2015 年 63 巻 11 号 p. 851-857
2015 年 63 巻 11 号 p. 851-857
In the present work the absorption of flutamide from suppositories containing hydrophilic tamarind alginate microparticles after rectal administration in rats was investigated with the purpose of enhancing bioavailability and to avoid hepatic toxicity. Microparticles were developed by ionic gelation method and optimized using one factorial design of response surface methodology. The optimized batch of microparticles had tamarind gum–sodium alginate (1 : 3) ratio and showed entrapment efficiency 94.969% and mucoadhesion strength 94.646% with desirability of 0.961. Suppositories loaded with microparticles were developed by fusion method using poloxamer 407 and poloxamer 188 in combination as suppository base. Kinetic analysis of the release data of microparticle-loaded suppositories showed time-independent release of drug. Higher values of ‘n’ (>0.89) represent Super Case II-type drug release. The pharmacokinetics of flutamide from flutamide tamarind alginate microparticle-loaded suppository were compared with oral suspension. Cmax of microparticle-loaded suppository was significantly larger than that of oral suspension (1.711 and 0.859 µg/mL, respectively).
Adenocarcinoma of the prostate is the second most common cause of death of men world-wide. A nonsteroidal antiandrogen flutamide could be used in combination with medical or surgical castration to provide superior care for patients with metastatic prostate cancer. Large studies have shown a longer disease-free interval and a short survival advantage with maximum androgen blockade compared with monotherapy, especially in younger, healthy patients with minimum metastatic disease.1)
Flutamide is a potent nonsteroidal antiandrogen drug that inhibits androgen uptake and/or nuclear binding of androgen in target tissues. It is chemically known as 2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]propanamide. Flutamide competes with testosterone and its powerful metabolite, dihydrotestosterone for binding to androgen receptors in the prostate gland.2)
The low oral bioavailability of flutamide may be due to poor wettability, low aqueous solubility, poor permeability, extensive first pass metabolism and low availability at absorption site. Several attempts have been made to improve oral bioavailability and to reduce side effects of flutamide such as nanoemulsions,3) hydroxypropyl-β-cyclodextrin (HP-βCD) inclusion complex,4) nanoparticles,5) lyophilised dispersions with βCD and HP-βCD,6) hydrophilic polymers,7) polyols and amino acids,8) biopolymeric microparticles combined with lyophilized monophase dispersions.9) However, rectal administration of flutamide in the interest of bioavailability enhancement has never been discussed or examined.
The advantage of rectal route over oral route of administration is avoidance of first pass metabolism and gastrointestinal side effects. For effective retention of medication on the rectal mucosa, the drug delivery system should have mucoadhesive property.
Microparticulate drug delivery system is one of the prospective drug delivery systems because it has advantages, such as effective and long lasting release of drug, protection of drug from inactivation, capacity to reduce drug toxicity, reduced number of doses required for treatment of the diseases, and smaller dose variations due to administration of large number of microparticulates. Mucoadhesive polymers make the microparticles able to promote intimate contact between the pharmaceutical form and targeted tissue and to prolong the residence time of drug at the site of administration.
Natural polymers are widely used as drug carriers as these have advantages like low cost, biocompatibility, and biodegradability.10) Alginate is an anionic copolymer of 1,4-linked β-D-mannuronic acid and α-L-guluronic acid, can be used for encapsulation of a wide range of drugs, with minimal use of organic solvents.
Tamarind kernel powder, derived from the seeds of Tamarindus indica. A common and most important tree of India and Southeast Asia belongs to the family Leguminosae. Tamarind kernel powder is composed of xyloglucan polysaccharide which is (1,4)-linked-β-D-glucan backbone chain, which has (1,6)-linked α-D-xylose branches that are partially substituted by (1,2)-linked β-D-galactoxylose. For use in drug-release studies polymer is partially degraded by β-galactosidase to eliminate 44% of the galactose residues.11)
Tamarind gels are non-toxic, cause no detectable damage to rectal mucosa and can be effectively used in rectal administration of drugs, are subject to extensive first pass metabolism.12) Clotrimazole-loaded polaxamer-propylene glycol based suppository which was effective rectal dosage form for the treatment of tumours and found to decrease the hepatotoxicity compared to oral administration in rat.13) Microcapsules of theophylline and oxyphenbutazone were prepared with different ratios of polyethylene to ethyl cellulose and incorporated in polyethylene glycol suppositories.14) Mucoadhesive microspheres based on gelatin and its admixtures with porcine mucin were enhanced the rectal availability of cefuroxime sodium through entrapment into the microspheres and also provides alternative route for administration.15) Ketoprofen chitosan granules loaded polyethylene glycol suppository was examined and compared with conventional form in rabbits.16)
Extensive survey of literature and patent databases did not reveal any microparticles loaded suppository formulation developed of flutamide. Based on these considerations, the main objectives of the present study were: i) To develop flutamide microparticles by ionic gelation method using natural hydrophilic polymers sodium alginate and tamarind kernel powder in combination. ii) To characterise the microparticles formulation in terms of size, entrapment efficiency, morphology, and physicochemical properties using X-ray diffraction (XRD), differential scanning calorimetry (DSC). iii) To develop suppository of optimised microparticles formulation. iv) To carry out in-vivo study.
ExperimentalMaterialsFlutamide was obtained as a gift sample from Cipla Ltd. (Mumbai, India). Tamarind kernel powder (TKP) was obtained from Hindustan Gums and Chemicals Pvt. Ltd. (Bhiwani, India).
Sodium alginate and calcium chloride were obtained as gratitude samples from Thomas Baker Chemicals Pvt. Ltd. (Mumbai, India) and Poloxamer 407 (P407) and poloxamer 188 (P188) from Mylan Laboratories (Nasik, India). Freshly excised rectal cavity was obtained from the local butcher shop (Nasik, India). Methanol and acetonitrile used was HPLC grade from Rankem Ltd., India. Water used was HPLC grade generated from Milli-Q purification system. All other chemicals were of reagent grade and were used as such.
MethodsPreparation of Microparticles by Ionic Gelation MethodAqueous solution of sodium alginate (1–3%, w/v) containing 1% aqueous gel of TKP and flutamide (1%, w/v) was added manually drop wise into calcium chloride (10%, w/v) solution through a syringe with a 26 gauge needle. The added droplets were retained in crosslinker solution for 15 min to complete curing reaction. The microparticles so prepared were collected by decantation technique and dried overnight at room temperature.17)
Experimental DesignThe preparation of flutamide-loaded alginate tamarind microparticles was carried out using Design Expert 8.0.7.1 software by one factorial design of response surface methodology. The concentration of sodium alginate was selected as independent variable based on preliminary studies. All other processing variables were kept invariant throughout the study. % Production yield, % entrapment efficiency and % mucoadhesive strength were taken as response variables. In all, 7 experimental runs were carried out as shown in Table 1.
| Formulation code | Tamarind kernel powder (TKP) (% w/v) | Sodium alginate (% w/v) | Y1 (% production yield) | Y2 (% EE) | Y3 (% mucoadhesive strength) |
|---|---|---|---|---|---|
| T1 | 1 | 0.5 | 90.033 | 92.773 | 93.333 |
| T2 | 1 | 1.0 | 93.104 | 94.969 | 94.646 |
| T3 | 1 | 0.0 | 81.195 | 96.039 | 90.667 |
| T4 | 1 | 1.0 | 90.317 | 95.470 | 93.333 |
| T5 | 1 | −1.0 | 85.400 | 83.242 | 81.333 |
| T6 | 1 | −0.5 | 78.087 | 82.793 | 90.667 |
| T7 | 1 | −1.0 | 88.683 | 81.803 | 85.333 |
Sodium alginate −1.0=1% w/v; −0.5=1.5% w/v; 0.0=2% w/v; 0.5=2.5% w/v; 1.0=3% w/v.
Production yields were determined using following formula.
 |
Fifty milligram sample of each batch of flutamide-loaded alginate tamarind microparticles was triturated in mortar and shaken vigorously with 50 mL of methanol and left for 12 h for complete drug extraction. This dispersion was then filtered through membrane filter, diluted to desired concentration with water and analyzed spectrophotometrically at 227 nm. % EE was calculated by using following formula.
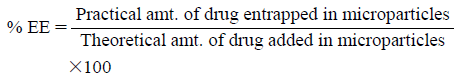 |
A freshly excised goat rectal mucosa was obtained from local slaughterhouse and was rinsed with normal saline. The mucosa was then pinned onto a polyethylene support inclined at an angle of 60°. A 25 number (No) of counted flutamide-loaded alginate tamarind microparticles formulated with various ratios of the polymers were placed on the trough of the mucosal surface, were allowed to hydrate with water for 15 min. A 50 mL of pH 6.8 phosphate buffer was allowed to flow over the tissue at the rate of 40 drops/min.18,19) The number of the microparticles adhering on mucosal surface (Ns) was counted. The adhesive strength was determined using formula given below.20)
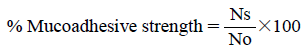 |
Optimized batch of flutamide-loaded alginate tamarind microparticles equivalent to 60 mg of flutamide was weighed accurately and subjected to release rate study in pH 6.8 phosphate buffer using USP dissolution test apparatus II (Model: Tablet Dissolution Test Apparatus, Labindia) with autosampler. Flutamide was used as control and subjected to release rate study by weighing 60 mg of it. Samples were periodically withdrawn and replaced with same volume of fresh buffer solution, and assayed using a spectrophotometer at 227.7 nm.
Particle Size AnalysisParticle size was analyzed by dispersing microparticles in immersion oil. Analysis was carried out using Motic DMW2-223 digital microscope (Motic Instruments Inc. Canada) equipped with a 1/399 CCD camera imaging accessory and computer controlled image analysis software (Motic images 2000, 1.3 version).
Zeta Potential StudyThe dispersion of microparticles in distilled water was filled in zeta cell and placed in the Zeta Sizer (Nano ZS90, Malvern Instruments, U.K.).
Scanning Electron Microscopy (SEM)The surface morphology of the optimized flutamide-loaded alginate tamarind microparticles was studied using a scanning electron microscope (JSM 6390, JEOL) operated at an accelerating voltage of 10 kV.
DSCThe thermal behavior of pure drug, flutamide-loaded alginate tamarind microparticles and blank microparticles were studied using a differential scanning calorimeter (Mettler-Toledo AG Analytical, Switzerland) at a heating rate of 10°C/min. The measurements were performed at a heating range of 40–300°C under nitrogen atmospheres.
XRDX-Ray diffractogram of pure drug, blank microparticles and flutamide-loaded alginate tamarind microparticles were recorded by Philips-PW-1050 scanner (PANalytical, the Netherlands) with filter Ni, Cu-Kα radiation, voltage 40 kV and a current of 30 mA. All samples were measured in the diffraction angle (2θ) range between 10°to 80° and 0.020 step size.
Formulation of Flutamide-Loaded Alginate Tamarind Microparticles Loaded SuppositoryOptimised batch of flutamide-loaded alginate tamarind microparticles was developed as suppository using different proportion of suppository bases Poloxamer 407–Poloxamer 188 (99 : 1, 98 : 2, 97 : 3). Suppository contains 5% microparticles and 95% suppository base. P407–P188 were mixed in different proportion and heated upto 55°C. Flutamide loaded alginate tamarind microparticles were then slowly added to the solution with continuous agitation. The resulting solution was then placed into the suppository mould and cooled down to 25°C.21)
In Vitro Release Study Flutamide-Loaded Alginate Tamarind Microparticles Loaded SuppositoryEach suppository containing required amount of flutamide-loaded alginate tamarind microparticles with different proportions of P407–P188 as 99 : 1, 98 : 2, 97 : 3 was inserted into semi permeable membrane tube. Both sides of the tube were closed by tying a thread to prevent leakage and then subjected to release rate study in pH 6.8 phosphate buffer using USP dissolution test apparatus II (Model: Tablet Dissolution Test Apparatus, Labindia) with autosampler. Sampling volume of 5 mL was withdrawn at predetermined time intervals and replaced by equal volumes of fresh dissolution medium. The drug concentration in the sample was determined from the standard curve of the drug in pH 6.8 phosphate buffer at 227.7 nm.
Kinetic Modelling of Release DataIn order to understand the drug release mechanisms from the polymer system, the power law (Peppas equation) is used to analyse the results.
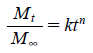 |
Stability study of optimized formulation of flutamide-loaded alginate tamarind microparticles was carried out for 3 months at 40±2°C/75±5%RH as per ICH guidelines. Microparticles of optimized batch were placed in sealed vials which were then stored at 40°C/75%RH for 90 d in stability chamber. The physical properties, % EE and drug release rate of the optimized batch were determined 1, 2, and 3 months interval.
For short term stability study of suppository, the suppositories were individually wrapped in aluminum foil and packed in cardboard boxes and were kept at refrigeration temperature (4°C) for 6 weeks. Samples are taken after 6 weeks for physical appearance and drug content estimation.24)
In Vivo StudyMale Sprague-Dawley Rats with body weights ranging from 350–400 g were fasted for 36 h before the experiment with free access to water. The animals were classified into 2 groups with 5 animals in each group. The protocol for this investigation was approved by the Institutional Animal ethics committee in accordance with the disciplinary principles and guidelines of CPCSEA (Committee for the purpose of control and supervision of experiments on animals). Oral suspension of flutamide and flutamide tamarind alginate microparticles loaded suppository with equivalent dose (15 mg/kg) were given separately to each group. After administration of different formulations blood sample (0.5 mL) will be collected through retro-orbital plexus at time intervals of 0, 30 min, 1, 2, 3, 4, 5 and 24 h.3,4)
The whole blood samples were centrifuged to obtain plasma, which was analysed for flutamide. The plasma was spiked with 0.4 mL of ethyl acetate, centrifuged at 4°C at 10000 rpm. After centrifugation, organic layer was isolated and evaporated under nitrogen. The tubes were reconstituted with respective mobile phases and the samples were used for chromatographic analyses. HPLC conditions were optimised to reduce interferences with other plasma proteins, if any. Chromatographic analyses were carried out using acetonitrile and 0.05 M KH2PO4 pH 4.5 (60 : 40) as the mobile phase, at 238 nm at flow rate of 1 mL/min. Column used was Kromasil C18, 150×4.6 mm.25)
Results and DiscussionFormulation of Flutamide-Loaded Alginate Tamarind MicroparticlesIt has been reported that polymers with positively or negatively charged groups interact with molecules of opposite charges to form three-dimensional (3-D) networks. The reaction of TKP and sodium alginate, with multivalent cation like calcium chloride (cation crosslinker) allows the formation of bridges between the polymeric chains and results in inter-cross-linking (by electrostatic interaction) between the polymer molecules, which might have eventually resulted in efficient adsorption of polymer (hydrophilic) on drug particles. Hence, in the present work, we prepared different polymeric-based microparticulate systems in order to improve the solubility, dissolution properties and hence absorption of flutamide.
Optimization Data Analysis and Model-ValidationFitting of Data to the ModelIn one factorial design different concentrations of sodium alginate (1, 1.5, 2, 2.5, 3%) were utilized. The ranges of responses Y1, Y2 and Y3 were 78.087–93.1, 81.803–96.04 and 81.33–94.66%, respectively (Table 1). All the responses observed for seven formulations prepared were fitted to various models using Design-Expert software. It was observed that the best fitted models were cubic for both production yield and entrapment efficiency, and linear for mucoadhesion strength. The values of R2, adjusted R2, predicted R2, standard deviation (S.D.) and %CV are given in Table 2 along with the regression equation generated for each response. The results of ANOVA for the dependent variables demonstrate that the model was significant for all response variables.
| Models | R2 | Adjusted R2 | Predicted R2 | S.D. | % CV |
|---|---|---|---|---|---|
| Response Y1 (% production yield) | 0.9581 | 0.9162 | 0.8276 | 1.40 | 1.62 |
| Cubic model | |||||
| Eqh Y1=80.91+11.72X+7.94X2−9.88X3 | |||||
| Response Y2 (% EE) | 0.9750 | 0.9500 | 0.7684 | 1.47 | 1.64 |
| Cubic model | |||||
| Eqh Y2=91.13−19.76X−0.444X2+26.05X3 | |||||
| Response Y3 (% mucoadhesion strength) | 0.7137 | 0.6564 | 0.4634 | 2.85 | 3.17 |
| Linear | |||||
| Eqh Y3=89.90+4.74X |
A numerical optimization technique with desirability approach was used to select the desired polymer combination under the constraints of maximizing both entrapment efficiency as well as mucoadhesion strength. Formulation with tamarind gum–sodium alginate (1 : 3) ratio has production yield 93.104%, entrapment efficiency 94.969% and mucoadhesion strength 94.646% with desirability of 0.961 was optimized.
Production Yield and Entrapment EfficiencyAs concentration of sodium alginate increases, production yield as well as % EE increases. This may be due to the greater and sufficient availability of alginate for cross linking with calcium binding sites. Decrease in concentration of sodium alginate results in insufficient cross linking with calcium binding sites and hence lowers both production yield and % EE.
% EE of the microparticles was dependent on aqueous solubility of drug, the concentration of sodium alginate, concentration and molecular weight of the mucoadhesive polymer used and size of microparticles. Also as flutamide is water insoluble, none or negligible diffusion of drug occurs in the aqueous calcium chloride solution which is used as manufacturing vehicle. Thus negligible amount of drug is leached during formulation and higher entrapment efficiency is achieved. Higher particle size also attributed to higher entrapment efficiency.
In Vitro Mucoadhesion StrengthMucoadhesion strength was determined to ensure the adhesion of formulation to the mucosa for a prolonged period of time at the site of administration. Results showed that flutamide-loaded alginate tamarind microparticles adequately adhered on rectal mucosa. Due to mucoadhesive properties of microparticles these would attach to the site of application, the mucous membrane of the lower rectum and do not reach to the end of the colon and hence avoids the drainage of the drug by the upper haemorrhoidal veins (leading to hepatic first pass effect).
Particle Size AnalysisSize ranges from 889.58–994.92 µm. Size of extrusion device and the viscosity of polymer solution were mainly affecting the particle size of microparticles prepared by ionic gelation method. Increase in the concentration of polymer, increases the viscosity of polymer solution, formed larger droplets and produce microparticles with large particle size. Decrease in concentration of polymer decreases the viscosity of polymer solution henceforth decreases particle size. Other researchers have reported similar results.26,27) Increasing the size of extrusion device increased the particle size of microparticles. Needle no. 26 was found suitable for the formulation of microparticles.
Zeta Potential StudyThe zeta potential of flutamide-loaded alginate tamarind microparticles and blank microparticles were −7.76 and −10, respectively. The microparticles prepared were negatively charged, indicating the presence of anionic polymer at the surface of all microparticles. Increase in zeta potential observed from blank to drug loaded microparticles i.e., from −10 to −7.76. Due to interaction between anionic moiety of TKP and alginate with cationic moiety of flutamide during microparticle formulation, increase in zeta potential may observed in drug loaded microparticles. Strong anionic charge on the polymer is one of the required characteristics for mucoadhesion.28)
Surface MorphologySEM analysis showed irregular shaped microparticles with rough and intact surface (Fig. 1). The rough surface may impart strong adhesion.18)

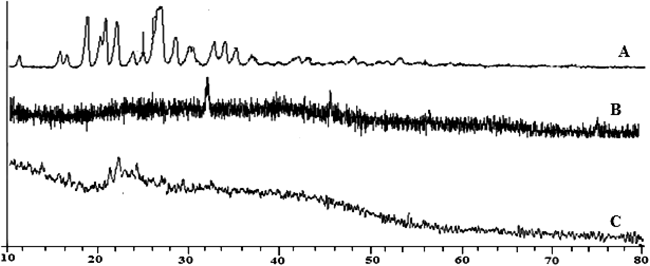
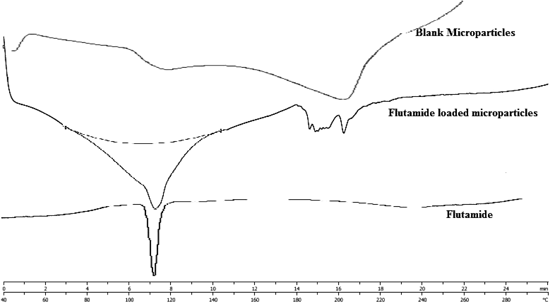
X-Ray diffractogram of flutamide indicates crystalline nature of drug. In contrast the blank and flutamide-loaded alginate tamarind microparticles, absence of crystalline peaks of drug in blank microparticles whereas presence of some peaks in drug loaded microparticles indicates the evidence of microencapsulation of drug with polymer (Fig. 2).
The thermogram of flutamide exhibited a sharp endothermic peak at 112.82°C indicated melting point which was reported in the literature. Characteristic sharp peak of flutamide was disappeared in the Flutamide loaded alginate tamarind microparticles. DSC studies revealed that flutamide were molecularly dispersed inside of the microparticles (Fig. 3).
Drug release from Flutamide loaded alginate tamarind microparticles was due to penetration of water in the matrix, hydration, swelling, diffusion of the dissolved drug (polymer hydro fusion), and/or the erosion of the gelatinous layer. Alginate degraded rapidly due to exchange of Ca2+ ions binding to carboxyl groups of alginate and phosphate ions of the medium.29) Considerable increase in dissolution rate of all suppositories was attributed to surfactant nature of poloxamers. Hydrophilic portions of the poloxamers probably contribute to increase the dissolution of flutamide. Due to the amphiphilic structure of poloxamers, these are developing the micellar core surface area accessible to flutamide molecules.30) However, different combinations of poloxamers as suppository bases did not show significant influence on the dissolution rates of flutamide from suppositories (Fig. 4).
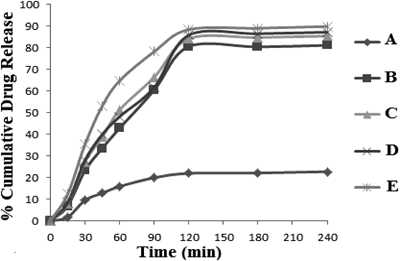
In vitro release study of flutamide-loaded alginate tamarind microparticles and microparticles loaded suppositories showed time independent release of drug. Higher values of ‘n’ (>0.89) represent Super Case II-type drug release and suggest that polymer relaxation occurs throughout the entire dissolution period (Table 3). This type of drug transport mechanism is associated with stresses in hydrophilic polymers which swell in water and biological fluids.
| Dissolution kinetic parameters | ||
|---|---|---|
| Formulation | Release exponent (n) | Correlation coefficient (r) |
| Flutamide loaded alginate tamarind microparticles | 1.124 | 0.9657 |
| Flutamide microparticles loaded suppository 1 P407/P188 (99/1%) | 1.094 | 0.9481 |
| Suppository 2 P407/P188 (98/2%) | 1.052 | 0.9506 |
| Suppository 3 P407/P188 (97/3%) | 0.9732 | 0.9254 |
Stability studies were carried out for optimized batch of flutamide-loaded alginate tamarind microparticles as per ICH guidelines. The entrapment efficiency of optimized batch of flutamide-loaded alginate tamarind microparticles after 3 months (91.034%) indicated that the drug was retained within the microparticles throughout the stability period. There was no change in drug content and indicated no significant change in the physical properties. The microparticles remained stable even after exposing to stress conditions of temperature and moisture. Stability studies of suppository showed that there was no significant change in physical characteristics and drug content of suppository after storing 6 weeks at refrigeration temperature.
Comparison of Plasma Concentration–Time Profiles of Flutamide after Administration of Oral Suspension and Flutamide Tamarind Alginate Microparticles Loaded SuppositoryPharmacokinetics parameters of flutamide after administration of oral suspension and suppository are shown in Table 4. Plasma flutamide levels after oral suspension reached a maximum in about 3 h and then declined rapidly. Plasma flutamide levels after 24 h of administration were as low as 0.4 µg/mL in average. After rectal suppository, plasma flutamide levels reached a maximum in 2–4 h (0.878–1.366 µg/mL in average) and declined slowly. Plasma flutamide levels after 24 h of administration were 0.728 µg/mL in average nearly half of Cmax. Increased plasma concentration after rectal administration of microencapsulated drug in the form of suppository could be attributed to avoidance of hepatic first pass effect, which was consequence of retaining the drug in the lower rectum i.e., by preventing the upward migration of the suppositories in the rectum by the aid of mucoadhesion. Higher absorption can also reveal the solubility-improving effect of microparticles. Area under curve (AUC) for oral suspension and flutamide tamarind alginate microparticles loaded suppository was found to be 15.58 and 18.35 µg·h/mL respectively. This elicits an increase in absorption from suppository than the oral suspension.
| Parameters | Flutamide oral suspension | Flutamide alginate tamarind microparticles loaded suppository |
|---|---|---|
| Cmax (µg/mL) | 0.859 | 1.711 |
| Tmax (h) | 3.5 | 4 |
| AUC (µg·h/mL) | 15.58 | 18.35 |
In this study we developed novel poloxamer suppository formulation for prostate cancer therapy by entrapment of flutamide into hydrophilic alginate tamarind microparticles. The developed novel poloxamer suppository gave significantly higher initial plasma concentrations, Cmax and AUC of flutamide than oral suspension, indicating that the drug could be more absorbed than that from oral suspension in rats. The developed microparticles loaded poloxamer suppository could be useful formulation for rectal administration of drugs which undergo extensive first pass metabolism or show hepatic side effects and which have low aqueous solubility and poor permeability.
The authors are grateful to Cipla Ltd., Mumbai, India for providing gift sample of flutamide.
The authors declare no conflict of interest.