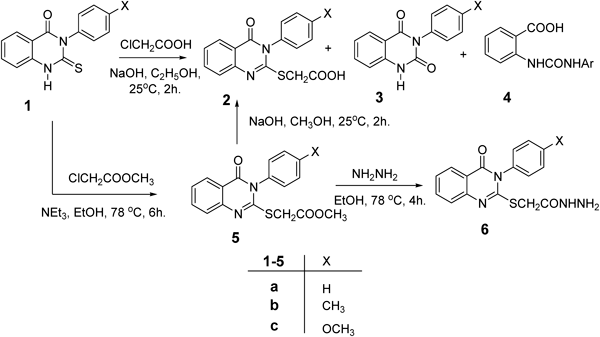
Our first choice for structure modification of quinazoline 1 by the introduction of acetyl amino acid esters at S-atom was the azide coupling method. The hydrazide 6 reacted with NaNO2 and HCl mixture at 0°C to presumably produce the azide 7, which was extracted with ethyl acetate. The in situ generated azide derivative 7 was treated with amino acid ester hydrochloride and NEt3. Unfortunately, the amino acid ester derivative 8 was not formed and instead an interesting rearrangement occurred to finally afford the dione 3 (Chart 2). The use of this rearrangement as an important synthetic method together with rational mechanism explanation will be discussed in another publication. Our alternative efforts for structure modification of quinazoline by the introduction of acetyl amino acid esters at S-atom was efficiently formed from the key substrate carboxylic acid derivative 2 via DCC coupling method.
The reaction pathway shows the reaction of hydrazide 6 with amino acid esters under azide coupling condition to afford quinazoline 3 via an interesting rearrangement.
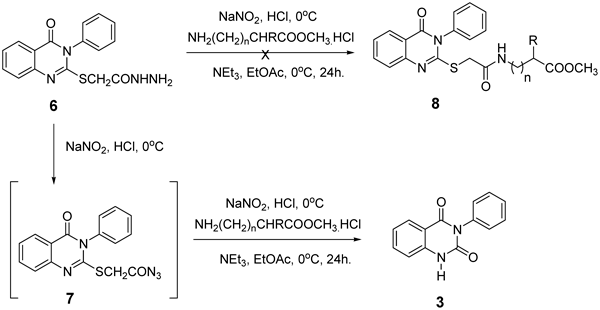
The DCC coupling is one of the major tools employed in literature to introduce peptide bonds by the reaction of carboxylic acid with amino acid methyl ester.27) Hydroxybenzotriazole (HOBT) is widely used as an additive to decrease racemization in the carbodiimide peptide coupling.28,29) Treatment of carboxylic acid 2a–c with the amino acid esters hydrochloride in the presence of coupling reagents DCC and HOBt afforded amino acid ester S-alkanamide 8–13, respectively in good yield (Chart 3).
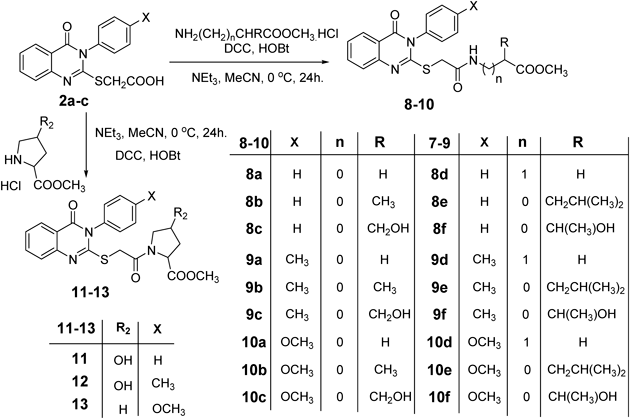
The reaction pathway shows quinazoline structure modification at sulfur atom by attachement of acetyl amino acid esters to produce 8–10 and 11–13. This was efficiently formed by the reaction of carboxylic acid derivative 2 with amino acid esters in the presence of HOBT and DCC.
Furthermore, our next target was the synthesis of dipeptide linked to quinazoline by thioacetyl spacer. The quinazoline amino acid esters 8a, 8d and 8e were boiled with hydrazine hydrate in absolute ethanol to afford the hydrazides 14a, 14d and 14e. The hydrazide derivatives 14a, 14d and 14e were reacted with NaNO2 and HCl mixture at 0°C to give the azide derivatives 15a, 15d and 15e, which was extracted with ethyl acetate. The in situ generated azide derivatives 15a, 15d and 15e solution in ethyl acetate was reacted with amino acid methyl ester hydrochloride in the presence of triethyl amine to afford the dipeptides derivatives 16a, 16d and 16e in good yield (Chart 4).
The reaction pathway shows quinazoline structure modification at sulfur atom by attachement of dipeptide linked with acetyl spacer. This was effeciently formed by the reaction of carboxylic hydrazides 14a, 14a and 14e with amino acid esters under azide coupling condition.
The enzyme free assay was performed on the most and least active compounds predicted by thermodynamic algorithms which showed the free energy of binding for compound 10a −207.19 kJ/mol and for compound 10e +63.48 kJ/mol. The results displayed that the percentage inhibition of the enzyme for compound 10a is 60±2/100 µM with inhibitory constant (Ki=25.2 µM). In contrary for compound 10e which exhibited stimulating platform for the enzyme catalysed reaction as shown in Table 1.
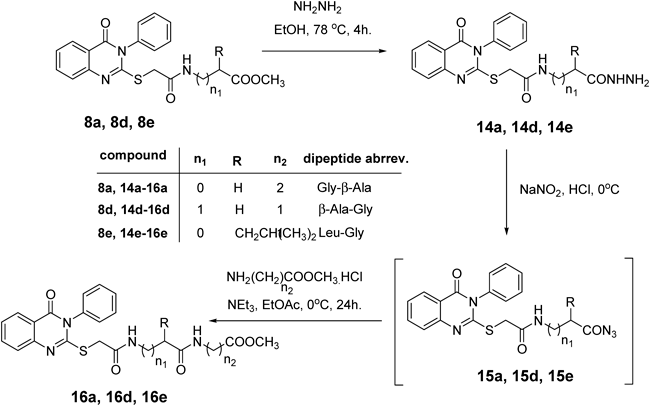
Molecular modeling investigations were done on the MTHF receptor and the designed inhibitors were docked in to the receptor on the same docking pose of the MTHF substrate (Fig. 1). The MTHF receptor is confined by 5 amino acids: aspartic acid 390, asparagine 411, aspartic acid 473, asparagine 508 and arginine 516. The receptor is considered one of the biggest receptors in size (20.7 Å×12.2 Å) (Fig. 1).
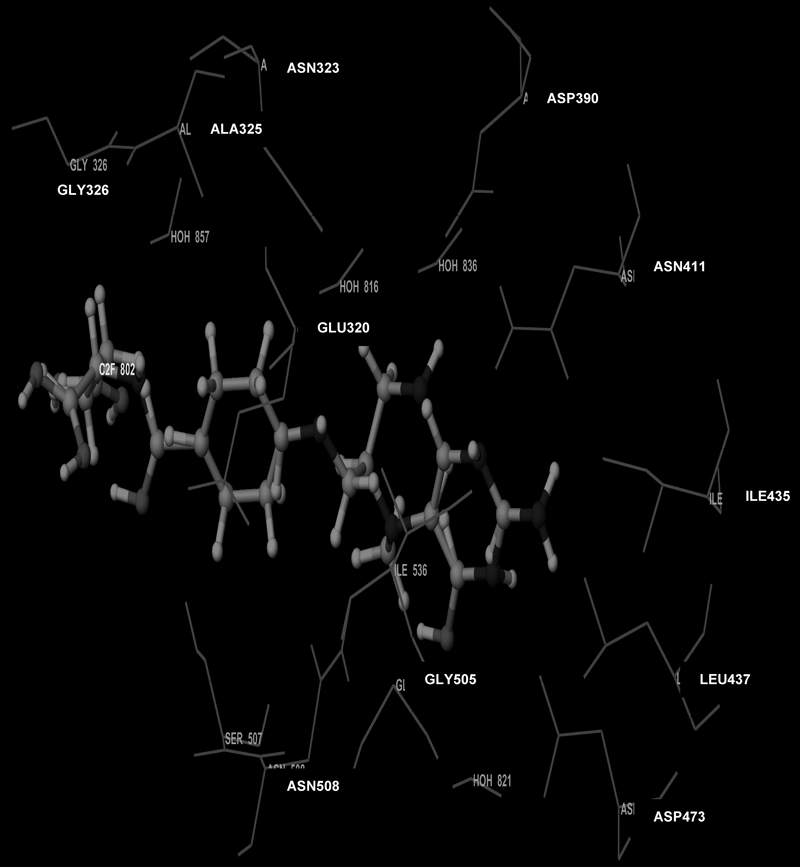
Image of MTHF docked in to its binding domain of MetS where pterine ring docked deep inside the receptor and methylamino-benzoylamino]pentanedioic acid side chain pointed towards the orifice of the receptor.
The ligand–protein complexes were subjected to intense dynamic work to obtain the most suitable and stable conformations and lowest in energy. The receptors do not recognize their ligands in terms of molecular frame work, atom positions or connectivities between them, but in terms of free energy difference between unassociated and associated states of the ligand with the receptor. Hydrogen bonds, electrostatic interactions, hydrophobic interactions and van der Waals interactions stabilize the associated ligand-receptor complex enthalpy.30) Subsequently the degree of freedom of the ligand and receptor is reduced. It is not desirable to get active unstable conformation with high energy content compared to the global minimum conformation. If the ligand structure is conformationally too unfavourable, the docking structure might become too unstable in total energy.31) Therefore, it is necessary to examine the relative stability of the generated conformation compared with all other possible conformations of the ligand. The internal energy content, conformation, desolvation and degree of freedom of the unassociated ligand and receptor will change after the docking occurred. Therefore, the free energy difference between the associated state and the unassociated state was calculated from Eq. 1. Each algorithm of the binding free energy for each ligand was compared to the IC50 value of each ligand obtained. The comparison was accomplished by measuring ΔEbind with the more negative value, the more favourable binding.11) If the molecule of interest fits the binding criteria and energy requests of the binding domain, then the receptor-molecule complex will have a lower energy than the sum of the energies of the un-complexed protein and molecule. The ligand showed a variation in the percentage inhibition associated with a variation in the total binding enthalpy as shown in Table 2.
| Ligand | Energy term (kJ/mol) | Sensitivity (PC-3) (inhibition %) | IC50 (µg/mL) PC-3 | RMSD (Å) | |||
|---|---|---|---|---|---|---|---|
| ΔEbind | ΔEcomplex | ΔEreceptor | ΔEligand | ||||
| MTHF | −122.36 | −255.00 | −190.64 | 58.00 | — | — | 1.41 |
| Lapatinib reference | — | — | — | — | — | 3.4 | — |
| 8a | −105.51 | −202.72 | −186.09 | 88.88 | 68.9 | 22.6 | 2.23 |
| 8d | −60.93 | −267.08 | −290.24 | 84.09 | — | — | 3.51 |
| 8f | −42.91 | −275.87 | −285.05 | 52.09 | — | — | 3.12 |
| 8e | −8.91 | −290.85 | −318.42 | 36.48 | — | — | 4.67 |
| 16a | −62.93 | −341.15 | −335.62 | 57.40 | — | — | 2.42 |
| 11 | −122.62 | −390.03 | −306.11 | 58.70 | 71.4 | — | 1.6 |
| 8b | −50.14 | −321.00 | −360.04 | 89.18 | — | — | 3.9 |
| 8c | −150.19 | −331.05 | −280.94 | 100.08 | 68 | 34 | 1.9 |
| 16e | +44.11 | −301.55 | −399.94 | 54.28 | — | — | 5.81 |
| 14e | −0.19 | −311.05 | −390.94 | 80.08 | — | — | 5.91 |
| 14a | −50.14 | −321.00 | −360.04 | 89.18 | — | — | 3.9 |
| 9a | −190.09 | −330.95 | −200.94 | 60.08 | 73.1 | 20.6 | 1.93 |
| 9d | −131.09 | −321.95 | −280.94 | 90.08 | — | — | 2.33 |
| 9f | −120.19 | −311.05 | −280.94 | 90.08 | — | — | 2.01 |
| 12 | −108.65 | −280.03 | −272.04 | 100.66 | 67.2 | — | 3.10 |
| 9b | −170.49 | −351.95 | −283.04 | 101.58 | — | — | 2.88 |
| 9c | −183.38 | −359.61 | −265.91 | 89.68 | 67.8 | 30.7 | 1.55 |
| 9e | +19.81 | −291.05 | −380.94 | 70.08 | — | — | 4.88 |
| 10a | −207.19 | −381.85 | −282.04 | 107.38 | 69.3 | 20 | 1.41 |
| 10d | −177.19 | −387.15 | −280.94 | 80.98 | — | — | 2.09 |
| 10f | −124.33 | −307.15 | −290.90 | 108.08 | 30.6 | — | 3.01 |
| 13 | −104.59 | −271.55 | −270.04 | 103.08 | — | — | 4.88 |
| 10b | −125.79 | −311.75 | −286.94 | 100.98 | — | — | 3.01 |
| 10c | −123.83 | −277.13 | −252.04 | 98.74 | 64.2 | 20.8 | 4.44 |
| 10e | +63.48 | −281.05 | −397.91 | 53.38 | — | — | 5.81 |
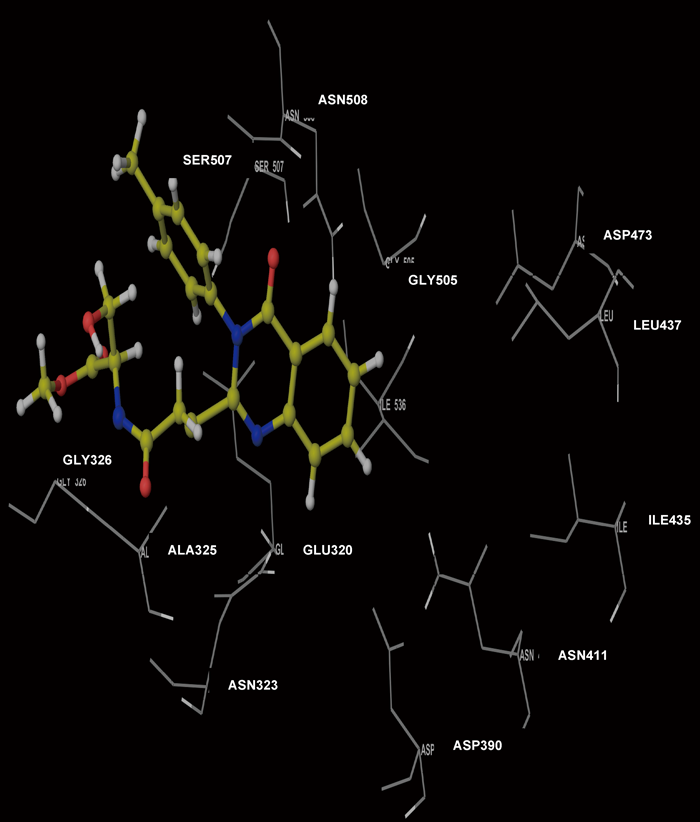
Compounds 8c, 9c and 10a have been docked on the same pose orientation of the natural substrate of the enzyme. The root mean square deviations (RMSD) for them were 1.9 Å, 1.55 Å and 1.41 Å, respectively (Figs. 2–4). This means that these compounds most likely will produce a competitive inhibition and gradient concentration of these compounds will displace MTHF from its domain and therefore inhibit the enzyme reaction.12)
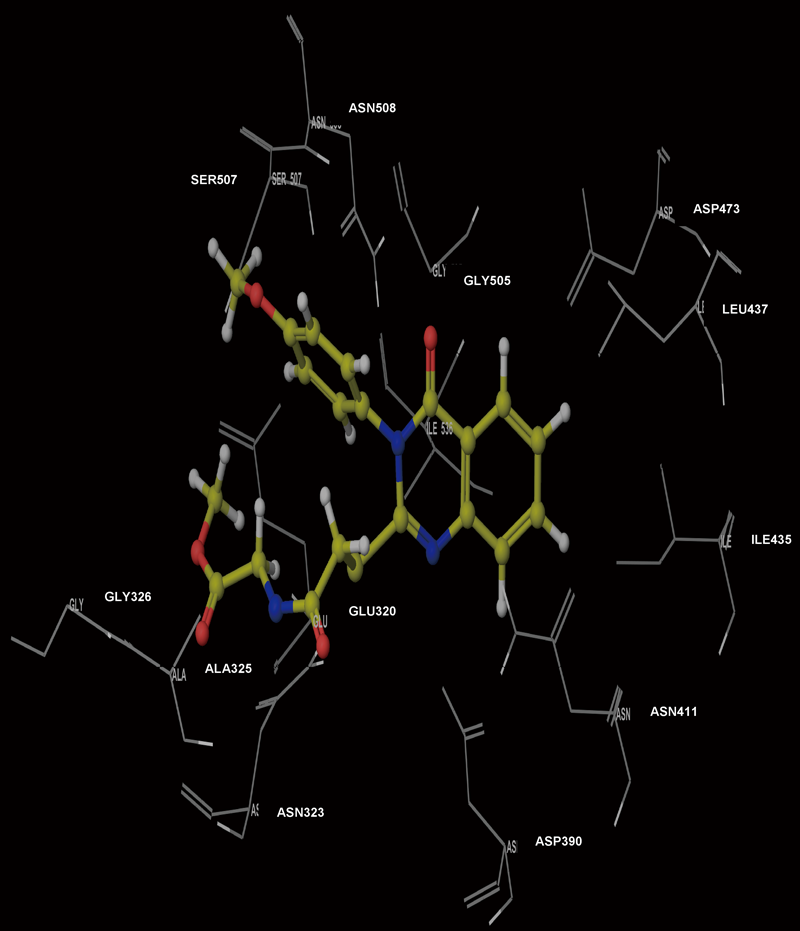
Image of compound 10a docked in to the MTHF binding domain of MetS. The left hand side represents the outermost part of the enzyme and the orifice of the receptor and the right hand site represents the innermost part of the enzyme.
The left hand side represents the outermost part of the enzyme and the orifice of the receptor and the right hand site represents the innermost part of the enzyme.
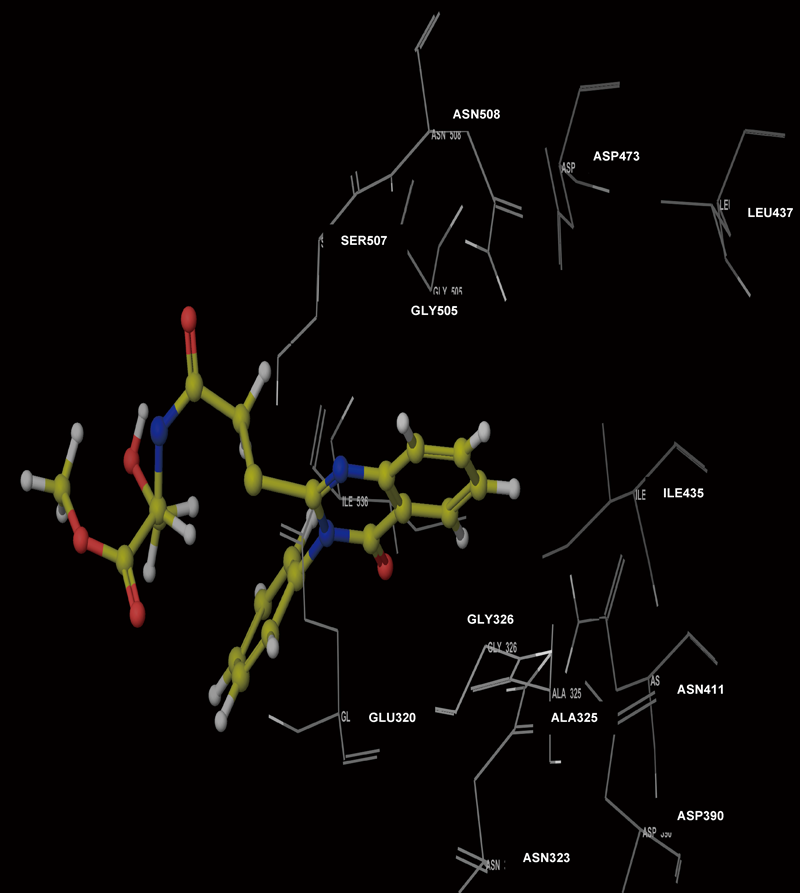
Image of compound 8c docked in to the MTHF binding domain of MetS in the primary structure of the enzyme. The left hand side represents the outermost part of the enzyme and the orifice of the receptor and the right hand site represents the innermost part of the enzyme.
Compound 10a docked vertically inside the pocket to avoid the van der Waals repulsive interactions (Figs. 2, 5). The plausible docking achieved RMSD value of 1.41 Å which is closer to the docking pose of the natural substrate MTHF. The compound achieved a low free energy of binding of −207.19 kJ/mol and IC50 value 20 µg/mL. This could be attributed to the ligand fitted plausibly the cavity of the receptor and produced favourable electrostatic interaction with serine 507, asparagine 323 and glutamic acid 320. This consequently contributed good in to the total free energy of binding and to the obtained IC50 value (Fig. 6). The docking pose of compound 9c showed that the ligand centre was slightly shifted outwards the binding domain. This probably could be ascribed to the free hydrophobic rotatable methyl group positioned at phenyl ring and the branched serinyl group positioned at the side chain of the quinazoline ring. This likely declined the Coulomb energy of the total free energy of binding (−183.38 kJ/mol) and IC50 value 30.7 µg/mL due to the high energy barrier among the methyl group and branched side chain of the ligand from one side and the polar amino acids exist at the orifice of the binding domain (Figs. 3, 7). Compound 8c was docked horizontally where the side chain and phenyl ring found at positions 2 and 3 of quinazoline ring protruded outside part of the receptor to avoid the steric clashes (Figs. 4, 8). The docking score started to diminish when the phenyl ring and the side chain of the ligand moved away and the van der Waals force decreased. There was an increase in steric clashes, which occurred with the polar parts of the amino acids serine 507, asparagine 323 and glutamic acid 320. The docking score started to decrease by 2 kJ/mol. Such an energy penalty resulted in the reduction in the binding affinity of the subsequent conformers of the ligand 8 and hence reflected on the values of total energy of binding (−150.19 kJ/mol) and IC50 value 34 µg/mL (Fig. 6).
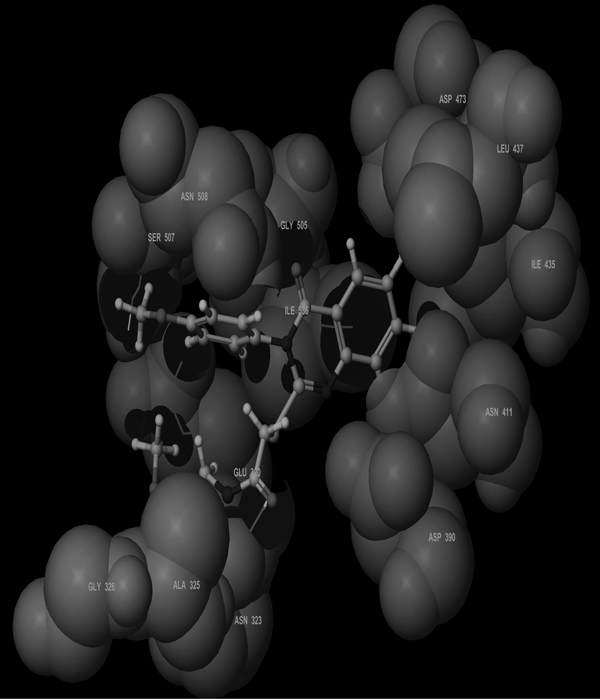
Image of compound 10a docked in to the MTHF binding domain of MetS with van der Waals radius. The left hand side represents the outermost part of the enzyme and the orifice of the receptor and the right hand site represents the innermost part of the enzyme.

The plots of concentarion gradients used (0, 5, 12.5, 25, 50 µg/mL) and the surviving fraction of the cancer cells.
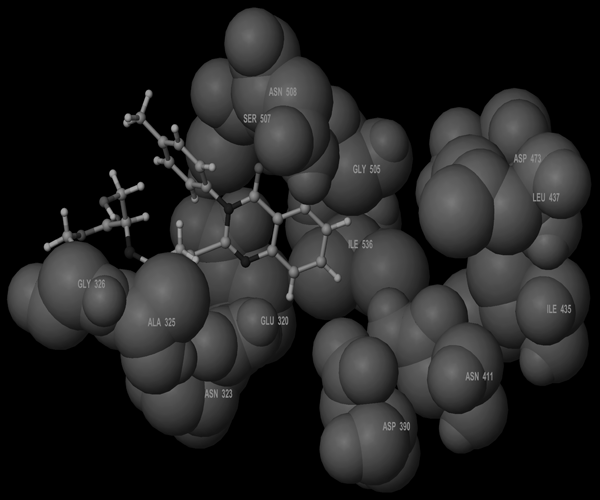
Image of compound 9c docked in to the MTHF binding domain of MetS with van der Waals radius showing spheres of nonbonding interactions of the atoms of the receptor and the ligand. The left hand side represents the outermost part of the enzyme and the orifice of the receptor and the right hand site represents the innermost part of the enzyme.
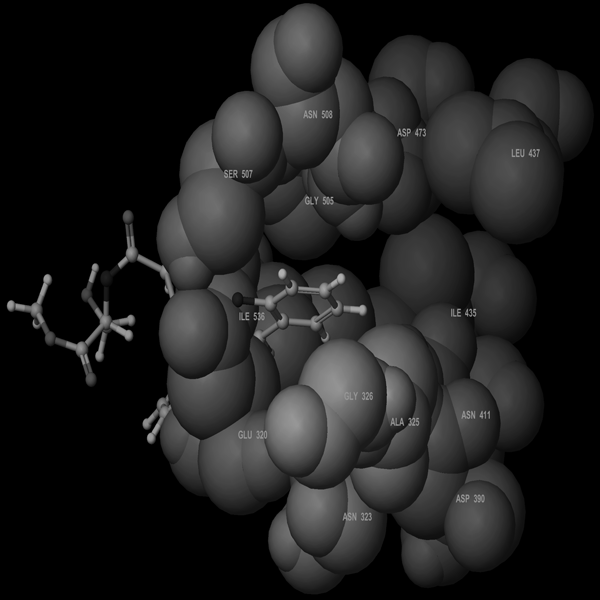
Image of compound 8c docked in to the MTHF binding domain of MetS. with van der Waals radius showing spheres of nonbonding interactions of the atoms of the receptor and the ligand. The left hand side represents the outermost part of the enzyme and the orifice of the receptor and the right hand site represents the innermost part of the enzyme. Compound 4 makes hydrogen bond with asparagine 508 and arginine 518.
The results of free cell assay suggested that the inhibitors may bind at other binding sites as well as at the desired 5-methyltetrahydrofolate site were listed in Table 1. Methionine synthase is a bisubstrate enzyme, it is possible that the inhibitors also compete for the homocysteine binding domain in addition to the 5-methyltetrahydrofolate binding domain, or even bind at the binding site of the S-adenosylmethionine reactivation cofactor. In this study we have only attempted the evaluation of the inhibitory activity against the 5-methyltetrahydrofolate binding site keeping all other parameters constant. The target compounds that were successfully synthesized were tested for their inhibitory activity against highly purified rat liver cobalamin-dependent methionine synthase. A pilot study was undertaken on the synthesized series of substituted quinazoline derivatives. The two compounds were tested using an established in vitro radiometric enzyme assay.32–34) The compounds were initially screened at 500 µM then at gradient concentrations of 250 µM and 100 µM. The tested compounds exhibited typical non-competitive (10a) and competitive (10e) inhibition patterns on Lineweaver–Burk plots (Eqs. 2, 3).
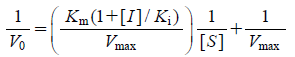
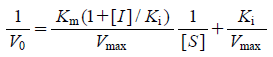

Represents the inhibition plots of the enzyme reaction with different inhibitor concentrations. The plots of compounds 10a and 10e showed non-competitive and competitive inhibitions, respectively.
| The structure of the compounds |
|---|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
The non-competitive inhibition pattern exhibited by compound 10a could be attributed to that ligand possibly bound the enzyme whether or not it has already bound the substrate. Meanwhile, the competitive inhibition pattern exhibited by compound 13 probably certified to that the ligand competed with the natural substrate (MTHF) to its binding domain.
MetS utilizes the most abundant form of the circulating folates, MTHF, as a substrate. MetS related pathways are important in many biological processes including the synthesis of proteins, lipids, polyamines and numerous AdoMet-dependent transmethylation reactions. Inhibition or impairment of the function of any one of these reactions may have fundamental effects on the functioning and growth of the cells. In particular, impaired conversion of homocysteine to methionine and MTHF to THF and could affect folate metabolism, DNA sythesis, methionine and AdoMet syhthesis. Some cancer cells showed methionine-dependence and depriving these cancer cells of methionine could affect their proliferation. Methionine synthase was found over expressed in 60% of the prostate cancer therefore it has a kind of a link between inhibiting the metabolic reaction of the enzyme and apoptosis.12) The synthesized inhibitors were analogues of quinazoline derivatives that inhibit the enzyme reaction and could be used as drug candidates.
Thanks for every person gave his valuable contribution to this article.