2015 Volume 63 Issue 11 Pages 935-944
2015 Volume 63 Issue 11 Pages 935-944
As one of our projects, we here report some new molecular modifications of 2,4,6-trichloro-1,3,5-triazine (TCTAZ: 1) to symmetrical 2,4,6-trialkoxy- or 2,4,6-triaryloxy-substituted 1,3,5-triazine (TAZ) molecules, as well as the results of anti-herpes simplex virus type 1 (anti-HSV-1) activity evaluation of synthesized 2,4,6-trisubstituted TAZ derivatives. Among the tested 2,4,6-trisubstituted TAZ derivatives, we reconfirmed that a C3-symmetrical TAZ derivative, 4e, shows the highest level of anti-HSV-1 activity with a good selectivity index. In this paper, we also report the results of the preparation of newly targeted TAZ derivatives and the structure–activity relationships (SARs) of these trialkoxy-substituted TAZ derivatives and related compounds. The sugar recognition properties of C3-symmetrical TAZ derivative 4e are also described.
The glycocalyx at the cell surface, containing glycoproteins, proteoglycans and glicolipids, plays an important role in various cell-to-cell communications. Specific interactions of these carbohydrates with lectins (protein receptors) are important biological processes, including the processes of bacterial or viral infection and tumor metastasis.1–3)
From the viewpoint of molecular symmetry, many host receptors that consist of homo-oligometric units (homo-multiligands) often construct symmetric macromolecule architectures such as C2- or C3-symmetrical geometry receptor systems. These phenomena of macromolecules connected with many biological stages have encouraged scientists to develop new multivalent symmetrical synthetic molecules to find new bioactive compounds or leads. Results of many works related to the above conception have been published over the past few decades.4–7) The terms identical twin-drugs and triplet-drugs (symmetrical bivalent and trivalent molecules) are now commonly used in medicinal chemistry and related scientific fields. In connection with our synthetic works on such symmetrical molecules, we have already designed and synthesized a few new symmetrical molecules and evaluated their bioactivities in order to find new types of bioactive compounds.8–17)
In connection with the above projects, we have recently reported some molecular modifications of 2,4,6-trichloro-1,3,5-triazine (TCT AZ) (1) to symmetrical 2,4,6-trisubstituted 1,3,5-triazine (TAZ) molecules and the results of biological evaluation of synthesized symmetrical 2,4,6-trisubstituted TAZ derivatives.8,9) Among previously targeted trisubstituted TAZ derivatives, we found that a C3-symmetrical TAZ derivative with three isopropoxy groups on a TAZ template such as compound 4e showed a high level of anti-herpes simplex virus (HSV)-1 activity and low cytotoxity, and it therefore seemed to be a potential lead in the search for preferred anti-HSV-1 activity with a good selectivity index (SI).
In this paper, we report the results of further preparation of newly targeted trisubstituted TAZ derivatives together with the results of biological evaluation of synthesized 2,4,6-trisubstituted TAZ derivatives. We also describe the structure–activity relationships (SARs) of 2,4,6-trialkoxy- or 2,4,6-triaryloxy-substituted TAZ derivatives and related compounds.
The main targeted symmetrical 2,4,6-trialkoxy-substituted TAZ derivatives with a TAZ template were prepared by a procedure with TCT AZ (1) as a starting material. The overall process for the preparation of target molecules involving three nucleophilic substitution reaction stages in one pot is shown in Chart 1. Preparation of all of the targeted symmetrical alkoxy- and/or aryloxy-trisubstituted TAZ derivatives (4) was conducted by nucleophilic substitutions of TCT AZ (1) with an alcohol (b–iH) or a phenol derivative (j–lH) (Chart 1). As we reported previously,8,9) the reactivity of chloro-substituted triazines was gradually decreased by the accumulation of electron-donating alcohol substituents (as with amine substituents). For the preparation of intermediate monoalkoxy-substituted TAZ (2) by alcohols (bH, e–iH), the following procedure is conventional. Thus, easy access for the target intermediates 2 was achieved from TCT AZ (1) with collidine as a base (Method C) under mild conditions (0°C to room temperature within 1 h). The generation of dialkoxy-substituted TAZ (3) needed harder reaction conditions (reflux for 1 d, and so on), especially for reactions with secondary alcohols. However, the use of n-BuLi in these reactions was effective for the formation of intermediate-disubstituted TAZ (3) and final trisubstituted TAZ derivatives (4). We consider that the generated alkoxide anions (RO−) may act as better nucleophiles in the substitution reactions. The reactions with n-BuLi are sometimes accompanied by the formation of undesired butyloxy-substituted TAZ derivatives, and the separation of target TAZ derivatives was not facile by either chromatography or recrystallization.8) Therefore, we used an alternative method with NaH for generation of RO− (Method A) for the preparation of target molecules, according to the method reported by Spielman et al.18)

The results (yields, reaction conditions and isolated products) for the preparation of target C3-symmetrical trisubstituted TAZ derivatives (4b–l) are summarized in Table 1. In order to find an alternative method to obtain trialkoxy TAZ (4f), we tried a procedure using triethylamine (TEA)19) (Method B, Entry 6); however, only undesired by-products (7–9) were obtained. This reaction can be assumed to result in the formation of quaternary N-triazinylammonium salts as reactive intermediates from the substitution reaction of TEA.20,21) Therefore, most of the reactions of TCT AZ (1) with various alcohols were performed by Method A, and trialkoxy TAZ derivatives (4g–i) were easily isolated by recrystallization after work-up in 35–45% yields (Entries 7–9).
 | |||||
|---|---|---|---|---|---|
| Entry | ROH | Method | Ratio of 1 : ROH : (Additive) | Conditions | Products (yield %)a) |
| 1 |  | A | 1 : bH : (NaH)=1 : 3.3 : (3.15) | 1) reflux 5 h, N2 | 4b (41) |
| 2) reflux 1 h, N2 | |||||
| 2 | 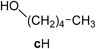 | A | 1 : cH : (NaH)=1 : 4.5 : (3.15) | 1) rt 15 min to reflux 4 h, Ar | 4c (74) |
| 2) reflux 1 h, Ar | |||||
| 3 |  | A | 1 : dH : (NaH)=1 : 4.5 : (3.15) | 1) rt 0.5 h to reflux 19 h, N2 | 4d (69) |
| 2) rt 20 min to reflux 1 h, N2 | |||||
| 4 | 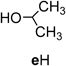 | A | 1 : eH : (NaH)=1 : 4.5 : (3.15) | 1) rt 15 min to reflux 1 h, N2 | 4e (77) |
| 2) rt 15 min to reflux 1 h, N2 | |||||
| 5 |  | A | 1 : fH : (NaH)=1 : 4.5 : (3.15) | 1) reflux 15 h, N2 | 4f (72) |
| 2) reflux 1 h, N2 | |||||
| 6 | B | 1 : fH : (TEA)=1 : 5 : (3.5) | rt 19 h, Ar, THFc) | 7 (30), 8 (8) | |
| 7 |  | A | 1 : gH : (NaH)=1 : 3.3 : (3.15) | 1) rt 0.5 h to reflux 12 h, N2 | 4g (38)b) |
| 2) rt 10 min to reflux 1 h, N2 | |||||
| 8 |  | A | 1 : hH : (NaH)=1 : 4.5 : (3.15) | 1) reflux 19 h, N2 | 4h (35)b) |
| 2) reflux 1 h, N2 | |||||
| 9 |  | A | 1 : iH : (NaH)=1 : 4.5 : (3.15) | 1) rt 15 min to reflux 14 h, N2 | 4i (45)b) |
| 2) rt 20 min to reflux 1 h, N2 | |||||
| 10 |  | C | 1 : jH : (collidine)=1 : 3.1 : (3.1) | rt 2 d | 4j (95) |
| 11 |  | B | 1 : kH : (TEA)=1 : 3.1 : (3.1) | rt 18 h, acetonec) | 4k (74) |
| 12 |  | B | 1 : lH : (TEA)=1 : 3.1 : (3.1) | reflux 19 h to reflux 0.5 h, CH2Cl2c) | 4l (40) |
a) Yield obtained from TCTAZ (1). b) Yield obtained after recrystallization. c) Solvent used.
In the case of reactions of TCT AZ (1) with some phenol derivatives (j–lH), the target C3-symmetrical derivatives (4j–l) were obtained by the procedures using TEA (Method B) and collidine (Method C). Results of our synthetic trials with phenol derivatives for targeted C3-symmetrical tri-substituted TAZ derivatives are also shown in Table 1.
We also synthesized CS-symmetrical aminoaryloxy-substituted TAZ derivatives (5, 6 in Chart 2) from the reactions of TCT AZ, phenol (kH or lH), and 4-piperidinemethanol (pH) stepwise using Method C with collidine in Step 1 (see Experimental).

As a result, we revealed that the reactions of TCT AZ (1) with various alcohols or phenols (b–lH) provide a conventional procedure for preparation of targeted C3-symmetrical trisubstituted TAZ molecules (4b–l). Some other isolated unexpected trisubstituted TAZ derivatives (7, 8) as by-products are also shown in Table 1.
Two CS-type compounds (4dde and 4ggg) in which one of the three alkoxy groups in the C3-type molecules 4d and 4g was replaced by one isopropoxy group were also prepared from reactions of 2e for a comparison of their geometrical features and antiviral activities (Chart 3 and see Experimental).

All structures of the synthesized compounds were easily confirmed by spectroscopic and analytical data. The geometries of symmetrical structures of target TAZ derivatives described in this article were also confirmed by 13C-NMR spectroscopic data (see Experimental for details).
The structures of targeted C3- and a few CS-symmetrical 2,4,6-trisubstituted TAZ derivatives obtained from TCT AZ and the results of their biological evaluations [anti-HSV-1 activities (EC50) by plaque reduction assays22) and cytotoxicities against Vero cells (IC50)] are summarized in Table 2 together with data for aciclovir.23) Calculated log P values24) for the compounds are also shown in Table 2. There was no distinct correlation between log P values and EC50 values or between log P values and IC50 values among the compounds listed in Table 2.
| Compound | EC50 (µM) | IC50 (µM) | Log Pa) | Compound | EC50 (µM) | IC50 (µM) | Log Pa) | ||
|---|---|---|---|---|---|---|---|---|---|
 | 4a | >100 | >100 | 1.39 |  | 4l | 85.0 | >200 | 9.15 |
 | 4b | >100 | >200 | 0.93 |  | 5jppc) | >6.3 | 42.2 | 3.52 |
 | 4c | >100 | >100 | 6.37 |  | 6jjpb) | 32.2 | 303.6 | 4.62 |
 | 4d | >100 | >100 | 8.87 |  | 5kpp | >25 | 25.5 | 4.67 |
 | 4eb) | 1.87 | 479.8 | 3.36 |  | 6kkp | >25 | 8.1 | 6.91 |
 | 4f | >100 | >200 | 6.28 |  | 5lpp | 35.4 | 37.1 | 4.67 |
 | 4g | >100 | >100 | 4.78 | 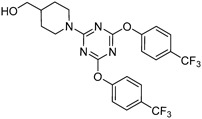 | 6llp | 63.9 | >200 | 6.91 |
 | 4h | >100 | >100 | 6.04 |  | 4dde | >100 | >200 | 7.04 |
 | 4i | >100 | >200 | 7.29 |  | 4egg | >100 | >200 | 4.31 |
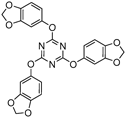 | 4j | >100 | >200 | 5.72 |  | 7 | >100 | >200 | 5.2 |
 | 4k | >100 | >200 | 9.15 | Aciclovir d) | 1.1 | >444 | −0.76 |
In supramolecular interactions of glycocalyx at the cell surface with trialkoxy TAZ derivatives, all three oxygen atoms of alkoxy groups on the TAZ template and three TAZ ring nitrogen lone pairs are expected to display the remarkable characteristics of hydrogen bonding acceptors or a Lewis base. We considered that a high level of antiviral activity of compound 4e is probably produced by the unique symmetrical structure and chemical property.
For the strategy of molecular modification, we considered a step-by-step modification approach for determining the relationship between the geometry and nature of the alkoxy group. C3-symmetrical molecule A, which has three 4-hydroxymethylpiperidine groups on the TAZ template, was used for a key starting structure for three-stage modification [A→B (CS-symmetry)→C (CS-symmetry)→D (C3-symmetry)] (see Chart 4). We carried out step-by-step introduction of the same alkoxy or related aryloxy functionality into the template instead of a 4-hydroxymethylpiperidine group involved in the key starting C3-symmetrical lead A.

For modification to the targeted molecular structure D, we carried out a few simple modifications of a C3-symmetrical TAZ derivative 4e with three isopropoxy groups on a TAZ template to other C3-symmmetrical trialkoxy TAZ derivatives such as compounds 4a–i. However, none of the C3-symmetrical trialkoxy-substituted TAZ molecules in which three isopropoxy moieties in the TAZ template had been replaced with other different alkoxy groups showed significant anti-HSV-1 activity at a dose of 100 µM, indicating no enhancement effect of this modification. Thus, C3-type compounds (4a–d) having aliphatic-chain alkoxy groups on the TAZ template showed no significant anti-HSV-1 activity at a dose of 100 µM. The size of cycloalkyloxy groups consisting of C5–C7 carbons (C3-type compounds 4g, 4h, and 4i) was also not a preferred structural feature for antiviral activity of C3-symmetrical trialkoxy-substituted TAZ derivatives. The trialkoxy-substituted C3-type derivatives prepared in this study, except for C3-symmetrical lead 4e, were inactive.
Comparing symmetrical geometric features, two CS-type compounds (4dde and 4egg) in which one of the three alkoxy groups in the C3-type molecules 4d and 4g is replaced by one isopropoxy group also showed no biological activities (anti-HSV-1 activity or cytotoxic activity) at a dose of 100 µM. Furthermore, two CS-type compounds (5epp8) and 6eep8) having one or two isopropoxy groups in the template A were also inactive at a dose of 100 µM (see ref. 8).
Regarding triaryloxy-substituted TAZ derivatives, three C3-type compounds (4j–l) showed lower levels of biological activitiy (anti-HSV-1 activity or cytotoxic activity) than those of the corresponding CS-type molecules (B: 5jpp,9) 5kpp, and 5lpp and C: 6jjp,8) 6kkp, and 6llp). However, these CS-symmetrical derivatives were not superior to the C3-type lead 4e in terms of both anti-HSV-activity and SI value. Derivatization to these C3-type compounds with the same three alkoxy substituents on the TAZ template was also found to be not effective for increasing anti-HSV-1 activity.
Through these modifications described above, we unfortunately could not find more antiviral active trialkoxy-substituted and triaryloxy-substituted C3-symmetrical TAZ derivatives than the original compound 4e.8) These results indicate that the molecule 4e is the best structural feature for anti-HSV-1 activity in the present modifications.
In calorimetric experiments for sugar recognition of C3-symmetrical antiviral active lead 4e, a few of the binding reactions that we have tried with sugar derivatives (see Experimental) were exothermic. Binding reactions with some monosaccharides obtained by calorimetric experiments showed interesting binding properties as listed in Table 3.
| Entry | Sugar* | Stoichiometry of binding (4e : Sugar) | Ka [M−1] | ΔG [kJ mol−1] | ΔH [kJ mol−1] | −TΔS [kJ mol−1] |
|---|---|---|---|---|---|---|
| 1 | MeO-α-Gal | 1 : 1 | 21,500 | −24.7 | −3.44 | −21.3 |
| 2 | MeO-β-Gal | 1 : 1 | 5,350 | −21.3 | −8.03 | −13.2 |
| 3 | MeO-α-Man | ND** | ND** | ND** | ND** | ND** |
| 4 | MeO-β-Man | 1 : 1 | 126,000 | −29.1 | −4.93 | −24.2 |
* MeO-α/β-Gal and MeO-α/β-Man stand for methyl α/β-D-galactopyranoside and methyl α/β-D-mannopyranoside, respectively. ** Not determined.
The Ka values of compound 4e were 2.15×104 M−1 for methyl α-D-galactopyranoside (MeO-α-Gal), 5.35×103 M−1 for methyl β-D-galactopyranoside (MeO-β-Gal), and 1.26×105 M−1 for methyl β-D-mannopyranoside (MeO-β-Man), and each of the binding reactions had a 1 : 1 stoichiometry. The reaction with MeO-β-Man showed a high value of Ka and thermodynamic parameters of ΔG=−29.1 kJ/mol, ΔH=−4.93 kJ/mol, and −TΔS=−24.2 kJ/mol. However, for the reaction of compound 4e with MeO-α-Man, no heat of binding was detected and the thermodynamic parameters (ΔG, ΔH, and −TΔS) including Ka could not be determined by repeated isothermal titration calorimetry (ITC) experiments as shown in Table 3. From a comparison of the Ka values of C3-symmetrical compound 4e, the binding affinity for MeO-α-Gal was about 4-times stronger than that for MeO-β-Gal. In the reactions of compound 4e with the set of MeO-α/β-Man, MeO-β-Man showed a high binding constant (Ka=1.26×105 M−1), but that of the reaction with MeO-α-Man was not exothermic as shown by ITC experiments. Furthermore, in both reactions of compound 4e with the set of methyl α/β-D-glucopyranoside (MeO-α/β-Glc), no heat of binding was detected by repeated ITC experiments. Thus, the results obtained for compound 4e by ITC experiments indicated that the binding reactions were very sensitive to the structural features of a monosaccharide moiety. These results (properties) are particularly interesting because our previously synthesized highly cytotoxic symmetrical lectin-like compound E (Fig. 1) showed β-anomer selectivity in its binding reactions with a few monosaccharides including MeO-α/β-Gal and MeO-α/β-Glc.10) We now consider that the selectivity of the symmetrical small molecule 4e between a few monosaccharides and the behaviors of these different sensitivities of these carbohydrates are characteristic and that this carbohydrate recognition property may be responsible for its high anti-HSV-1 activity, providing a large SI value for compound 4e.

Among the symmetrical TAZ derivatives prepared in this study, none of the synthesized symmetrical TAZ derivatives showed higher levels of anti-HSV-1 activity than that of the 2,4,6-triisopropoxy derivative 4e. Although more precise evaluation regarding the peculiarity of three isopropoxy groups on the TAZ template is needed, the reported compatibility of the isopropyl group on a few benzene-based symmetrical artificial receptors25) with some monosaccharides seems to indicate the possibility of important hydrophobic interactions of this side chain as a recognition site for carbohydrates through a combination of hydrogen bonding, CH–π interaction and van der Waals contacts.26) The results of calorimetric experiments indicate that binding interactions of compound 4e with these sugars (MeO-α/β-Gal and MeO-β-Man) have both favorable hydrophobic interaction and hydrogen bonding formation. In addition, in calorimetric experiments of an anti-HSV-1 inactive C3-type molecule 4g that has three cyclopentyloxy groups on the TAZ template with MeO-α/β-Gal, no heat of binding was detected, indicating that the three isopropoxy groups in the C3-type compound 4e may play an important role in these exothermic binding interactions with carbohydrates such as MeO-α/β-Gal. Applications of C3-symmetrical functional receptor molecules in the area of molecular recognition have led to significant advances, and it is also expected that the use of C3-symmetrical molecules will lead to many developments in molecular recognition stages.27) Regarding carbohydrate recognition by C3-type symmetrical small molecules, we consider that the results obtained for diastereo-selective sugar recognition in some monosaccharides such as MeO-α/β-Gal by compound 4e may provide useful information for research on carbohydrates recognition, especially by an achiral small molecule with three-fold (C3-geometrical) symmetry.28)
On the basis of this information on sugar recognition of C3-symmetrical antiviral active TAZ derivative 4e, we are also planning to carry out further molecular modifications to the related trisubstituted TAZ derivatives with branched C3–C5 alkoxy groups similar to the isopropoxy group. Further details of an SAR study and additional calorimetric experiments including other prepared TAZ derivatives will be described separately.
Melting points were determined using a micro melting point apparatus (Yanaco MP-S3) without correction. IR spectra were measured by a Shimadzu FTIR-8100 IR spectrophotometer. Low- and high-resolution mass spectra (LR-MS and HR-MS) were obtained by a JEOL JMS HX-110 double-focusing model equipped with an FAB ion source interfaced with a JEOL JMA-DA 7000 data system. lH- and 13C-NMR spectra were obtained by JEOL JNM A-500. Chemical shifts were expressed in δ ppm downfield from an internal TMS signal for lH-NMR and the carbon signal of the corresponding solvent [chloroform-d3 (CDCl3) (77.00 ppm), methanol-d4 (CD3OD) (49.00 ppm)] for 13C-NMR. The abbreviations qu=quintet, dd=double doublets, and dm=double multiplets are used for the multiplicity of 1H-NMR data. The signal assignments were confirmed by two-dimensional (2D)-NMR analyses: 1H–1H 2D correlation spectroscopy (COSY), lH–l3C heteronuclear multiple-quantum coherence (HMQC), and 1H–l3C heteronuclear multiple-bond connectivity (HMBC). Microanalyses were performed with a Yanaco MT-6 CHN corder. Routine monitoring of reactions was carried out using precoated Kieselgel 60F254 plates (E. Merck). Open and flash column chromatography separations of the reaction products were performed on silica gel (Kanto 60N) with a UV detector. Commercially available starting materials and compound 4a were used without further purification, and dry solvents were used in all reactions.
General Procedure for the Preparation of Trialkoxy-1,3,5-triazine Derivatives (Method A18)): Example: Preparation of 2,4,6-Tris(1-heptyloxy)-1,3,5-triazine (4d) (Entry 3): (Step 1)To a solution of n-heptanol (dH, 5.23 g, 45.0 mmol) in dry benzene (10 mL) was added NaH (60% in mineral oil, 1.26 g, 31.5 mmol) at room temperature under an N2 atmosphere. A suspension of sodium n-heptyloxide was prepared by stirring at room temperature for 0.5 h and then refluxing for 19 h. (Step 2) After cooling to room temperature, compound 1 (1.84 g, 10.0 mmol) in dry benzene (20 mL) was added dropwise with stirring. After stirring for 20 min at room temperature, the reaction mixture was refluxed for 1 h. After cooling to room temperature, the resulting mixture was acidified with acetic acid (ca. 2 mL) and then diluted with water (ca. 20 mL). The separated organic layer was washed with water (ca. 20 mL) and dried over magnesium sulfate (MgSO4). Evaporation of the solvent gave a pale yellow residual oil. To this residue was added n-hexane (ca. 10 mL), and insoluble solids were removed. The filtrate was purified by flash chromatography (n-hexane : EtOAc=97 : 3→95 : 5) to give 4d (2.91 g, 69%) as a colorless oil.
4d: IR (NaCl) cm−1: 1566 (C=N) 1145, 1118 (C–O of ether). 1H-NMR (CDCl3) δ: 0.88 (9H, t, J=7.0 Hz, H7′), 1.26–1.37 (18H, m, H4′, 5′, 6′), 1.38–1.46 (6H, m, H3′), 1.75–1.81 (6H, m, H2′), 4.38 (6H, t, J=6.7 Hz, H1′). 13C-NMR (CDCl3) δ: 13.98 (C7′), 22.53 (C6′), 25.76 (C3′), 28.64 (C2′), 28.91 (C4′), 31.69 (C5′), 68.45(C1′), 173.16 (C=N). Positive-ion FAB-MS m/z: 424 (M+H+). HR-FAB-MS m/z: 424.3547 (Calcd for C24H46N3O3: 424.3539). Anal. Calcd for C24H45N3O3: C, 68.04; H, 10.71; N, 9.92. Found: C, 67.88; H, 10.88; N, 9.91.
2,4,6-Tris(2-methoxyethoxy)-1,3,5-triazine (4b) (Entry 1)This compound was prepared from the reaction of compound 1 with 2-methoxyethanol (bH) by using Method A under the conditions shown in Table 1. Separation of the products by flash chromatography (n-hexane : EtOAc=35 : 65→30 : 70) gave 4b (41%) as a pale yellow oil.
4b: IR (NaCl) cm−1: 1569 (C=N), 1151, 1122 (C–O of ether). 1H-NMR (CDCl3) δ: 3.40 (9H, s, OCH3), 3.72 (6H, t, J=4.9 Hz, H2′), 4.54 (6H, t, J=4.9 Hz, H1′). 13C-NMR (CDCl3) δ: 58.91 (OCH3), 67.23 (C1′), 70.04 (C2′), 172.97 (C=N). Positive-ion FAB-MS m/z: 304 (M+H+). HR-FAB-MS m/z: 304.1498 (Calcd for C12H22N3O6: 304.1509). Anal. Calcd for C12H21N3O6·0.2H2O: C, 49.96; H, 7.03; N, 13.69. Found: C, 49.95; H, 6.93; N, 13.72.
2,4,6-Tris(1-pentyloxy)-1,3,5-triazine (4c)18) (Entry 2)This compound was prepared from compound 1 and 1-pentanol (cH) (Method A) under the conditions shown in Table 1. Separation of the reaction products by flash chromatography (n-hexane : EtOAc=93 : 7→95 : 5) gave 4c (74%) as a colorless oil.
4c: IR (NaCl) cm−1: 1567 (C=N), 1144, 1116 (C–O of ether). 1H-NMR (CDCl3) δ: 0.91 (9H, t, J=7.0 Hz, H5′), 1.32–1.45 (12H, m, H3′, 4′), 1.75–1.82 (6H, m, H2′), 4.38 (6H, t, J=6.7 Hz, H1′). 13C-NMR (CDCl3) δ: 13.85 (C5′), 22.30 (C4′), 27.91 (C3′), 28.30 (C2′), 68.40 (C1′), 173.14 (C=N). Positive-ion FAB-MS m/z: 340 (M+H+). HR-FAB-MS m/z: 340.2601 (Calcd for C18H34N3O3: 340.2600). Anal. Calcd for C18H33N3O3·0.4H2O: C, 62.36; H, 9.83; N, 12.12. Found: C, 62.47; H, 9.87; N, 12.34.
Preparation of 2,4,6-Tris(2-propoxy)-1,3,5-triazine (4e)8,18) (Entry 4)This compound was obtained in 77% yield from the reaction of compound 1 with isopropanol (eH) by using Method A under the conditions shown in Table 1. Recrystallization from EtOH gave analytically pure 4e as colorless crystals, mp 106–108°C (from EtOH). Spectral data (IR, NMR, and MS) and elemental analysis data of the product 4e were consistent with those of the authentic sample.8)
2,4,6-Tris(3-pentyloxy)-1,3,5-triazine (4f)18) (Entry 5)This compound was prepared from compound 1 and 3-pentanol (fH) (Method A) under the conditions shown in Table 1. Separation of the reaction products by flash chromatography (n-hexane : EtOAc=98 : 2→95 : 5) afforded 4f (72%) as colorless solids. An analytical sample of 4f was obtained by recrystallization from water as colorless crystals.
4f: mp 66–67°C (from H2O). IR (KBr) cm−1: 1553 (C=N), 1137, 1100 (C–O of ether). 1H-NMR (CD3OD) δ: 0.95 (18H, t, J=7.5 Hz, CH3), 1.69–1.76 (12H, m, CH2), 5.08 (3H, qu, J=6.1 Hz, CH). 13C-NMR (CD3OD) δ: 9.84 (CH3), 27.33 (CH2), 82.30 (CH), 174.60 (C=N). Positive-ion FAB-MS m/z: 340 (M+H+). HR-FAB-MS m/z: 340.2602 (Calcd for C18H34N3O3: 340.2600). Anal. Calcd for C18H33N3O3: C, 63.68; H, 9.80; N, 12.38. Found: C, 63.53; H, 9.84; N, 12.35.
Reaction of Compound 1 with 3-Pentanol (fH): Formation of N2,N2,N4,N4-Tetraethyl-6-(pentan-3-yloxy)-1,3,5-triazine-2,4-diamine (7), N2,N2,N4,N4,N6,N6-Hexaethyl-1,3,5-triazine-2,4,6-triamine (8),20) and N2,N2,N4,N4-Tetraethyl-6-methoxy-1,3,5-triazine-2,4-diamine (9)29) (Method B) (Entry 6)To a solution of compound 1 (1.84 g, 10.0 mmol) in dry tetrahydrofuran (THF) (20 mL) was added 3-pentanol (fH, 4.41 g, 50.0 mmol) and TEA (3.54 g, 35.0 mmol) at room temperature in an atmosphere of argon. After stirring for 19 h at room temperature, the resulting precipitates (TEA·HCl salt) were removed by filtration. After evaporation of the solvent, the products were separated by flash chromatography (n-hexane : EtOAc=99 : 1→95 : 5) to give 8 (8%) as a colorless oil, 7 (30%) as a colorless oil, and 9 (2%) as a pale yellow oil.
7: IR (NaCl) cm−1: 1568 (C=N), 1225, 1096 (C–O of ether). 1H-NMR (CDCl3) δ: 0.93 (6H, t, J=7.5 Hz, H1″, 5″), 1.16 (12H, t, J=7.0 Hz, H2′), 1.60–1.76 (4H, m, H2″, 4″), 3.56 (8H, q, J=7.0 Hz, H1′), 4.97–5.02 (1H, m, H3″). 13C-NMR (CDCl3) δ: 10.03 (C1″, 5″), 13.35 (C2′), 26.72 (C2″, 4″), 41.24 (C1′), 78.23 (C3″), 165.62 (C2, 4), 170.90 (C6). Positive-ion FAB-MS m/z: 310 (M+H+). HR-FAB-MS m/z: 310.2577 (Calcd for C16H32N5O: 310.2607). Anal. Calcd for C16H31N5O·0.5H2O: C, 60.34; H, 10.13; N, 21.99. Found: C, 60.37; H, 10.11; N, 22.10.
8: 1H-NMR (CDCl3) δ: 1.14 (18H, t, J=7.0 Hz, H2′), 3.53 (12H, q, J=7.0 Hz, H1′). 13C-NMR (CDCl3) δ: 13.47 (C2′), 40.98 (C1′), 164.75 (C=N). Positive-ion FAB-MS m/z: 295 (M+H+). HR-FAB-MS m/z: 295.2611 (Calcd for C15H31N6: 295.2610).
9: 1H-NMR (CDCl3) δ: 1.16 (12H, t, J=7.0 Hz, H2′), 3.56 (8H, qu, J=7.0 Hz, H1′), 3.87 (3H, s, OCH3). 13C-NMR (CDCl3) δ: 13.26 (C2′), 41.28 (C1′), 53.27 (OCH3), 165.54 (C4, 6), 170.92 (C2). Positive-ion FAB-MS m/z: 254 (M+H+). HR-FAB-MS m/z: 254.1982 (Calcd for C12H24N5O: 254.1981).
2,4,6-Tris(cyclopentyloxy)-1,3,5-triazine (4g) (Entry 7)This compound was obtained from the reaction of compound 1 with cyclopentanol (gH) (Method A) under the conditions shown in Table 1. After work-up of the reaction mixture, crude orange solid material 4g was obtained. Recrystallization from EtOAc gave 4g (38%) as colorless crystals.
4g: mp 146–148°C (from EtOAc). IR (KBr) cm−1: 1561 (C=N), 1129 (C–O of ether). 1H-NMR (CDCl3) δ: 1.60 (6H, m, H3′, 4′), 1.78–1.90 (12H, m, H3′, 4′, 2′, 5′), 1.92–2.00 (6H, m, H2′, 5′), 5.46 (3H, qu, J=3.1 Hz, H1′). 13C-NMR (CDCl3) δ: 23.77 (C3′, 4′), 32.71 (C2′, 5′), 80.59 (C1′), 172.68 (C=N). Positive-ion FAB-MS m/z: 334 (M+H+). HR-FAB-MS m/z: 334.2130 (Calcd for C18H28N3O3: 334.2131). Anal. Calcd for C18H27N3O3: C, 64.84; H, 8.16; N, 12.60. Found: C, 64.71; H, 8.21; N, 12.54.
2,4,6-Tris(cyclohexyloxy)-1,3,5-triazine (4h) (Entry 8)This compound was obtained from compound 1 and cyclohexanol (hH) (Method A) under the conditions shown in Table 1. After work-up of the reaction mixture, crude pale yellow solid material 4h was obtained. Recrystallization from EtOH afforded 4h (35%) as colorless crystals.
4h: mp 230–233°C (from EtOH). IR (KBr) cm−1: 1561 (C=N), 1126, 1010 (C–O of ether). 1H-NMR (CDCl3) δ: 1.24–1.33 (3H, m, H4′), 1.35–1.44 (6H, m, H3′, 5′), 1.54–1.62 (9H, m, H4′, 2′, 6′), 1.76–1.83 (6H, m, H3′, 5′), 1.97–2.03 (6H, m, H2′, 6′), 5.03–5.10 (3H, m, H1′). 13C-NMR (CDCl3) δ: 23.79 (C3′, 5′), 25.39 (C4′), 31.58 (C2′, 6′), 76.14 (C1′), 172.71 (C=N). Positive-ion FAB-MS m/z: 376 (M+H+). HR-FAB-MS m/z: 376.2602 (Calcd for C21H34N3O3: 376.2600). Anal. Calcd for C21H33N3O3: C, 67.17; H, 8.86; N, 11.19. Found: C, 67.11; H, 8.87; N, 11.15.
2,4,6-Tris(cycloheptyloxy)-1,3,5-triazine (4i) (Entry 9)This compound was prepared from the reaction of compound 1 and cycloheptanol (iH) by using Method A under the conditions shown in Table 1. After work-up of the reaction mixture, crude yellow solid material 4i was obtained. Recrystallization from EtOH gave 4i (45%) as colorless crystals.
4i: mp 175–177°C (from EtOH). IR (KBr) cm−1: 1562 (C=N), 1129 (C–O of ether). 1H-NMR (CDCl3) δ: 1.44–1.51 (6H, m, H3′, 6′), 1.56–1.62 (12H, m, H4′, 5′), 1.69–1.76 (6H, m, H3′, 6′), 1.79–1.86 (6H, m, H2′, 7′), 2.01–2.07 (6H, m, H2′, 7′), 5.23 (3H, m, H1′). 13C-NMR (CDCl3) δ: 22.80 (C3′, 6′), 28.36 (C4′, 5′), 33.66 (C2′, 7′), 78.63 (C1′), 172.58 (C=N). Positive-ion FAB-MS m/z: 418 (M+H+). HR-FAB-MS m/z: 418.3075 (Calcd for C24H40N3O3: 418.3070). Anal. Calcd for C24H39N3O3: C, 69.03; H, 9.41; N, 10.06. Found: C, 69.08; H, 9.61; N, 10.07.
Procedure for the Synthesis of 2,4,6-(1,3-Benzodioxol-5-yloxy)-1,3,5-triazine (4j) (Method C) (Entry 10)To a solution of compound 1 (922 mg, 5.0 mmol) in dry acetone (25 mL) was added sesamol (jH, 2.14 g, 15.5 mmol) and collidine (1.88 g, 15.5 mmol) at room temperature. After stirring for 2 d at room temperature, the resulting precipitates were collected and the obtained material was added to CH2Cl2 (ca. 60 mL). The resulting mixture was stirred for 30 min at room temperature, and then the insoluble precipitates were collected and the obtained material was added to EtOH (ca. 150 mL). After stirring for 1 h at room temperature, the insoluble crude precipitates 4j were collected as a white solid (2.31 g, 95%). An analytical sample of 4j was obtained by recrystallization from acetone as colorless crystals.
4j: mp 249–250°C (from acetone). IR (KBr) cm−1: 1579 (C=N), 1175, 1140, 1040 (C–O). 1H-NMR (CDCl3) δ: 5.98 (6H, s, H2), 6.58 (3H, dd, J=8.2, 2.4 Hz, H6), 6.63 (3H, d, J=2.4 Hz, H4), 6.75 (3H, d, J=8.2 Hz, H7). 13C-NMR (CDCl3) δ: 101.80 (C2), 103.68 (C4), 107.92 (C7), 113.84 (C6), 145.63 (C7a), 146.00 (C5), 148.04 (C3a), 174.05 (C=N). Positive-ion FAB-MS m/z: 490 (M+H+). HR-FAB-MS m/z: 490.0888 (Calcd for C24H16N3O9: 490.0887). Anal. Calcd for C24H15N3O9: C, 58.90; H, 3.09; N, 8.59. Found: C, 58.92; H, 3.14; N, 8.61.
Procedure for the Synthesis of 2,4,6-[3-(Trifluoromethyl)phenoxy]-1,3,5-triazine (4k)30) (Method B) (Entry 11)A mixture of compound 1 (922 mg, 5.0 mmol), 3-(trifluoromethyl)phenol (kH, 2.51 g, 15.5 mmol), and TEA (1.57 g, 15.5 mmol) in dry acetone (20 mL) was stirred for 18 h at room temperature. After evaporation of the solvent, CH2Cl2 (ca. 100 mL) and 10% HCl (100 mL) were added to the reaction mixture. The separated organic layer was washed with brine (40 mL) and dried over MgSO4. Evaporation of the solvent gave crude white solid material 4k. Recrystallization from 2-PrOH gave analytically pure 4k (74%) as a colorless amorphous solid.
4k: mp 155–158°C (from 2-PrOH). IR (KBr) cm−1: 1572 (C=N), 1321 (C–F), 1183, 1124 (C–O). 1H-NMR (CDCl3) δ: 7.30 (3H, dm, J=7.6 Hz, H6′), 7.37 (3H, br s, H2′), 7.45–7.50 (6H, m, H4′, 5′). 13C-NMR (CDCl3) δ: 118.76 (q, J=4.1 Hz, C2′), 123.18 (q, J=4.1 Hz, C4′), 123.33 (q, J=272.1 Hz, CF3), 124.89 (C6′), 130.17 (C5′), 132.16 (q, J=33.1 Hz, C3′), 151.43 (C1′), 173.50 (C=N). Positive-ion FAB-MS m/z: 562 (M+H+). HR-FAB-MS m/z: 562.0823 (Calcd for C24H13F9N3O3: 562.0813). Anal. Calcd for C24H12F9N3O3: C, 51.35; H, 2.15; N, 7.49. Found: C, 51.11; H, 2.24; N, 7.57.
2,4,6-[4-(Trifluoromethyl)phenoxy]-1,3,5-triazine (4l) (Entry 12)This compound was prepared from the reaction of compound 1 and 4-(trifluoromethyl)phenol (lH) by using Method B under the conditions shown in Table 1. The resulting precipitates (TEA·HCl salt) were removed by filtration. After evaporation of the solvent, the products were separated by flash chromatography (n-hexane : CH2Cl2=4 : 6→1 : 9) to give 4l (33%) as a white solid. An analytical sample of 4l was obtained by recrystallization from 2-PrOH gave 4l as colorless needles.
4l: mp 217–218°C (from 2-PrOH). IR (KBr) cm−1: 1577 (C=N), 1331 (C–F), 1119, 1066 (C–O). 1H-NMR (CDCl3) δ: 7.23 (6H, d, J=8.5 Hz, H2′, 6′), 7.63 (6H, d, J=8.5 Hz, H3′, 5′). 13C-NMR (CDCl3) δ: 121.96 (C2′, 6′), 123.65 (q, J=272.0 Hz, CF3), 126.94 (q, J=4.1 Hz, C3′, 5′), 128.87 (q, J=33.1 Hz, C4′), 153.74 (C1′), 173.39 (C=N). Positive-ion FAB-MS m/z: 562 (M+H+). HR-FAB-MS m/z: 562.0807 (Calcd for C24H13F9N3O3: 562.0813). Anal. Calcd for C24H12 F9N3O3: C, 51.35; H, 2.15; N, 7.49. Found: C, 51.55; H, 2.15; N, 7.49.
General Procedure for the Preparation of Aryloxy-amino-1,3,5-triazine Derivatives (Method C): Example: Preparation of 1,1′-[6-[3-(Trifluoromethyl)phenoxy]-1,3,5-triazine-2,4-diyl]bis-4-piperidinemethanol (5kpp)(Step 1) A mixture of compound 1 (922 mg, 5.0 mmol), phenol (kH, 973 mg, 6.0 mmol), and collidine (727 mg, 6.0 mmol) in dry acetone (10 mL) was stirred for 10 min at 0°C and kept for 15 min at room temperature. The resulting colorless precipitated material (collidine·HCl) was removed by filtration and then the solvent was evaporated to afford a yellow solid. (Step 2) To this material in dry CH3CN (10 mL) was added 4-piperidinemethanol (pH, 2.30 g 20.0 mmol), and the resulting mixture was stirred for 1 h at room temperature. After evaporation of the solvent, a solvent (ca. 10 mL of n-hexane : 2-PrOH=80 : 20) was added to the residue, and then the insoluble materials were filtered to give crude 5kpp (1.92 g, 4.1 mmol, 81%) as a white solid. Recrystallization from propionitrile (EtCN) gave an analytically pure product 5kpp.
5kpp: mp 126–128°C (from EtCN). IR (KBr) cm−1: 1580 (C=N), 1323 (C–F), 1107, 1029 (C–O). 1H-NMR (CDCl3) δ: 1.10–1.25 (4H, m, H3′β, 5′β), 1.58 (2H, br s, OH), 1.68–1.82 (6H, m, H4′α, 3′α, 5′α), 2.72–2.85 (4H, m, H2′β, 6′β), 3.50 (4H, d, J=5.8 Hz, H1), 4.50–4.82 (4H, m, H2′α, 6′α), 7.36 (1H, dm, J=7.9 Hz, H5‴), 7.41–7.47 (2H, m, J=7.6 Hz, H4‴, 6‴) 7.52–7.54 (1H, m, H2‴). 13C-NMR (CDCl3) δ: 28.50 (C3′, 5′), 39.03 (C4′), 43.37 (C2′, 6′), 67.55 (C1), 119.61 (q, J=4.1 Hz, C2‴), 121.40 (q, J=4.1 Hz, C4‴), 123.82 (q, J=272.6 Hz, CF3), 125.19 (C6‴), 129.35 (C5‴), 131.15 (q, J=32.4 Hz, C3‴), 152.82 (C1‴), 165.78 (C2″, 4″), 170.60 (C6″). Positive-ion FAB-MS m/z: 468 (M+H+). HR-FAB-MS m/z: 468.2229 (Calcd for C22H29F3N5O3: 468.2222). Anal. Calcd for C22H28F3N5O3 : C, 56.52; H, 6.04; N, 14.98. Found: C, 56.52; H, 5.90; N, 14.99.
[1-[4,6-Bis[3-(trifluoromethyl)phenoxy)]-1,3,5-triazin-2-yl]piperidin-4-yl]methanol (6kkp)This compound was prepared from phenol (kH) and amine (pH) by using Method C at room temperature for 1 h in Step 1 and then at room temperature for 18 h in Step 2 with the ratio of TCT AZ : kH : collidine : pH=1 : 2.4 : 2.4 : 2. Purification of products by flash chromatography (n-hexane : EtOAc=85 : 15→70 : 30) gave 6kkp (69%) and 5kpp (29%). An analytical sample of 6kkp was obtained by recrystallization from diisopropyl ether as colorless crystals.
6kkp: mp 135.0–136.5°C (from diisopropyl ether). IR (KBr) cm−1: 1596 (C=N), 1326 (C–F), 1123, 1068 (C–O). 1H-NMR (CDCl3) δ: 1.12–1.22 (4H, m, H3′β, 5′β), 1.53 (1H, br s, OH), 1.74–1.80 (3H, m, H4′α, 3′α, 5′α), 2.84 (2H, dt, J=13.4, 2.4 Hz, H2′β, 6′β), 3.51 (2H, d, J=5.8 Hz, H1), 4.57 (2H, dm, J=13.4 Hz, H2′α, 6′α), 7.30–7.34 (2H, m, H5‴), 7.43–7.48 (6H, m, H2‴, 4‴, 6‴). 13C-NMR (CDCl3) δ: 28.37 (C3′, 5′), 38.71 (C4′), 43.88 (C2′, 6′), 67.19 (C1), 119.20 (q, J=4.1 Hz, C2‴), 122.19 (q, J=4.1 Hz, C4‴), 123.58 (q, J=273.1 Hz, CF3), 125.15 (C6‴), 129.74 (C5‴), 131.65 (q, J=32.8 Hz, C3‴), 152.12 (C1‴), 166.21 (C2″, 4″), 171.89 (C6″). Positive-ion FAB-MS m/z: 515 (M+H+). HR-FAB-MS m/z: 515.1524 (Calcd for C23H21F6N4O3: 515.1518). Anal. Calcd for C23H20F6N4O3: C, 53.70; H, 3.92; N, 10.89. Found: C, 53.63; H, 3.76; N, 10.86.
1,1′-[6-(4-Trifluoromethyl)pheoxy)-1,3,5-triazine-2,4-diyl]bis-4-piperidinemethanol (5lpp) and [1-[4,6-Bis[4-(trifluoromethyl)phenoxy]-1,3,5-triazin-2-yl]piperidin-4-yl]methanol (6llp)These compounds were prepared from phenol (lH) and amine (pH) by Method C at room temperature for 1 h in Step 1 and then at room temperature for 0.5 h in Step 2 with the ratio of TCT AZ : lH : collidine : pH=1 : 1.8 : 1.8 : 2. Purification of products by flash chromatography (CH2Cl2 : EtOH=97 : 3→93 : 7) gave 6llp (73%) and 5lpp (19%). Analytical samples of 5lpp and 6llp were obtained as colorless crystals by recrystallization from EtCN and EtOH, respectively.
5lpp: 162–164°C (from EtCN). IR (KBr) cm−1: 1583, 1527 (C=N), 1328 (C–F), 1064, 1038 (C–O). 1H-NMR (CDCl3) δ: 1.10–1.25 (4H, m, H3′β, 5′β), 1.44 (2H, br s, OH), 1.72–1.86 (6H, m, H4′α, 3′α, 5′α), 2.74–2.87 (4H, m, H2′β, 6′β), 3.51 (4H, d, J=6.1 Hz, H1), 4.50–4.86 (4H, m, H2′α, 6′α), 7.30 (2H, d, J=8.4 Hz, H2‴, 6‴), 7.61 (2H, d, J=8.4 Hz, H3‴, 5‴). 13C-NMR (CDCl3) δ: 28.53 (C3′, 5′), 39.04 (C4′), 43.39 (C2′, 6′), 67.57 (C1), 122.28 (C2‴, 6‴), 124.17 (q, J=33.1 Hz, CF3), 126.16 (q, J=3.1 Hz, C3‴, 5‴), 126.77 (q, J=33.1 Hz, C4‴), 155.38 (C6″), 165.82 (C2′, 4″), 170.57 (C1″). Positive-ion FAB-MS m/z: 468 (M+H+). HR-FAB-MS m/z: 468.2226 (Calcd for C22H29F3N5O3: 468.2222). Anal. Calcd for C22H28F3N5O3: C, 56.52; H, 6.04; N, 14.98. Found: C, 56.44; H, 5.93; N, 14.99.
6llp: mp 196–199°C (from EtOH). IR (KBr) cm−1: 1562, 1540 (C=N), 1329 (C–F), 1060, 1047 (C–O). 1H-NMR (CDCl3) δ: 1.14–1.24 (2H, m, H3′β, 5′β), 1.47 (1H, br s, OH), 1.73–1.84 (3H, m, H4′α, 3′α, 5′α), 2.85 (2H, dt, J=11.6, 2.1 Hz, H2′β, 6′β), 3.51 (2H, d, J=5.8 Hz, H1), 4.59 (2H, dm, H2′α, 6′α), 7.45 (4H, d, J=5.8 Hz, H2‴, 6‴), 7.61 (4H, d, J=8.5 Hz, H3‴, 5‴). 13C-NMR (CDCl3) δ: 28.41 (C3′, 5′), 39.69 (C4′), 43.91 (C2′, 6′), 67.19 (C1), 122.20 (C2‴, 6‴), 123.91 (q, J=272.1 Hz, CF3), 126.54 (q, J=4.1 Hz, C3‴, 5‴), 127.82 (q, J=33.1 Hz, C4‴), 154.53 (C4″, 6″), 166.32 (C2″), 171.80 (C1‴). Positive-ion FAB-MS m/z: 515 (M+H+). HR-FAB-MS m/z: 515.1523 (Calcd for C23H21F6N4O3: 515.1518). Anal. Calcd for C23H20F6N4O3: C, 53.70; H, 3.92; N, 10.89. Found: C, 53.77; H, 4.06; N, 10.81.
2,4-Bis(heptyloxy)-6-isopropoxy-1,3,5-triazine (4dde) and 2,4-Bis(cyclopentyloxy)-6-isopropoxy-1,3,5-triazine (4egg)The intermediate 2e for the preparation of compounds 4dde and 4egg was prepared from the reaction of compound 1 and 2-PrOH (eH) by using Method C at room temperature for 2.5 h with the ratio of TCT AZ : eH : collidine=1 : 2.6 : 2. After removal of the precipitated material (collidine·HCl), purification of the obtained crude product by open column chromatography (n-hexane : EtOAc=90 : 10) gave 2e8) (83%). Title two compounds were prepared from the reactions of compound 2e with alcohol dH or gH by using Method A under the same conditions as those for the preparation of 4e (Entry 4 in Table 1) with the ratio of 2e : dH or gH : NaH=1 : 4 : 2. Separation of the products by flash chromatography (n-hexane : EtOAc=95 : 5→93 : 7) gave 4dde (75%) as colorless oil or 4egg (42%) as a white solid, respectively. Recrystallization from 2-PrOH–H2O gave 4egg as white crystals.
4dde: IR (NaCl) cm−1: 1560 (C=N), 1146, 1102 (C–O). 1H-NMR (CDCl3) δ: 0.88 (6H, t, J=7.0 Hz, H7′), 1.26–1.36 (12H, m, H4′, 5′, 6′), 1.38 (6H, d, J=6.1 Hz, H1″, 3″), 1.40–1.46 (4H, m, H3′), 1.78 (4H, qu, J=6.7 Hz, H2′), 4.37 (4H, t, J=6.7 Hz, H1′), 5.36 (1H, qu, J=6.1 Hz, H2″). 13C-NMR (CDCl3) δ: 13.92 (C7′), 21.69 (C1″, 3″), 22.47 (C6′), 25.71 (C3′), 28.60 (C2′), 28.86 (C4′), 31.64 (C5′), 68.31 (C1′), 71.43 (C2″), 172.52 (C6), 173.14 (C2, 4). Positive-ion FAB-MS m/z: 368 (M+H+). HR-FAB-MS m/z: 368.2909 (Calcd for C20H38N3O3: 368.2913). Anal. Calcd for C20H37N3O·0.2H2O: C, 64.73; H, 10.16; N, 11.32. Found: C, 64.71; H, 10.15; N, 11.43.
4egg: mp 94–97°C (from 2-PrOH–H2O). IR (KBr) cm−1: 1562 (C=N), 1133, 1101 (C–O). 1H-NMR (CDCl3) δ: 1.37 (6H, d, J=6.1 Hz, H1″, 3″), 1.56–1.65 (4H, m, H3′, 4′), 1.78–1.89 (8H, m, H2′, 3′, 4′, 5′), 1.92–2.00 (4H, m, H2′, 5′), 5.34 (1H, qu, J=6.1 Hz, H2″), 5.46 (2H, qu, J=3.1 Hz, H1′). 13C-NMR (CDCl3) δ: 21.79 (C1″, 3″), 23.79 (C3′, 4′), 32.71 (C2′, 5′), 71.27 (C2″), 80.61 (C1′), 172.48 (C6), 172.80 (C2, 4). Positive-ion FAB-MS m/z: 308 (M+H+). HR-FAB-MS m/z: 308.1977 (Calcd for C16H26N3O3: 308.1974). Anal. Calcd for C16H25N3O3: C, 62.52; H, 8.20; N, 13.67. Found: C, 62.57; H, 8.22; N, 13.55.
Antiviral Activity Assay and Cytotoxicity of Synthesized Trisubstituted TAZ DerivativesThe anti-HSV-1 activities (EC50) of the synthesized TAZ derivatives were measured by using a plaque reduction assay22) and their cytotoxicity against Vero cells (IC50) was also evaluated. The results are summarized in Table 2 together with data for aciclovir.23) Calculated log P values24) for the compounds are also shown in Table 2. There were few distinct correlations between log P values and EC50 values or between log P values and IC50 values among the compounds listed in Table 2.
Calorimetric ExperimentsThe heat of binding between a tripodal receptor-type TAZ derivative 4e and sugars was measured in aqueous 25% 2-propanol solution at 298.15 K by using an isothermal titration calorimeter (Thermal Activity Monitor 2270). Titrations were performed by stepwise injection of a sugar-containing solution (3.5–4.6 mM, or ca. 5 mg/mL of dermatan sulfate or heparin sulfate) into a reaction cell (3 mL) loaded with the compound 4e solution (ca. 310–460 µM). Many commercial sugar derivatives of monosaccharide, disaccharide, trisaccharide, tetrasaccharide, oligosaccharide, aminosugar and some sulfated glycosaminoglycans were used for calorimetric experiments; however, observed reactions of compound 4e with many sugar derivatives were not exothermic. Three typical examples of exothermic binding reactions for methyl esters of monosaccharide (MeO-α/β-Gal and MeO-α/β-Man) are shown in Table 3. The data obtained were analyzed by NanoAnalyze™ Software (TA Instruments, U.S.A). Binding stoichiometry, Ka and ΔH are shown in Table 3. The values of ΔG and −TΔS were also calculated from the equation ΔG=−RT ln Ka=ΔH−TΔS (where R is the gas constant and T is absolute temperature). The heat of binding between a tripodal receptor-type TAZ derivative 4g and MeO-α/β-Gal was also measured in aqueous 25% 2-propanol solution at 298.15 K.
The authors would like to thank Ms. Izumi Sakai, Ms. Marina Sano, Ms. Saki Sawai, and Ms. Yuuna Kawaguchi for their valuable technical assistance.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.
