2016 年 64 巻 11 号 p. 1630-1640
2016 年 64 巻 11 号 p. 1630-1640
We previously identified 3-chloro-N-{(S)-[3-(1-ethyl-1H-pyrazol-4-yl)phenyl][(2S)-piperidine-2-yl]methyl}-4-(trifluoromethyl)pyridine-2-carboxamide (5, TP0439150) as a potent and orally available glycine transporter 1 (GlyT1) inhibitor. In this article, we describe our identification of 1-methyl-N-(propan-2-yl)-N-({2-[4-(trifluoromethoxy)phenyl]pyridin-4-yl}methyl)-1H-imidazole-4-carboxamide (7n) as a structurally diverse back-up compound of 5, using central nervous system multiparameter optimization (CNS MPO) as a drug-likeness guideline. Compound 7n showed a higher CNS MPO score and different physicochemical properties as compared to 5. Compound 7n exhibited potent GlyT1 inhibitory activity, a favorable pharmacokinetics profile, and elicited an increase in the cerebrospinal fluid (CSF) concentration of glycine in rats.
Hypofunction of the N-methyl-D-aspartate (NMDA) receptor is implicated in the pathophysiology of schizophrenia, based on the results of clinical observation and pre-clinical studies.1–4) Stimulation of the NMDA receptor may improve the symptoms of schizophrenia, but direct activation of the glutamate-binding site has been reported to induce neurotoxicity.5) Under this circumstance, it was considered that elevating the extracellular levels of glycine would be an effective alternative approach, as glycine is an important co-agonist of the NMDA receptor. In fact, co-administration of glycine, or of the glycine site agonist D-serine, with atypical antipsychotics has been reported to improve schizophrenia.6)
Elevating glycine levels in the brain can be achieved by inhibition of the glycine transporter 1 (GlyT1) which is co-expressed with the NMDA receptor.7,8) This approach is supported by the results of clinical studies on sarcosine (N-methylglycine, a prototypical weak GlyT1 inhibitor); administration of sarcosine with atypical antipsychotics has been shown to improve the positive, negative, as well as cognitive symptoms in schizophrenic patients.9)
Many research groups have reported the development of GlyT1 inhibitors, as illustrated in Fig. 1: 1 (RG1678),10) 2 (DCCCyB),11) and 3a (PF-0346275).12) Compound 413) has been also reported as the hybrid compound of 3a and Merck’s sulfonamide derivatives.14,15) Recently, our group discovered 5 (TP0439150), and reported its pharmacological profile.16) Compound 5 shows potent in vitro GlyT1 inhibitory activity with an IC50 value of 1.8 nM; oral dosing at 1 mg/kg elicited an increase in the concentration of glycine in the cerebrospinal fluid (CSF) in rats. Furthermore, at doses of 0.1 and 0.3 mg/kg, respectively, 5 significantly improved cognitive deficit induced by MK-801 in the social recognition test in rats and reversed the reduction in social interaction induced by repeated phencyclidine treatment in mice. These results suggest that 5 would be useful for the treatment of the cognitive dysfunction and negative symptoms of schizophrenia. In parallel with further pharmacological studies of 5, our medicinal chemistry efforts have been focused on the identification of structurally diverse back-up compounds.

There are several drug-likeness guidelines for enhancing the probability of success in drug discovery.17,18) Wager et al. at Pfizer have proposed the Central Nervous System Multiparameter Optimization (CNS MPO) as a drug-likeness guideline for the development of drugs acting on the CNS.19) The CNS MPO score is calculated using six physicochemical parameters: (1) calculated distribution coefficient (c Log P); (2) calculated distribution coefficient at pH 7.4 (c Log D); (3) molecular weight (MW); (4) topological polar surface area (tPSA); (5) number of hydrogen bond donor (HBD); (6) pKa of the most basic center; it has been reported that a higher CNS MPO score may increase of the probability of success. While the CNS MPO scores of 74% of the CNS drugs launched in the market are more than 4.0, the calculated score of 5 is 3.17 (Fig. 2). Precise analysis of its each parameter indicated that this relatively low score was due to its high MW, lipophilic characteristic (c Log P), and basicity (pKa).
In this article, we report our exploration and identification of the structurally diverse GlyT1 inhibitor 7n, based on the CNS MPO guideline.

(a) Value of each parameter and CNS MPO score of each component; (b) Radar chart based on each component of CNS MPO.
Compounds were prepared as shown in Charts 1 to 3. Compounds with various alkyl groups at the nitrogen atom of the amide bond were prepared according to Chart 1. Condensation of 1-methyl-1H-imidazole-4-carboxylic acid with 3-(trifluoromethoxy)benzylamine 8 yielded amide intermediate 9, which was alkylated with alkyl halide using sodium hydride to obtain 6a–c and e. The introduction of secondary alkyl groups, such as the i-propyl, cyclohexyl, or tetrahydro-2H-pyran-4-yl groups, was conducted by reductive amination of the corresponding ketones with 8. Intermediates 10a–c were condensed with 1-methyl-1H-imidazole-4-carboxylic acid to obtain 6d, f, and g.

Reagents and conditions: (a) 1-Methyl-1H-imidazole-4-carboxylic acid, HOBt, EDC, acetonitrile; (b) NaH, R-I, DMF; (c) Acetone or cyclohexanone or tetrahydro-2H-pyran-4-one, NaBH(OAc)3, CHCl3.
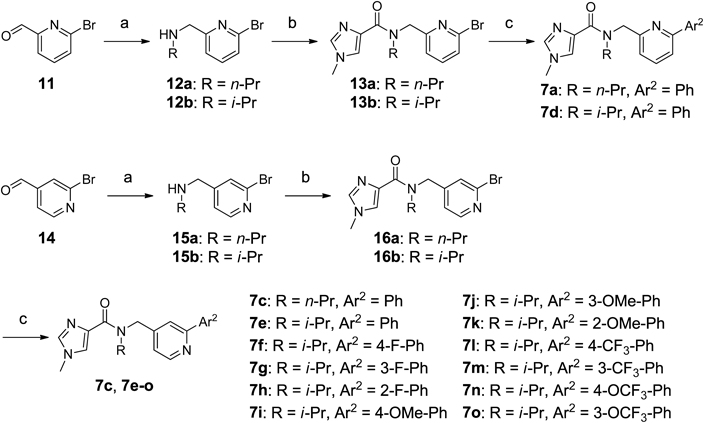
Reagents and conditions: (a) 1-Aminopropane or 2-aminopropane, NaBH(OAc)3, CHCl3; (b) 1-Methyl-1H-imidazole-4-carboxamide, HOBt, EDC, acetonitrile; (c) (Condition A) Ar2-B(OH)2, Pd(PPh3)4, K2CO3, DMF, EtOH, 150°C, microwave irradiation (condition B) Ar2-B(OH)2, Pd(PPh3)4, Cs2CO3, toluene, EtOH, H2O, 150°C, microwave irradiation.

Reagents and conditions: (a) 1-Aminopropane, HOBt, EDC, acetonitrile; (b) PhB(OH)2, Pd(PPh3)4, K2CO3, DMF, EtOH, 150°C, microwave irradiation; (c) LiAlH4, THF, 70°C; (d) 1-Methyl-1H-imidazole-4-carboxamide, HOBt, EDC, acetonitrile.
The synthetic routes for compounds 7a and c–o are depicted in Chart 2. Reductive amination of 2-bromopyridine-6-carboxaldehyde 11 with 1-aminopropane or 2-aminopropane yielded 12a and b, which were condensed with 1-methyl-1H-imidazole-4-carboxylic acid to obtain intermediates 13a and b, respectively. Subsequently, the Suzuki coupling reaction with phenylboronic acid yielded 7a and d, respectively. In a similar manner, 2-phenyl-4-pyridyl derivatives, 7c and e–o, were synthesized using 2-bromopyridine-4-carboxaldehyde 14 as the starting material.
The synthesis of compound 7b is shown in Chart 3. Condensation of 4-bromopyridine-2-carboxylic acid 17 with 1-aminopropane yielded 18, which was converted to 19 by the Suzuki coupling reaction with phenylboronic acid. The amide moiety of 19 was reduced by lithium aluminium hydride, and subsequent condensation with 1-methyl-1H-imidazole-4-carboxylic acid yielded 7b.
As mentioned above, we placed a high priority on the CNS MPO score for exploration of our back-up lead compound. Through these efforts, we found that 6a, which was designed by removal of the basic amine substructure of 3b,12) retained moderate GlyT1 inhibitory activity with a CNS MPO score of 5.96 (Fig. 3). This high score was due to the different physicochemical characteristics of 6a ((1) low molecular weight (=313), (2) low lipophilicity (c Log P=2.0), and (3) weak basicity (calculated pKa=4.2)) as compared to those of GlyT1 inhibitor 5 reported by us previously. Thus, we decided to optimize 6a to obtain a back-up candidate with a higher CNS MPO score than 5.

Firstly, the alkyl group at the nitrogen atom of the amide linker was explored (Table 1). While elongation of the methyl group to ethyl group was associated with retention of equivalent activity (6b: IC50=76 nM), the n-propyl derivative 6c showed higher activity (6c: IC50=10 nM). Moreover, introduction of a branch alkyl substituent, such as an i-propyl or i-butyl group, increased the inhibitory activity (6d: IC50=2.7 nM, 6e: IC50=2.8 nM). More bulky substituents, such as a cyclohexyl or tetrahydro-2H-pyran-4-yl group, were also tolerated (6f: IC50=1.6 nM, 6g: IC50=4.7 nM), however, their CNS MPO scores were lower than the score of 6d, due to their higher molecular weight. Since 6d exhibited a CNS MPO score of more than 5.0, with maintenance of potent inhibitory activity, we selected the n-propyl or i-propyl group as the alkyl group at the nitrogen atom of the amide bond for further structure–activity relationship (SAR) studies.
 | |||
|---|---|---|---|
| No. | R | IC50 (nM) | CNS MPO |
| 6a | Me | 67 | 5.96 |
| 6b | Et | 76 | 5.78 |
| 6c | n-Pr | 10 | 5.58 |
| 6d | i-Pr | 2.7 | 5.66 |
| 6e | i-Bu | 2.8 | 5.23 |
| 6f | c-Hex | 1.6 | 4.55 |
| 6g | Tetrahydro-2H-pyran-4-yl | 4.7 | 5.54 |
Secondly, modification of the side chain moieties was investigated to introduce further structural novelty into the lead compound 6d. The left-hand side moiety (left hand, LH), 1-methyl-1H-imidazole-4-carboxamide, was essential for maintenance of the inhibitory activity (data not shown). Thus, we modified the right-hand side chain moiety (right hand, RH). The strategy for optimization of RH is summarized in Fig. 4. From the perspective of feasibility of synthesis, we initially designed a biphenyl derivative, however, its CNS MPO score was lower than that of 6d due to its relatively high lipophilicity. Therefore, to modulate the lipophilicity, we designed phenylpyridine derivatives, which exhibited almost equivalent CNS MPO scores to the score of 6d. The results are shown in Tables 2 and 3.

 | ||||
|---|---|---|---|---|
| No. | R | Ar1 | IC50 (nM) | CNS MPO |
| 7a | n-Pr |  | 140 | 5.78 |
| 7b | n-Pr |  | 1400 | 5.78 |
| 7c | n-Pr |  | 39 | 5.78 |
| 7d | i-Pr |  | 100 | 5.81 |
| 7e | i-Pr |  | 20 | 5.81 |
 | |||
|---|---|---|---|
| No. | Ar2 | IC50 (nM) | CNS MPO |
| 7e |  | 20 | 5.81 |
| 7f |  | 18 | 5.85 |
| 7g |  | 16 | 5.84 |
| 7h |  | 43 | 5.76 |
| 7i |  | 34 | 5.93 |
| 7j |  | 37 | 5.94 |
| 7k |  | 190 | 5.96 |
| 7l |  | 37 | 5.00 |
| 7m |  | 8.2 | 5.00 |
| 7n |  | 7.3 | 4.75 |
| 7o |  | 7.9 | 4.75 |
Among phenylpyridine derivatives (7a–c), it was found that the 2-phenyl-4-pyridyl derivative 7c showed moderate inhibitory activity. Replacement of the n-propyl with an i-propyl group resulted in a slight increase of the inhibitory activity (7a vs. d, and 7c vs. e). All the phenylpyridine derivatives listed in Table 2 showed high CNS MPO scores; therefore, our efforts were focused on optimization of the substituents on the phenyl ring.
Introduction of the electron-withdrawing fluoro atom at the para- and meta-positions of the benzene ring were tolerated in terms of the inhibitory activity, while ortho-fluoro substitution resulted in a slight decrease of the activity (7f–h). Introduction of the electron-donating methoxy group was associated with a similar tendency (7i–k). In comparison with the fluoro atom and methoxy group, fluoro-substituted derivatives showed higher activities at any position (7f vs. i, 7g vs. j); thus, other electron-withdrawing substituents at the para- and meta-positions were examined for increasing the activity.
Although introduction of a trifluoromethyl group at the para-position resulted in a decrease of the activity as compared to a fluoro substitution, meta-trifluoromethyl was associated with an increase of the activity (7l: IC50=37 nM, 7m: IC50=8.2 nM). In the case of the trifluoromethoxy group, both the para- and meta-substituted analogs showed good inhibitory activities (7n: IC50=7.3 nM, 7o: IC50=7.9 nM). Although both trifluoromethyl and trifluoromethoxy groups have a relatively high molecular weight and lipophilicity, these compounds retained high CNS MPO scores of around 5.0.
Having obtained potent compounds with structurally novel side chains, the metabolic stabilities of 7m–o in human microsomes were evaluated. We found that the para-substituted derivative 7n exhibited higher microsomal stability (% metabolized after 15 min’ incubation with human microsomes, 7n: 5.6%) than the meta-substituted derivatives 7m and o (7m: 25%, 7o: 25%). Furthermore, 7n did not significantly inhibit the major CYP450 enzymes (3A4, 2D6, 2C9, 2C19, and 1A2) at a concentration of 10 µM. From these results, we selected 7n for further in vivo evaluation. The results are summarized in Fig. 5.

(a) PK parameters of 7n in rats. (b) Effects of 7n on the CSF glycine levels in rats. Data represent the mean±S.E.M. (n=6–7). Vehicle: 0.5% methylcellulose, *** p<0.001, n.s.: not significant (p>=0.05) versus the vehicle-treated group (Dunnett’s test). (c) CNS MPO scores of each component and radar chart based on each component of CNS MPO.
The pharmacokinetic (PK) parameters of 7n in rats are shown in Fig. 5(a). In the intravenous administration, the systemic clearance of 7n was about a half of the hepatic blood flow, and the oral bioavailability was 42% at the dose of 2.55 mg/kg. Furthermore, 7n showed higher maximum plasma exposure and a higher plasma to brain penetration ratio than 5 (7n (2.55 mg/kg): Cmax=0.64 µM, Tmax=0.42 h, B/P ratio=0.90, 5 (10 mg/kg): Cmax=0.24 µM, Tmax=4.0 h, B/P ratio=0.52). These PK results prompted us to conduct an in vivo study of 7n using a rat model with measurement of the CSF concentration of glycine, which is considered to reflect the extracellular glycine concentration in the brain. As shown in Fig. 5(b), significant increase of the CSF glycine concentration was observed in a dose-dependent manner from 3 mg/kg oral dosing, indicating that 7n blocked GlyT1 in vivo and may be expected to exhibit efficacy in rodent models of schizophrenia. The minimum effective dose (MED) of 7n was 3 mg/kg per os (p.o.), which was almost close efficacy comparing with that of 5 (1 mg/kg p.o.). The slight difference in MED could be partially explained by the difference in in vitro activity. Detail PK/pharmacodynamics (PD) studies will be needed for further comparison of two compounds.
In this article, we have described the identification of 7n as a structurally diverse back-up compound of 5. Compound 7n exhibited potent GlyT1 inhibitory activity, good metabolic stability, and a favorable PK profile. Furthermore, in vivo evaluation showed that 7n significantly increased the CSF glycine concentration in rats following oral dosing at 3 mg/kg. Compound 7n also showed a higher CNS MPO score and different physicochemical properties as compared to 5 (5: 3.17, 7n: 4.75); thus, 7n could be a suitable back-up candidate to 5. Further in vivo pharmacological evaluation will be reported in due course.
1H- and 13C-NMR spectra were recorded on a JEOL JNM-ECA600 or JEOL JNM-ECA500, and the chemical shifts were expressed in δ (ppm) values with trimethylsilane as an internal reference (s=singlet, d=doublet, t=triplet, q=quartet, m=multiplet, and br=broad peak). Some NMR signals doubled and broadened due to the existence of rotamers caused by the sterically hindered amide moiety. MS were recorded on a micromass Platform LC or Shimadzu LCMS-2010EV. High resolution (HR) mass spectral data were acquired using a Shimadzu LCMS-IT-TOF equipped with an electrospray ionization (ESI)/atmospheric pressure chemical ionization dual ion source. The purities of the final compounds were confirmed using LC-MS on an Agilent instrument with ESI. The LC-MS conditions were as follows: Agilent 1290 infinity and Agilent 6150; column Waters Acquity CSH C18, 1.7 µm, 2.1 mm ×50 mm; eluent A, water +0.1% formic acid; eluent B, acetonitrile +0.1% formic acid; 20–99% B for 1.2 min, 99% B for 0.2 min; flow rate 0.8 mL/min; UV detection, λ=254 nm.
1-Methyl-N-{[3-(trifluoromethoxy)phenyl]methyl}-1H-imidazole-4-carboxamide (9)To a solution of 1-methyl-1H-imidazole-4-carboxylic acid (0.50 g, 4.0 mmol), 1-hydroxybenztriazole monohydrate (0.74 g, 4.8 mmol), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (0.92 g, 4.8 mmol) in acetonitrile (10 mL) was added a solution of 3-(trifluoromethoxy)benzylamine 8 (0.84 g, 4.4 mmol) in acetonitrile (10 mL), and the mixture was stirred at room temperature for overnight. The reaction mixture was concentrated in vacuo, and saturated aqueous NaHCO3 solution was added to the residue. After extraction with ethyl acetate, the organic layer was dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was purified using silica gel column chromatography (0–10% MeOH in CHCl3) to obtain 9 (1.18 g, 98%) as a colorless powder. 1H-NMR (600 MHz, CDCl3) δ: 3.73 (3 H, s), 4.62 (2 H, d, J=6.0 Hz), 7.08–7.13 (1 H, m), 7.18 (1 H, s), 7.26–7.29 (1 H, m), 7.31–7.37 (2 H, m), 7.44–7.51 (1 H, m), 7.55 (1 H, d, J=1.4 Hz); MS ESI: m/z 300 [M+H]+.
N,1-Dimethyl-N-{[3-(trifluoromethoxy)phenyl]methyl}-1H-imidazole-4-carboxamide (6a)To a solution of 9 (50 mg, 0.17 mmol) in N,N-dimethylformamide (1 mL) was added sodium hydride (60% in oil, 8 mg, 0.20 mmol), and the mixture was stirred at room temperature for 10 min. To the reaction mixture was added methyl iodide (12 µL, 0.19 mmol), and the mixture was stirred for an additional 30 min. The reaction was quenched with saturated aqueous NaHCO3 solution and extracted with ethyl acetate. The organic layer was washed with water and brine, dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was purified using silica gel column chromatography (0–2% MeOH in CHCl3) to obtain 6a (42 mg, 80%) as a colorless powder. 1H-NMR (600 MHz, DMSO-d6) δ: 2.83 and 3.38 (3H, br s), 3.69 (3H, s), 4.67 and 5.37 (2H, br s), 7.23–7.32 (3H, m), 7.48 (1H, t, J=7.8 Hz), 7.64 (1H, br s), 7.69–7.72 (1H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 33.12, 33.46, 36.23, 50.32, 52.21, 119.38, 119.64, 119.82, 120.03 (q, J=256.7 Hz), 126.53, 130.37, 136.84, 137.42, 140.96, 141.54, 148.52, 163.37; HR-MS: Calcd for C14H14F3N3O2 [M+H]+ 314.1111. Found 314.1089.
N-Ethyl-1-methyl-N-{[3-(trifluoromethoxy)phenyl]methyl}-1H-imidazole-4-carboxamide (6b)Compound 6b (73%, colorless powder) was obtained in a similar manner to that described for 6a. 1H-NMR (600 MHz, DMSO-d6) δ: 0.99–1.18 (3H, m), 3.29 and 3.92 (2H, br s), 3.69 (3H, s), 4.67 and 5.37 (2H, br s), 7.23–7.31 (2H, m), 7.33 (1H, d, J=7.8 Hz), 7.47 (1H, t, J=7.9 Hz), 7.61–7.69 (1H, m), 7.71 (1H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 12.32, 14.39, 33.10, 40.45, 42.54, 47.81, 49.68, 119.26, 119.78, 120.04 (q, J=259.72 Hz), 126.43, 130.21, 137.06, 137.51, 141.79, 142.05, 148.46, 162.73, 163.27; HR-MS: Calcd for C15H16F3N3O2 [M+H]+ 328.1267. Found 328.1261.
1-Methyl-N-propyl-N-{[3-(trifluoromethoxy)phenyl]methyl}-1H-imidazole-4-carboxamide (6c)Compound 6c (73%, colorless oil) was obtained in a similar manner to that described for 6a. 1H-NMR (600 MHz, DMSO-d6) δ: 0.73–0.84 (3H, m), 1.45–1.62 (2H, m), 3.22 and 3.87 (2H, br s), 3.70 (3H, s) 4.68 and 5.39 (2H, br s), 7.23–7.34 (3H, m), 7.46 (1H, t, J=8.0 Hz), 7.59–7.68 (1H, m), 7.70 (1H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.75, 11.13, 20.04, 21.86, 33.10, 47.31, 48.31, 49.28, 50.16, 119.26, 119.74, 120.04 (q, J=255.19 Hz), 126.39, 126.63, 127.20, 130.21, 137.04, 137.44, 139.35, 141.69, 142.05, 148.46, 163.39; HR-MS: Calcd for C16H18F3N3O2 [M+H]+ 342.1424. Found 342.1404.
1-Methyl-N-(2-methylpropyl)-N-{[3-(trifluoromethoxy)phenyl]methyl}-1H-imidazole-4-carboxamide (6e)Compound 6e (40%, colorless oil) was obtained in a similar manner to that described for 6a. 1H-NMR (499 MHz, CDCl3) δ: 0.82–0.98 (6H, m), 1.94–2.13 (1H, m), 3.21–3.28 and 3.88–3.98 (2H, m), 3.71 (3H, br s), 4.76 and 5.43 (2H, br s), 7.05–7.24 (3H, m), 7.29–7.35 (1H, m), 7.35–7.43 (1H, m), 7.57 (1H, d, J=1.4 Hz); 13C-NMR (126 MHz, CDCl3) δ: 19.90, 20.27, 26.71, 33.68, 51.58, 53.13, 119.41, 120.01, 126.37, 129.80, 136.47, 149.46, 164.04; HR-MS: Calcd for C17H20F3N3O2 [M+H]+ 356.1580. Found 356.1551.
N-{[3-(Trifluoromethoxy)phenyl]methyl}propan-2-amine (10a)To a solution of acetone (0.38 mL, 5.2 mmol) and 8 (1.0 g, 5.2 mmol) in CHCl3 (10 mL) was added sodium triacetoxyborohydride (2.2 g, 10 mmol), and the mixture was stirred at room temperature for 45 min. The mixture was quenched with saturated aqueous NaHCO3 solution and extracted with CHCl3. The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified with silica gel column chromatography (0–10% MeOH in CHCl3) to obtain 10a (0.68 g, 56%) as a colorless oil. 1H-NMR (600 MHz, CDCl3) δ: 1.10 (6H, d, J=6.4 Hz), 2.84 (1H, spt, J=6.3 Hz), 3.80 (2H, s), 7.09 (1H, d, J=7.3 Hz), 7.20 (1H, s), 7.30–7.36 (1H, m); MS (ESI): m/z 234 [M+H]+.
N-{[3-(Trifluoromethoxy)phenyl]methyl}cyclohexanamine (10b)Compound 10b (quant., colorless oil) was obtained in a similar manner to that described for 10a. 1H-NMR (600 MHz, CDCl3) δ: 1.08–1.27 (4H, m), 1.56–1.66 (2H, m), 1.67–1.80 (2H, m), 1.82–1.99 (2H, m), 2.43–2.51 (1H, m), 3.83 (2H, s), 7.07–7.12 (1H, m), 7.20–7.28 (2H, m), 7.30–7.38 (1H, m); MS (ESI): m/z 274 [M+H]+.
N-{[3-(Trifluoromethoxy)phenyl]methyl}oxan-4-amine (10c)Compound 10c (82%, colorless oil) was obtained in a similar manner to that described for 10a. 1H-NMR (600 MHz, CDCl3) δ: 1.40–1.49 (2H, m) 1.82–1.89 (2H, m) 2.66–2.76 (1H, m) 3.36–3.44 (2H, m) 3.85 (2H, s) 3.94–4.02 (2H, m) 7.08–7.38 (4H, m); MS (ESI): m/z 276 [M+H]+.
1-Methyl-N-(propan-2-yl)-N-{[3-(trifluoromethoxy)phenyl]methyl}-1H-imidazole-4-carboxamide (6d)Compound 6d (93%, colorless powder) was obtained from 10a in a similar manner to that described for 9. 1H-NMR (600 MHz, DMSO-d6) δ: 1.02–1.15 (6H, m), 3.69 (3H, br s), 4.44–4.71 and 5.21–5.40 and 5.54–5.67 (3H, m), 7.18 (1H, br d, J=8.3 Hz), 7.22 (1H, br s), 7.31 (1H, d, J=7.8 Hz), 7.42 (1H, s), 7.54–7.72 (2H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 19.89, 21.16, 33.08, 42.91, 46.68, 48.17, 118.65, 119.20, 120.02 (q, J=258.21), 125.79, 125.93, 126.51, 126.69, 129.85, 137.34, 143.39, 148.28, 156.52, 163.85; HR-MS: Calcd for C16H18F3N3O2 [M+H]+ 342.1424. Found 342.1398.
N-Cyclohexyl-1-methyl-N-{[3-(trifluoromethoxy)phenyl]methyl}-1H-imidazole-4-carboxamide (6f)Compound 6f (9%, colorless powder) was obtained from 10b in a similar manner to that described for 9. 1H-NMR (600 MHz, DMSO-d6) δ: 0.96–1.09 (1H, m), 1.13–1.59 (6H, m), 1.61–1.73 (3H, m), 3.70 (3H, br s), 4.12–4.32 and 4.54–4.76 and 5.06–5.46 (3H, m), 7.17 (1H, d, J=8.3 Hz), 7.20 (1H, s), 7.29 (1H, br d, J=7.4 Hz), 7.38–7.44 (1H, m), 7.48–7.72 (2H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 24.72, 25.44, 30.02, 31.41, 33.08, 43.95, 46.79, 54.78, 56.31, 118.57, 119.12, 119.94, 120.01 (q, J=256.7 Hz), 125.75, 126.03, 126.63, 129.79, 130.15, 137.34, 137.67, 143.41, 144.04, 148.24, 148.42, 163.27, 164.05; HR-MS: Calcd for C19H22F3N3O2 [M+H]+ 382.1737. Found 382.1716.
1-Methyl-N-(oxan-4-yl)-N-{[3-(trifluoromethoxy)phenyl]methyl}-1H-imidazole-4-carboxamide (6g)Compound 6g (14%, colorless powder) was obtained from 10c in a similar manner to that described for 9. 1H-NMR (600 MHz, CDCl3) δ: 1.56–1.82 (4H, m), 3.45 (2H, br t, J=11.8 Hz), 3.71 (3H, br s), 3.95 (2H, br dd, J=11.6, 4.1 Hz), 4.60–4.86 and 5.28–5.66 (3H, m), 7.05 (1H, br d, J=7.8 Hz), 7.12 (1H, br s), 7.18–7.23 (1H, m), 7.26–7.46 (1H, m), 7.36–7.40 (1H, m), 7.57 (1H, br s); 13C-NMR (151 MHz, DMSO-d6) δ: 30.62, 32.06, 33.57, 44.98, 47.46, 52.54, 54.19, 67.47, 119.00, 119.53, 120.43 (q, J=259.7 Hz), 125.13, 126.48, 129.55, 136.63, 138.40, 142.18, 142.94, 149.31, 164.58; HR-MS: Calcd for C18H20F3N3O3 [M+H]+ 384.1530. Found 384.1507.
N-[(6-Bromopyridin-2-yl)methyl]propan-1-amine (12a)Compound 12a (99%, yellow oil) was obtained from 2-bromopyridine-6-carboxaldehyde 11 and 1-aminopropane in a similar manner to that described for 10a. 1H-NMR (600 MHz, CDCl3) δ: 0.94 (3H, t, J=7.3 Hz), 1.52–1.59 (2H, m), 2.59–2.64 (2H, m), 3.89 (2H, s), 7.29 (1H, d, J=7.4 Hz), 7.36 (1H, d, J=7.8 Hz), 7.51 (1H, t, J=7.5 Hz); MS (ESI): m/z 229 [M+H]+.
N-[(6-Bromopyridin-2-yl)methyl]propan-2-amine (12b)Compound 12b (49%, pale yellow oil) was obtained from 11 and 2-aminopropane in a similar manner to that described for 10a. 1H-NMR (600 MHz, CDCl3) δ: 1.11 (6H, d, J=6.0 Hz), 2.84 (1H, spt, J=6.3 Hz), 3.88 (2H, s), 7.29 (1H, d, J=7.3 Hz), 7.33–7.36 (1H, m), 7.49 (1H, t, J=7.6 Hz); MS (ESI): m/z 229 [M+H]+.
N-[(6-Bromopyridin-2-yl)methyl]-1-methyl-N-propyl-1H-imidazole-4-carboxamide (13a)Compound 13a (67%, pale yellow oil) was obtained from 12a in a similar manner to that described for 9. 1H-NMR (600 MHz, CDCl3) δ: 0.83–0.93 (3H, m), 1.61–1.71 (2H, m), 3.34–4.05 (5H, m), 4.72–5.51 (2H, m), 7.29–8.11 (5H, m); MS (ESI): m/z 337 [M+H]+.
N-[(6-Bromopyridin-2-yl)methyl]-1-methyl-N-(propan-2-yl)-1H-imidazole-4-carboxamide (13b)Compound 13b (95%, colorless powder) was obtained from 12b in a similar manner to that described for 9. 1H-NMR (600 MHz, CDCl3) δ: 1.09–1.24 (6H, m), 3.64–3.76 (3H, m), 4.70–4.88 and 5.29–5.36 and 5.68–5.75 (3H, m), 7.26–7.34 (2H, m), 7.41–7.47 (2H, m), 7.51–7.59 (1H, m); MS (ESI): m/z 337 [M+H]+.
N-[(2-Bromopyridin-4-yl)methyl]propan-1-amine (15a)Compound 15a (37%, pale yellow oil) was obtained from 2-bromopyridine-4-carboxaldehyde 14 and 1-aminopropane in a similar manner to that described for 10a. 1H-NMR (600 MHz, CDCl3) δ: 0.94 (1H, t, J=7.3 Hz), 1.50–1.55 (2H, m), 2.57 (2H, t, J=7.3 Hz), 3.79 (2H, s), 7.23 (1H, dd, J=5.0, 1.4 Hz), 7.49–7.50 (1H, m), 8.29 (1H, d, J=5.0 Hz); MS (ESI): m/z 229 [M+H]+.
N-[(2-Bromopyridin-4-yl)methyl]propan-2-amine (15b)Compound 15b (74%, pale yellow oil) was obtained from 14 and 2-aminopropane in a similar manner to that described for 10a. 1H-NMR (600 MHz, CDCl3) δ: 1.09 (6 H, d, J=6.4 Hz), 2.83 (1 H, spt, J=6.3 Hz), 3.78 (2 H, s), 7.23 (1 H, dd, J=5.0, 0.9 Hz), 7.50–7.51 (1 H, m), 8.28 (1 H, d, J=5.0 Hz); MS (ESI): m/z 229 [M+H]+.
N-[(2-Bromopyridin-4-yl)methyl]-1-methyl-N-propyl-1H-imidazole-4-carboxamide (16a)Compound 16a (81%, pale yellow oil) was obtained from 15a in a similar manner to that described for 9. 1H-NMR (600 MHz, CDCl3) δ: 0.84–0.97 (3H, m), 1.59–1.73 (2H, m), 3.29–4.04 (5H, m), 4.60–4.73 (1H, m), 5.30–5.50 (1H, m), 7.12–7.64 (4H, m), 8.28 (1H, d, J=5.0 Hz); MS (ESI): m/z 337 [M+H]+.
N-[(2-Bromopyridin-4-yl)methyl]-1-methyl-N-(propan-2-yl)-1H-imidazole-4-carboxamide (16b)Compound 16b (98%, pale yellow oil) was obtained from 15b in a similar manner to that described for 9. 1H-NMR (600 MHz, CDCl3) δ: 1.14–1.23 (6H, m), 3.62–3.77 (3H, m), 4.49–4.90 and 5.22–5.34 and 5.75–5.84 (3H, m), 7.17 (1H, br s), 7.27–7.31 (1H, m), 7.37–7.45 (2H, m), 7.57 (1H, br s), 8.24 (1H, br d, J=5.0 Hz); MS (ESI): m/z 337 [M+H]+.
1-Methyl-N-[(6-phenylpyridin-2-yl)methyl]-N-propyl-1H-imidazole-4-carboxamide (7a)To a solution of 13a (150 mg, 0.44 mmol) in N,N-dimethylformamide (2 mL) and ethanol (1 mL) were added phenylboronic acid (107 mg, 0.88 mmol), potassium carbonate (122 mg, 0.88 mmol), and tetrakis(triphenylphosphine)palladium(0) (51 mg, 0.044 mmol), and the reaction mixture was stirred at 150°C under microwave irradiation for 10 min. Water was added, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and brine, dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was purified using silica gel column chromatography (0–5% MeOH in CHCl3) and NH-silica gel column chromatography (67–100% ethyl acetate in hexane) to obtain 7a (98 mg, 67%) as a colorless oil. 1H-NMR (499 MHz, CDCl3) δ: 0.84–0.95 (3H, m), 1.64–1.82 (2H, m), 3.46 (1H, br t, J=7.4 Hz), 3.68 and 3.72 (3H, br s), 4.07 (1H, br t, J=7.2 Hz), 4.93 (1H, br s), 5.53 (1H, s), 7.23–7.33 (2H, m), 7.38–7.49 (3H, m), 7.56–7.61 (2H, m), 7.68 (1H, t, J=7.7 Hz), 7.98–8.03 (2H, m); 13C-NMR (126 MHz, CDCl3) δ: 11.04, 11.45, 20.49, 22.24, 33.54, 48.56, 50.40, 52.01, 53.99, 118.65, 118.72, 119.71, 120.09, 125.90, 126.05, 126.95, 128.67, 128.77, 128.87, 136.58, 137.27, 138.61, 139.33, 139.51, 156.69, 158.14, 158.84, 164.20; HR-MS: Calcd for C20H22N4O [M+H]+ 335.1866. Found 335.1855.
1-Methyl-N-[(2-phenylpyridin-4-yl)methyl]-N-propyl-1H-imidazole-4-carboxamide (7c)Compound 7c (74%, colorless oil) was obtained from 16a in a similar manner to that described for 7a. 1H-NMR (499 MHz, CDCl3) δ: 0.83–0.98 (3H, m), 1.63–1.76 (2H, m), 3.41 (1H, br s), 3.69 and 3.72 (3H, br s), 3.99 (1H, br s), 4.79 (1H, br s), 5.49 (1H, br s), 7.09–7.20 (1H, m), 7.28–7.48 (4H, m), 7.57–7.66 (2H, m), 7.95 (2H, br d, J=6.9 Hz), 8.61 (1H, d, J=4.8 Hz); 13C-NMR (126 MHz, CDCl3) δ: 11.01, 11.42, 20.54, 22.28, 33.59, 48.49, 49.06, 50.13, 51.06, 119.06, 119.41, 120.76, 120.98, 126.50, 127.01, 128.66, 128.91, 136.65, 138.32, 139.33, 148.15, 148.96, 149.74, 157.69, 163.87, 164.32; HR-MS: Calcd for C20H22N4O [M+H]+ 335.1866. Found 335.1856.
1-Methyl-N-[(6-phenylpyridin-2-yl)methyl]-N-(propan-2-yl)-1H-imidazole-4-carboxamide (7d)Compound 7d (52%, colorless oil) was obtained from 13b in a similar manner to that described for 7a. 1H-NMR (499 MHz, CDCl3) δ: 1.14–1.30 (6H, m), 3.65 and 3.73 (3H, br s), 4.86 (2H, br s), 5.43 and 5.72 (1H, br s), 7.28 (1H, br d, J=7.5 Hz), 7.37–7.49 (4H, m), 7.53–7.57 (2H, m), 7.62–7.67 (1H, m), 8.00 (2H, d, J=7.5 Hz); 13C-NMR (126 MHz, CDCl3) δ: 20.23, 21.50, 33.57, 46.88, 47.26, 48.97, 49.99, 118.37, 119.77, 120.03, 125.86, 126.98, 128.43, 128.53, 128.64, 129.47, 131.91, 131.93, 132.05, 132.13, 136.57, 136.83, 137.11, 156.41, 159.28, 164.48; HR-MS: Calcd for C20H22N4O [M+H]+ 335.1866. Found 335.1855.
1-Methyl-N-[(2-phenylpyridin-4-yl)methyl]-N-(propan-2-yl)-1H-imidazole-4-carboxamide (7e)Compound 7e (58%, colorless amorphous) was obtained from 16b in a similar manner to that described for 7a. 1H-NMR (499 MHz, CDCl3) δ: 1.14–1.30 (6H, m), 3.66 and 3.72 (3H, br s), 4.69 and 5.37 (2H, br s), 4.78–4.94 and 5.74–5.86 (1H, m), 7.14–7.25 (1H, m), 7.35–7.49 (4H, m), 7.56–7.59 (1H, m), 7.64 (1H, br s), 7.94 (2H, br d, J=7.5 Hz), 8.57 (1H, br d, J=4.5 Hz); 13C-NMR (126 MHz, CDCl3) δ: 20.37, 21.61, 33.60, 43.88, 46.98, 47.27, 48.85, 109.90, 118.88, 120.51, 126.36, 127.01, 128.64, 128.81, 136.64, 138.37, 139.53, 149.51, 149.74, 150.96, 157.43, 164.48; HR-MS: Calcd for C20H22N4O [M+H]+ 335.1866. Found 335.1851.
N-{[2-(4-Fluorophenyl)pyridin-4-yl]methyl}-1-methyl-N-(propan-2-yl)-1H-imidazole-4-carboxamide Bishydrochloride (7f)To a solution of 16b (1.50 g, 4.4 mmol) in N,N-dimethylformamide (10 mL) and ethanol (5 mL) were added 4-fluorophenylboronic acid (1.24 g, 8.9 mmol), potassium carbonate (1.23 g, 8.9 mmol), and tetrakis(triphenylphosphine)palladium(0) (0.51 g, 0.45 mmol), and the reaction mixture was stirred at 150°C under microwave irradiation for 30 min. Water was added, and the mixture was extracted with CHCl3. The organic layer was dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was purified using NH-silica gel column chromatography (25–100% ethyl acetate in hexane) and silica gel column chromatography (0–10% MeOH in CHCl3). The fractions including the product were collected and concentrated in vacuo. The residue was dissolved in ethyl acetate (10 mL), and 4 mol/L HCl in ethyl acetate (1.9 mL) was added to the solution. After stirring at room temperature for 4.5 h, the resultant precipitate was collected by filtration to obtain 7f (1.41 g, 79%) as a colorless powder. 1H-NMR (600 MHz, DMSO-d6) δ: 1.11–1.31 (6H, m), 3.75 and 3.92 (3H, br s), 4.61 and 4.89 and 5.12 (3H, br s), 7.46 (2H, t, J=8.7 Hz), 7.73 (1H, br s), 8.18–8.28 (4H, m), 8.72 (1H, d, J=5.8 Hz), 9.20 (1H, br s); 13C-NMR (151 MHz, DMSO-d6) δ: 20.90, 35.57, 44.09, 49.94, 116.14, 116.28, 121.37, 122.23, 124.02, 126.61, 129.61, 130.57, 137.10, 144.08, 151.33, 156.70, 159.43, 163.05, 164.70; HR-MS: Calcd for C20H21FN4O [M+H]+ 353.1772. Found 353.1784.
N-{[2-(3-Fluorophenyl)pyridin-4-yl]methyl}-1-methyl-N-(propan-2-yl)-1H-imidazole-4-carboxamide (7g)Compound 7g (49%, colorless amorphous) was obtained from 16b in a similar manner to that described for 7a. 1H-NMR (499 MHz, CDCl3) δ: 1.14–1.30 (6H, m), 3.68 and 3.73 (3H, br s), 4.61–4.92 and 5.24–5.46 and 5.81 (3H, m), 7.08 (1H, td, J=8.2, 2.4 Hz), 7.20 (1H, br s), 7.33–7.50 (2H, m), 7.55–7.65 (2H, m), 7.65–7.74 (2H, m), 8.57 (1H, br d, J=4.8 Hz); 13C-NMR (126 MHz, CDCl3) δ: 20.40, 21.61, 33.62, 43.85, 46.98, 47.32, 48.89, 113.86, 114.04, 115.55, 115.73, 118.94, 121.06, 122.54, 126.41, 128.44, 128.54, 130.06, 130.13, 136.67, 141.79, 149.57, 156.03, 162.27, 164.21; HR-MS: Calcd for C20H21FN4O [M+H]+ 353.1772. Found 353.1784.
N-{[2-(2-Fluorophenyl)pyridin-4-yl]methyl}-1-methyl-N-(propan-2-yl)-1H-imidazole-4-carboxamide Bishydrochloride (7h)To a solution of 16b (150 mg, 0.44 mmol) in toluene (1.5 mL), ethanol (1.5 mL), and water (1.0 mL) were added 2-fluorophenylboronic acid (93 mg, 0.66 mmol), cesium carbonate (217 mg, 0.67 mmol), and tetrakis(triphenylphosphine)palladium(0) (26 mg, 0.022 mmol), and the reaction mixture was stirred at 150°C under microwave irradiation for 30 min. Water was added, and the mixture was extracted with ethyl acetate. The organic layer was dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was purified using silica gel column chromatography (0–10% MeOH in CHCl3). The fractions including the product were collected and concentrated in vacuo. The residue was dissolved in MeOH (2 mL), and 2 mol/L HCl in MeOH (0.8 mL) was added to the solution. The solution was concentrated in vacuo to obtain 7h (165 mg, 87%) as a colorless amorphous. 1H-NMR (600 MHz, DMSO-d6) δ: 1.21–1.30 (6H, m), 3.92 (3H, br s), 4.54–4.69 and 4.87–5.19 (3H, m), 7.42–7.50 (2H, m), 7.63–7.70 (1H, m), 7.81–7.98 (2H, m), 8.05 (1H, br s), 8.22–8.37 (1H, m), 8.83 (1H, d, J=5.8 Hz), 9.06–9.35 (1H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 20.88, 35.65, 44.11, 48.53, 50.00, 116.42, 116.58, 123.02, 123.96, 124.42, 125.11, 126.11, 128.72, 128.80, 131.50, 132.04, 133.16, 137.14, 144.40, 148.02, 158.53, 159.19, 160.20; HR-MS: Calcd for C20H21FN4O [M+H]+ 353.1772. Found 353.1784.
N-{[2-(4-Methoxyphenyl)pyridin-4-yl]methyl}-1-methyl-N-(propan-2-yl)-1H-imidazole-4-carboxamide Bishydrochloride (7i)Compound 7i (73%, colorless powder) was obtained from 16b in a similar manner to that described for 7h. 1H-NMR (600 MHz, DMSO-d6) δ: 1.07–1.24 (6H, m), 3.66–3.81 (3H, m), 3.83 (3H, s), 4.73 (2H, br s), 5.26 (1H, br s), 7.08–7.12 (2H, m), 7.34 (1H, br d, J=5.4 Hz), 7.74–7.90 (1H, m), 7.93 (1H, s), 8.03 (2H, br d, J=8.7 Hz), 8.27 (1H, br s), 8.57 (1H, d, J=5.4 Hz); 13C-NMR (151 MHz, DMSO-d6) δ: 19.79, 21.08, 34.08, 43.55, 48.88, 55.37, 114.43, 119.00, 120.62, 125.35, 128.20, 128.60, 137.38, 146.41, 153.72, 160.92, 162.10; HR-MS: Calcd for C21H24N4O2 [M+H]+ 365.1972. Found 365.1961.
N-{[2-(3-Methoxyphenyl)pyridin-4-yl]methyl}-1-methyl-N-(propan-2-yl)-1H-imidazole-4-carboxamide Bishydrochloride (7j)Compound 7j (59%, colorless powder) was obtained from 16b in a similar manner to that described for 7h. 1H-NMR (600 MHz, DMSO-d6) δ: 1.10–1.30 (6H, m), 3.66–3.93 (3H, m), 3.88 (3H, s), 4.64–5.21 (3H, m), 7.12–7.18 (1H, m), 7.51 (1H, t, J=7.8 Hz), 7.58–7.68 (2H, m), 7.70–7.75 (1H, m), 8.16 (2H, s), 8.70 (1H, d, J=5.8 Hz), 8.98 (1H, br s); 13C-NMR (151 MHz, DMSO-d6) δ: 19.69, 20.94, 35.19, 43.91, 49.64, 55.43, 112.73, 116.70, 119.82, 121.03, 122.01, 124.36, 126.33, 130.29, 135.42, 137.20, 144.92, 152.56, 159.73, 160.07; HR-MS: Calcd for C21H24N4O2 [M+H]+ 365.1972. Found 365.1959.
N-{[2-(2-Methoxyphenyl)pyridin-4-yl]methyl}-1-methyl-N-(propan-2-yl)-1H-imidazole-4-carboxamide Bishydrochloride (7k)Compound 7k (86%, colorless powder) was obtained from 16b in a similar manner to that described for 7h. 1H-NMR (600 MHz, DMSO-d6) δ: 1.15–1.30 (6H, m), 3.85 (3H, s), 3.92 (3H, br s), 4.64 and 4.96 (3H, br s), 7.20 (1H, t, J=7.4 Hz), 7.30 (1H, d, J=8.3 Hz), 7.61–7.73 (2H, m), 7.96 (1H, br s), 8.12 (1H, s), 8.26 (1H, br s), 8.83 (1H, d, J=5.8 Hz), 9.19 (1H, br s); 13C-NMR (151 MHz, DMSO-d6) δ: 20.84, 35.55, 44.29, 49.92, 56.01, 112.34, 119.86, 121.01, 122.97, 124.18, 125.25, 126.59, 131.28, 133.49, 137.18, 141.34, 148.94, 159.15, 159.45; HR-MS: Calcd for C21H24N4O2 [M+H]+ 365.1972. Found 365.1962.
1-Methyl-N-(propan-2-yl)-N-({2-[4-(trifluoromethyl)phenyl]pyridin-4-yl}methyl)-1H-imidazole-4-carboxamide Bishydrochloride (7l)Compound 7l (98%, colorless amorphous) was obtained from 16b in a similar manner to that described for 7f. 1H-NMR (600 MHz, DMSO-d6) δ: 1.15–1.30 (6H, m), 3.75 and 3.92 (3H, br s), 4.62 and 4.83 and 5.04 (3H, br s), 7.55 (1H, br s), 7.92 (2H, m, J=8.3 Hz), 8.11 (1H, s), 8.26 (1H, br s), 8.32 (2H, m, J=8.3 Hz), 8.72 (1H, d, J=5.4 Hz), 9.20 (1H, br s); 13C-NMR (151 MHz, DMSO-d6) δ: 20.96, 35.57, 43.81, 49.90, 120.32, 122.15, 123.92, 124.13 (q, J=247.8 Hz), 125.79, 126.67, 128.02, 129.73, 129.95, 137.10, 140.24, 147.49, 152.90, 159.29; HR-MS: Calcd for C21H21F3N4O [M+H]+ 403.1740. Found 403.1743.
1-Methyl-N-(propan-2-yl)-N-({2-[3-(trifluoromethyl)phenyl]pyridin-4-yl}methyl)-1H-imidazole-4-carboxamide Bishydrochloride (7m)Compound 7m (81%, colorless amorphous) was obtained from 16b in a similar manner to that described for 7h. 1H-NMR (600 MHz, DMSO-d6) δ: 1.17–1.30 (6H, m), 3.76 and 3.93 (3H, br s), 4.59 and 4.87 and 5.06 (3H, br s), 7.63 (1H, br s), 7.80–7.86 (1H, m), 7.92 (1H, br d, J=7.4 Hz), 8.23 (1H, s), 8.28 (1H, br s), 8.43 (1H, d, J=8.3 Hz), 8.47 (1H, s), 8.73 (1H, d, J=5.4 Hz), 9.26 (1H, br s); 13C-NMR (151 MHz, DMSO-d6) δ: 20.94, 35.63, 43.83, 49.98, 120.84, 122.24 (q, J=273.3 Hz), 122.25, 123.86, 124.00, 126.37, 126.75, 127.01, 129.76 (q, J=30.2 Hz), 130.21, 131.42, 136.32, 137.08, 146.43, 152.00, 153.94, 159.23; HR-MS: Calcd for C21H21F3N4O [M+H]+ 403.1740. Found 403.1742.
1-Methyl-N-(propan-2-yl)-N-({2-[4-(trifluoromethoxy)phenyl]pyridin-4-yl}methyl)-1H-imidazole-4-carboxamide Bishydrochloride (7n)Compound 7n (95%, colorless amorphous) was obtained from 16b in a similar manner to that described for 7f. 1H-NMR (600 MHz, DMSO-d6) δ: 1.14–1.30 (6H, m), 3.76 and 3.93 (3H, br s), 4.60 and 4.87 and 5.08 (3H, br s), 7.59 (2H, br d, J=8.3 Hz), 7.68 (1H, br s), 8.15 (1H, s), 8.23–8.29 (3H, m), 8.73 (1H, d, J=5.4 Hz), 9.23 (1H, br s); 13C-NMR (151 MHz, DMSO-d6) δ: 20.94, 35.61, 43.97, 49.94, 119.16, 120.88, 120.97, 121.37, 122.27, 123.94, 126.51, 129.91, 137.10, 145.46, 149.89, 151.79, 159.31; HR-MS: Calcd for C21H21F3N4O2 [M+H]+ 419.1689. Found 419.1690.
1-Methyl-N-(propan-2-yl)-N-({2-[3-(trifluoromethoxy)phenyl]pyridin-4-yl}methyl)-1H-imidazole-4-carboxamide Bishydrochloride (7o)Compound 7o (60%, colorless amorphous) was obtained from 16b in a similar manner to that described for 7h. 1H-NMR (600 MHz, DMSO-d6) δ: 1.15–1.30 (6H, m), 3.77 and 3.93 (3H, br s), 4.61 and 4.86 and 5.02 (3H, br s), 7.51–7.66 (2H, m), 7.73 (1H, t, J=8.1 Hz), 8.12 (1H, s), 8.15–8.18 (2H, m), 8.27 (1H, br s), 8.72 (1H, d, J=5.8 Hz), 9.25 (1H, br s); 13C-NMR (151 MHz, DMSO-d6) δ: 20.94, 35.63, 43.87, 49.96, 119.84, 120.10 (q, J=258.2 Hz), 120.72, 122.25, 122.61, 123.86, 126.49, 131.13, 137.10, 137.59, 146.43, 148.88, 151.88, 154.01, 159.19; HR-MS: Calcd for C21H21F3N4O2 [M+H]+ 419.1689. Found 419.1691.
4-Bromo-N-propylpyridine-2-carboxamide (18)Compound 18 (84%, colorless oil) was obtained from 4-bromopyridine-2-carboxylic acid 17 and 1-aminopropane in a similar manner to that described for 9. 1H-NMR (600 MHz, CDCl3) δ: 1.00 (3H, t, J=7.3 Hz), 1.62–1.69 (2H, m), 3.41–3.46 (2H, m), 7.57–7.60 (1H, m), 7.92–8.01 (1H, m), 8.35 (1H, d, J=5.0 Hz), 8.38 (1H, d, J=1.8 Hz); MS (ESI): m/z 243 [M+H]+.
4-Phenyl-N-propylpyridine-2-carboxamide (19)Compound 19 (88%, pale yellow oil) was obtained from 18 in a similar manner to that described for 7a. 1H-NMR (600 MHz, CDCl3) δ: 1.02 (3H, t, J=7.3 Hz), 1.65–1.72 (2H, m), 3.43–3.51 (2H, m), 7.28–8.60 (9H, m); MS (ESI): m/z 241 [M+H]+.
N-[(4-Phenylpyridin-2-yl)methyl]propan-1-amine (20)To a solution of 19 (0.35 g, 1.5 mmol) in tetrahydrofuran (10 mL) was added lithium aluminium hydride (0.28 g, 7.3 mmol), and the mixture was stirred at 70°C for 2 h. The reaction was quenched with 10% aqueous NaOH solution under ice cooling, dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was purified using NH-silica gel column chromatography (50–100% ethyl acetate in hexane) to obtain 20 (107 mg, 32%) as a yellow oil. 1H-NMR (600 MHz, CDCl3) δ: 0.95 (3H, t, J=7.6 Hz), 1.53–1.62 (2H, m), 2.65–2.69 (2H, m), 3.97 (2H, s), 7.38 (1H, dd, J=5.0, 1.8 Hz), 7.42–7.46 (1H, m), 7.46–7.51 (2H, m), 7.54 (1H, s), 7.63–7.67 (2H, m), 8.60 (1H, d, J=5.0 Hz); MS (ESI): m/z 227 [M+H]+.
1-Methyl-N-[(4-phenylpyridin-2-yl)methyl]-N-propyl-1H-imidazole-4-carboxamide (7b)Compound 7b (28%, colorless oil) was obtained in a similar manner to that described for 9. 1H-NMR (499 MHz, CDCl3) δ: 0.83–0.95 (3H, m), 1.64–1.75 (2H, m), 3.45 (1H, br t, J=7.4 Hz), 3.68 and 3.71 (3H, br s), 4.04 (1H, br t, J=6.9 Hz), 4.92 (1H, br s), 5.55 (1H, br s), 7.28–7.47 (5H, m), 7.54–7.63 (4H, m), 8.58 (1H, br d, J=4.8 Hz); 13C-NMR (126 MHz, CDCl3) δ: 11.01, 11.44, 20.45, 22.22, 33.55, 48.55, 50.26, 51.77, 53.85, 119.34, 119.92, 120.09, 120.22, 126.21, 127.07, 127.16, 129.00, 136.62, 138.31, 138.48, 149.05, 149.24, 149.36, 149.51, 158.79, 159.57, 164.01, 164.20; HR-MS: Calcd for C20H22N4O [M+H]+ 335.1866. Found 335.1858.
Calculation of CNS MPOThe CNS MPO score was calculated according to a previously reported method.19) The c Log P value was calculated by a software from Daylight Chemical Information Systems, Inc. The c Log D, pKa, and tPSA were calculated usign the software from ACD/Laboratories.
BiologyGlycine Uptake Inhibitory AssayGlioma T98G cells expressing human GlyT1 were used. The T98G cells were seeded at a density of 2.0×104 cells/well onto a 96-well plate and cultured overnight in a carbon dioxide incubator. The test compound was dissolved in 100% DMSO and then dissolved in 10 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES) buffer solution (pH 7.4) containing 150 mM sodium chloride, 1 mM calcium chloride, 5 mM potassium chloride, 1 mM magnesium chloride, 10 mM glucose, and 0.2% bovine serum albumin. After the medium for the cell culture was removed, the test compound and [3H]glycine (final concentration, 250 nM) were added to the cells and reacted at room temperature for 15 min. After the completion of the reaction, the labeled glycine solution was aspirated with a manifold. The cells were then lysed with 0.5 mol/L sodium hydroxide solution. The amount of intracellular glycine was determined by measuring the radio activity in the cell lysate using a liquid scintillation counter. The quantity of glycine uptake in the presence of 10 µM (N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl])sarcosine (ALX5407) was defined as nonspecific uptake, and the specific uptake amount was determined by subtracting the nonspecific uptake amount from the total uptake amount in the absence of 10 µM ALX5407. The glycine uptake inhibitory activity (IC50 value) was calculated from an inhibition curve for test compound concentrations of 10−9 to 10−5.
Measurement of Glycine Concentration in CSF of RatsRats were anesthetized by isoflurane 1 h after oral administration of vehicle or 7n, and were sacrificed by cutting of an abdominal aorta. Then, CSF samples were collected from the cisterna magna using a 29-gauge needle. The CSF samples were centrifuged at 21500×g for 10 min at 4°C. The glycine standard and the CSF samples were derivatized with 1.33 mM of o-phthaldialdehyde at 10°C for 2.5 min, then injected into a high-performance liquid chromatography device equipped with an electrochemical detection system, ECD-300 (Eicom, Kyoto, Japan). Derivatized glycine was separated on a reversed phase Octa-Decyl-Silyl (ODS) column, Eicompak SC-5ODS (3.0 mm, i.d.×150 mm, Eicom, Kyoto, Japan), at 35°C with a flow rate of 0.5 mL/min. The electrode potential for the electrochemical detector was set at +600 mV against Ag/AgCl reference electrode. The mobile phase consisted of 0.1 M phosphate buffer solution (pH 6.0) containing 10.0% v/v acetonitrile and 5.0 mg/L ethylenediaminetetraacetic acid disodium salt.
All the studies were reviewed by the Taisho Pharmaceutical Co., Ltd., Animal Care Committee.
The authors would like to thank Dr. Shigeyuki Chaki for helpful discussion: Dr. Takashi Yoshizumi for carefully reading the manuscript and for making useful suggestion to improve it: Dr. Atsushi Okada and Mr. Akira Higuchi for analytical and spectrum studies.
All authors are employees of Taisho Pharmaceutical Co., Ltd.