2016 年 64 巻 12 号 p. 1659-1665
2016 年 64 巻 12 号 p. 1659-1665
The stomatitis caused by anticancer agents and radiation therapy deteriorates patient QOL, potentially causing eating disorders as a result of pain. Although gargling and ointments can be used in the treatment of stomatitis, patients must spit out mouthwash after use, while ointment application requires a finger to be inserted into the oral cavity. In contrast, sprays eliminate these potential compliance problems. Therefore, we developed a stomatitis spray that remains on the oral mucosa. It has been reported that irsogladine maleate (IM) is effective against stomatitis via oral administration. IM is water insoluble; thus, it was dissolved with various cyclodextrins (CDs). Furthermore, we examined combination with gum ghatti (GG), a mucoadhesive polymer. The interaction between mucin and GG was examined by Quartz Crystal Microbalance with Dissipation monitoring. We found that GG exhibited mucoadhesion. Furthermore, we examined the healing effects of IM on stomatitis in a stomatitis model hamster. We found that stomatitis healed after direct application of IM. However, the model used in this experiment is not based on stomatitis caused by anticancer agents. Further study is therefore necessary.
Aphthous stomatitis is a common complication of chemotherapy and radiation therapy. In particular, 100% of patients administered high-dose chemotherapy develops stomatitis. Furthermore, in 5-fluorouracil (5-FU)-based chemotherapy regimens, 40% of patients experience stomatitis with conventional-dose chemotherapy.1–6) With regard to stomatitis caused by anticancer agents, severe cases are often accompanied by pain.1) As a result, patients are apt to reduce their appetite and body weight; thus, QOL is reduced.1) Chemotherapy is thought to initiate stomatitis directly by causing DNA strand breaks, through the generation of reactive oxygen species, or through enzymatic or transcription factor activation in multiple cellular elements within the mucosa.7) Although various treatments for stomatitis have been attempted, they primarily comprise basic oral care, bland oral rinses, analgesics, cryotherapy, topical anesthetics, antimicrobial agents, and anti-inflammatory agents.8)
In general, mouthwashes and oral ointments are administered for remedying stomatitis. However, mouthwashes must be spit out which can induce nausea and vomiting via gargling.9) Furthermore, oral ointments can also induce nausea as a result of finger insertion at the time of application. Therefore, we attempted to develop a therapeutic spray for stomatitis in an effort to overcome these shortcomings.
The surface of mucosa in the oral cavity is covered by mucin which is the main component, we took into account for the interaction between mucin and water-soluble polymers. Because if mucin interacts with contents of spray, the active pharmaceutical ingredient in spray formulation could retain in the oral cavity. A large number of polysaccharides such as amylose, guar gum chitosan pectin, cyclodextrins (CDs), and locust bean gum have been investigated for use in sustained drug delivery systems.10) These polymers have hydrophobic and hydrophilic parts and free hydroxyl groups in the polymeric molecule.10) Therefore, water-soluble macromolecules were selected. We focused on gum ghatti (GG), which is a water-soluble polysaccharide. The water solubility of GG is >90 g/100 mL. GG mainly consists of a calcium salt (on occasions, it may occur as a magnesium salt) of high molecular weight polysaccharides (arabinose, galactose, mannose, xylose, and glucronic acid via hydrolysis).11) GG is approved as a food additive, and although there are no examples of its use as an additive in pharmaceutical products in Japan, it has been used as a pharmaceutical additive in other countries. In this study, GG was used as base for the sprays.
Irsogladine maleate (IM) is a drug with gastric mucosal protective properties that is often used in Japan, South Korea, and China. IM increases intracellular cAMP levels in the gastric mucosa and activates communication between cells.12,13) IM also inhibits the production of reactive oxygen species (ROS) by neutrophils, as well as acting as an ROS scavenger.14) It has been reported that oral administration of IM reduces the incidence of 5-FU-based chemotherapy-induced stomatitis.14) Therefore, we hypothesized that with external application of IM could be efficacious.
However, IM has a bitter taste and is only slightly soluble in water. Therefore, IM was subjected to bitter masking and solubilization with various CDs in this study, as CDs have been used for similar applications.15,16)
In the present study, we prepared and evaluated the oral sprays for stomatitis containing IM and GG as “patient-friendly” dosage form.
IM was purchased from Tokyo Kasei Co., Ltd. (Tokyo, Japan). β-CD, hydroxypropyl-β-CD (HP-β-CD), 2,6-di-O-methyl-β-CD (DM-β-CD), and sulfobutyl ether-β-CD (SBE-β-CD) were used as solubilizing agents. β-CD and DM-β-CD were purchased from Nacalai Tesque Co., Ltd. (Kyoto, Japan). HP-β-CD was purchased from Nihon Shokuhin Kako Co., Ltd. (Tokyo, Japan). SBE-β-CD was purchased from CyDex Pharmaceuticals, Inc. (Lenexa, KS, U.S.A.). Pentobarbital was purchased from Kyoritsu Seiyaku Co. (Tokyo, Japan). Sodium carboxymethyl cellulose (CMC-Na) and acetic acid were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). GG for spray base was provided by San-ei gen F.F.I., Inc. (Osaka, Japan).
All other chemicals were of analytical reagent grade.
AnimalsGolden Syrian hamsters (male; weight, approximately 100 g) were used for evaluation of stomatitis.
The conditions in the laboratory animal facility were: 24±1°C and 55±5% humidity. Hamsters were housed in a 12-h automatic light–dark cycle. Food and water were given ad libitum.
The present study was conducted according to the guidelines and ethics of Toho University and Meiji Pharmaceutical University. All animal experiments were performed on the basis of sufficient knowledge and information from the scientific literature at adequate laboratories with respect to animal welfare.
Solubilization with CDsAt first, we performed examination of solubilizing of the IM in this study.
In order to examine the improvements in IM solubility by the addition of β-CDs, data were plotted in a phase solubility diagram.
Various amounts of CDs were weighed in an L-shaped tube. An excess amount of IM was then added to each L-shaped tube. L-Shaped tubes were shaken at constant rate (171 min−1) for 5 h at 30°C. After the equilibrium, samples were passed through a membrane filter (0.2 µm).
IM concentration was measured by HPLC (LC-20AD; Shimadzu Corporation, Kyoto, Japan).
Samples were injected onto a C18 column [Shodex® (C18M 4D), 4.6×150 mm; Showa Denko K.K., Tokyo, Japan] at 40°C. The flow rate was set at 1.0 mL/min for all separations using a mobile phase composed of methane sulfonic acid solution (1→1000) and methanol (4 : 1). λmax was 254 nm.
The mean of 3 samples was calculated.
Stability constant of the complex was calculated using the following equation.
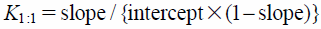 | (1) |
Acetic acid solution (10%, 25 µL) was injected into the mucous membrane of the center of the hamster’s cheek pouch sandwiched between ring forceps 5 mm in inner diameter under anesthesia by the pentobarbital, which induced stomatitis, 2 d before applying IM (shown as “−2” in the measurement schedule) (Fig. 1).
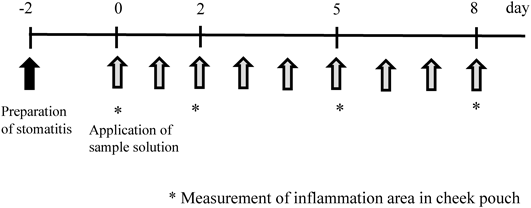
IM was applied 2 d after injecting 10% acetic acid, and the major and minor axis of the injury area part were measured using the vernier caliper on days 0, 2, 5, and 8 (Fig. 4). Then, surface area of injury area was calculated as a surface area of ellipse from the measurement value. IM solution (200 µL) or solvent was applied onto stomatitis in hamster cheek pouches. The injury area was determined by measuring the major axis (a) and minor axis (b) of the lesion using a Shinwa Digital Caliper under pentobarbital anesthesia on days 0, 2, 5 and 8, and calculated as an elliptic area (a×b×π/4).
Statistical AnalysisNumerical results were expressed as the mean±standard error (S.E.), and data were analyzed using Student’s test and Welch’s t test. Differences at p<0.05 were considered to be statistically significant.
Preparation of Sample SolutionsThe composition of sample solutions is shown in Table 1.
| IM | HP-β-CD | GG | |
|---|---|---|---|
| IM solution | 80 mg | 4.0 g | — |
| Oral spray solution of IM | 80 mg | 4.0 g | 0.5 g |
Composition per 10 mL.
IM solution included IM 80 mg dissolved in refined water and 4 g of HP-β-CD per 10 mL, and the sprays contained 5 g of GG in IM solution.
Viscosity Measurement of SolutionsThe viscosity of various solutions was measured using a viscometer (TVE-35; Toki Sangyo Co., Ltd., Tokyo, Japan). A cone rotor (1°34′× R24; shear rate coefficient: 3.83) was used for measurements, which were performed at 25±1°C.
Evaluation of Spray ContainerThe sprayability of sample solutions was evaluated using a plastic spray container.
The spray container was provided by Aptar Pharm Japan (Tokyo, Japan). The volume of the container was 20 mL, the length of the nozzle was 65 mm and the diameter of the pore of spray was 1 mm (Fig. 2). We chose the length of the nozzle because it was considered that this length of the nozzle was enough length to reach depths of the oral cavity. The formulation or purified water (20 mL) was placed into the spray container. It was reported that the power of the tip of a finger to push was from 20 to 50 N, therefore it was sprayed at constant pressure and speed.17,18) It was 30 N and 10 mm/s, respectively.

Data were obtained using a creep meter (Rheoner II; Yamaden Co., Ltd., Tokyo, Japan). The amount of solution sprayed from the nozzle was measured as weighing in the balance of the amount of spray before and after. The difference of the quantity was considered to be quantity of mist per the once of the spray.
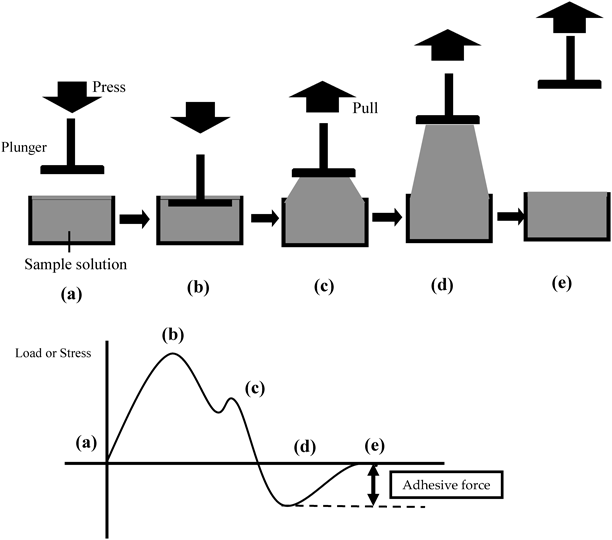
Measurement of Adhesive Strength of Sample SolutionAdhesive strength of the sample solution was measured using a creep meter (Rheoner II; Yamaden Co., Ltd.).19) The method is summarized in Fig. 3. Thirty milliliters of sample solution were poured in a container. The container’s diameter and depth were 45 mm and 25 mm, respectively. This evaluation used a plunger made of Teflon® to simulate oral mucosa.19) The plunger’s diameter was 20 mm, and the surfaces of the plunger and sample solution came into contact with one another. The top of the plunger was dipped to a depth of 1 mm, and the plunger was pulled up at a speed of 1 mm/s.19) The adhesive force was measured when the plunger was completely separated from the surface of the sample solution.19)
The interaction between mucin and sample solution was measured by QCM-D (Quartz Crystal Microbalance with Dissipation monitoring) (Q-sense; Meiwafosis Co., Ltd., Tokyo, Japan).20)
Mucin derived from pig’s stomach (Sigma-Aldrich, Inc., St. Louis, MO, U.S.A.) was used as alternative of a transmembrane oral model.
Au-coated sensors were used for measurement of QCM-D. Experiments were performed at 37.0±0.1°C. AT cut quartz crystals oscillated at 4.95±0.05 MHz, and the oscillation was monitored. The measurement frequency was measured at 3, 5, 7, 9, 11 and 13 times of 4.95±0.05 MHz. Dissipation (D value) is related to the viscoelasticity of an adsorption layer formed by the surface of sensor. Change in shift (Δf) is related to the amount of substance adsorbed on the surface of the sensor, while dissipation shift (ΔD) reflects the viscoelastic properties of the adsorption layer.
Immobilized mucin on the gold sensor flowed at 0.1% (w/w) mucin aqueous solution at 100 µL/min. After flowing of mucin, purified water flow was started for fixation of mucin. Mucin was progressively adsorbed onto the gold surface. When IM solution flow was started, purified water flow was started for fixation of IM solution (Fig. 4). Both Δf and ΔD were monitored.20)

(a) Flow of mucin solution, (b) Flow of purified water (=Fixation of mucin), (c) Flow of IM solution, (d) Flow of purified water (=Fixation of IM).
β-CDs have been widely used in pharmaceutical applications because of its ready availability and a cavity size suitable a wide range of drugs.21) In this study, various β-CDs were studied.
Figure 5 shows phase solubility diagrams for IM/CD systems at 30°C. In β-CD, HP-β-CD and DM-β-CD, the solubility of IM increased linearly and showed AL type in a classification of Higuchi and Connors.22) The order of the increase was β-CD<HP-β-CD<DM-β-CD. On the other hand, the solubility of IM increased in SBE-β-CD curvilinearly and showed AN type.

■, β-CD; ▲, HP-β-CD; ●, DM-β-CD; ◆, SBE-β-CD.
The stability constants (K′) of IM and various β-CD complexes were listed in Table 2. The IM/DM-β-CD complex demonstrated a high stability constant of 824 M−1. However, in vitro, hemolytic activity of CDs has been reported, with the potency being reported as DM-β-CD>HP-β-CD.23)
| CD | K′ (M−1) |
|---|---|
| β-CD | 83.90 |
| HP-β-CD | 99.89 |
| DM-β-CD | 824.24 |
| SBE-β-CD | 310.59 |
Binding of guest to HP-β-CD is very similar to β-CD.21) Furthermore, HP-β-CD is widely used as a pharmaceutical solubilization agent, and is known to have high safety.21) Subsequently, HP-β-CD was used as a solubilizing agent in this study. The K′ of IM and HP-β-CD was 99.89 M−1. As a result, IM was dissolved in HP-β-CD, as shown in Table 1.
Evaluation in AnimalsIt has been reported that IM reduces stomatitis after oral administration.24,25) However, there is no such precedent for its use as a medicine for external application. Therefore, the effects of its use against stomatitis after direct application were investigated in this study.
Chemotherapy-induced stomatitis is due to direct DNA damage caused by chemotherapy causing and generation of ROS that can indirectly damage to the connective tissue of oral mucosa. IM produces the increase of intracellular cAMP, and inhibits the ROS production in neutrophils by the increase of cAMP content by phosphodiesterase type 4 (PDE4) inhibition. The inhibition of the ROS production in neutrophils may result in the reduction of chemotherapy and/or radiotherapy-induced damage.14) Furthermore, the proinflammatory cytokine tumor necrosis factor (TNF)-α is a necessary factor in the chain of pathophysiological events leading to inflammation. IM inhibits the release of TNF-α.26)
Figure 6 shows the effects of IM on stomatitis in hamster cheek pouches. When IM solution was administered onto stomatitis in hamster cheek pouches, the area of stomatitis significantly decreased as compared to that in the control group. This indicates that IM affects stomatitis on the hamster’s cheek pouch.

Change in stomatitis area; ◆, control group; ▲, IM solution group. Means±S.E. (n=9), statistical analysis was performed by t-test. * p<0.05 vs. control group.
The results shown in Fig. 6 were due to the application of a solution containing IM alone.
In this study, the model of stomatitis was prepared by injection of 10% acetic acid solution, inducing inflammatory stomatitis. The effects of IM were thus the result of direct action on the induced inflammation. The scavenging action of IM on ROS has been reported; it will therefore be necessary to examine its effects against stomatitis derived from ROS during treatment with anticancer agents such as 5-FU in future study.
Viscosity Measurement of SolutionsFigure 7 shows rheograms of various sample solutions. As CMC-Na solution has been widely used as a dispersing agent in hospital preparations, it was used as a control solution.
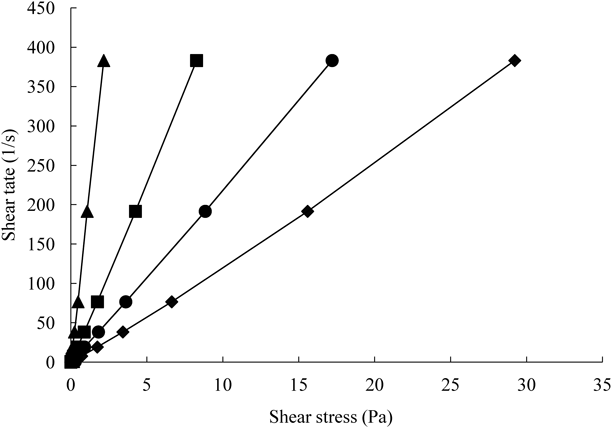
■, GG 5% (w/w); ◆, GG 10% (w/w); ▲, CMC-Na 1% (w/w); ●, IM/HP-β-CD+GG 5% (w/w).
In Fig. 7, all rheograms of GG solution showed Newtonian flow characteristics, and their viscosity was higher than that of CMC-Na solution. The viscosity of GG solution increased with GG concentration. As shown in Fig. 7, viscosity of polymer solution increased with the addition of HP-β-CD. Sekhara et al. demonstrated that the apparent viscosities were enhanced with increase in the proportion of CD to xanthan gum (0.1%) or guar gum (0.5%) solutions.27) They considered that the viscosity enhancement might be due to the formation of bridges between adjacent polymer chains in strong association network. This suggests that there are some interactions, such as hydrogen bonding, between GG and HP-β-CD.
Evaluation of Spray ContainerFigure 8 shows the changes in the amount of sample solution sprayed from the spray nozzle.

◆, purified water, ■, IM solution.
In the case of purified water, the average amount sprayed was 50 µL, which was the amount stated for the spray container. On the other hand, in the case of IM solution, the average amount sprayed was about 60 µL.
This difference was due to differences in viscosity, which can affect the flow of solution through the nozzle. According to the package insert of IM, the daily dose of IM is 4 mg, and as the quantity of one push from the spray nozzle was 60 µL, the concentration of IM was set at 80 mg/mL.
Measurement of Adhesive Strength of Sample SolutionThe adhesive properties of GG solution on oral mucosa were examined using a creep meter. A 1% (w/v) CMC-Na solution was used as a control solution.
As shown in Fig. 9, the adhesive force of 5 and 10% (w/v) GG solution was higher as compared with CMC-Na solution. However, there was no difference in adhesive force between 5 and 10% (w/v) GG.
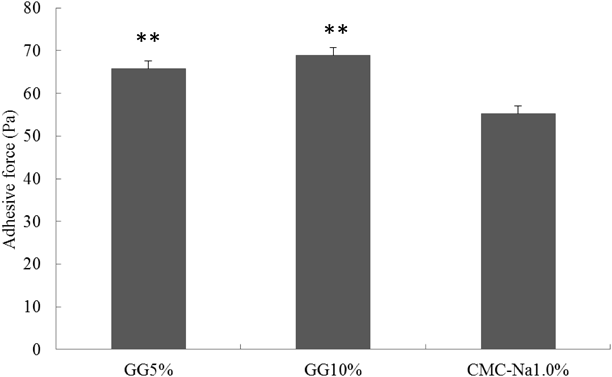
Means±S.D. (n=3), statistical analysis was performed by Scheff’s F test. ** p<0.01 vs. CMC-Na 1.0% (w/v).
Therefore, the 5% GG solution was used as a basis of the spray in this study.
Retention of Artificial MucosaThe interaction between mucin and GG were subsequently measured. QCM-D was used to evaluate the adhesiveness of GG to mucin as the artificial mucosa model.
QCM-D is equipment for simultaneously measuring change of mass (Δf) and thickness (ΔD) on a surface of a quartz sensor, which induce a decrease in f value and an increase in D value. These changes provide the mass and thickness of the adsorbed layer. When molecules are adsorbed on a sensor, f values decrease, and D values increase.
Figures 10(a)–(c) show the changes in f value and D value in the time. Figure 10(a) shows to remain amount of IM on QCM-D sensor. The Y-axis on the left edge of the graph shows f value that is change in mass, and the Y-axis on the right edge of the graph shows D value that is change in thickness. The X-axis shows progress of the time. Each line shows the difference of the frequencies in Figs. 10(a)–(c). With progress of the time, Δf refers to change in mass, and ΔD refers to changes in thickness on surface of the gold sensor, respectively. The surface of the gold sensor vibrates at a definite frequency. At the same time, a change in D value, which refers to thickness, might be also observed. If mucin and sample solution interact, it is considered that D values increase and f values decrease. Interactions between mucin and sample solution were assessed by measuring the changes in f value and D value in this study.
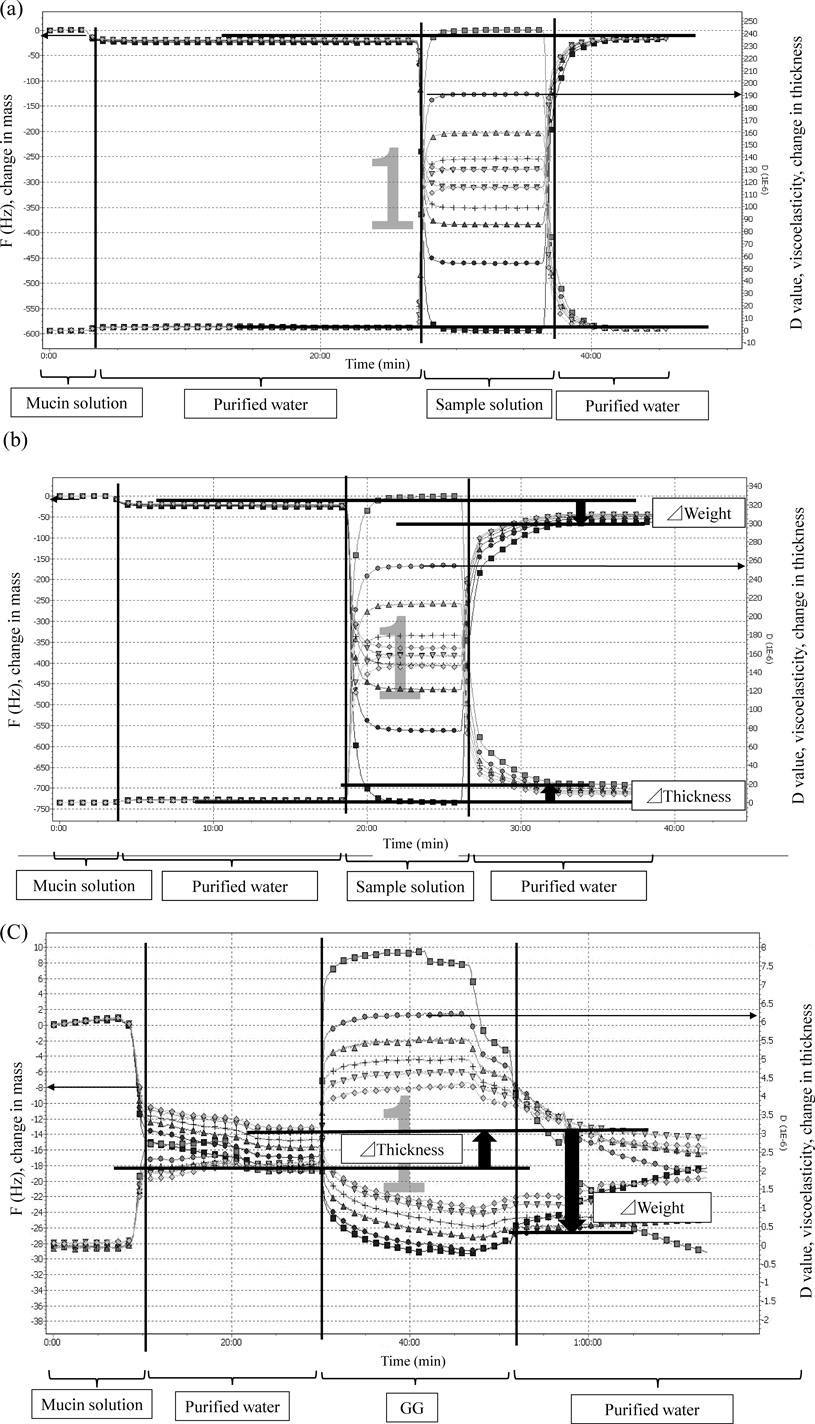
(a) *, 8 mg/mL, diluted 10-fold; ■, 3 times of 4.95±0.05 MHz; ●, 5 times of 4.95±0.05 MHz; ▲, 7 times of 4.95±0.05 MHz; +, 9 times of 4.95±0.05 MHz; ▼, 11 times of 4.95±0.05 MHz; ◆, 13 times of 4.95±0.05 MHz; (b) ■, 3 times of 4.95±0.05 MHz; ●, 5 times of 4.95±0.05 MHz; ▲, 7 times of 4.95±0.05 MHz; +, 9 times of 4.95±0.05 MHz; ▼, 11 times of 4.95±0.05 MHz; ◆, 13 times of 4.95±0.05 MHz; (c) ■, 3 times of 4.95±0.05 MHz; ●, 5 times of 4.95±0.05 MHz; ▲, 7 times of 4.95±0.05 MHz; +, 9 times of 4.95±0.05 MHz; ▼, 11 times of 4.95±0.05 MHz; ◆, 13 times of 4.95±0.05 MHz.
Figure 10(a) shows remaining amount of IM on QCM-D sensor. In other words, Fig. 10(a) shows interaction of between IM and mucin. In the case of this study, in Fig. 10(a), when the mucin solution was circulated on the surface of a gold senor, sudden decreases in Δf due to the adsorption of mucin were observed. These changes were still observed after rinsing the surface of the gold sensor with purified water, this indicates that mucin was strongly adsorbed on the surface of gold sensor.
Subsequently, IM solution containing HP-β-CD was circulated on the surface of the mucin coated gold sensor, changes in f or D value, which indicate the adsorption of IM solution on mucin were observed. Furthermore, purified water flow carried the IM solution away; f or D values recovered by rinsing the surface of the gold sensor with purified water, this suggested that only IM molecules were removed and mucin was kept adsorbing on the gold sensor. This demonstrates that IM solution did not interact with mucin.
Figures 10(b) and (c) show the effect of adsorption of GG on the changes in f or D value. Figure 10(b) shows remaining amount of IM solution containing HP-β-CD and GG on QCM-D sensor, and Fig. 10(c) shows remaining amount of GG on QCM-D sensor.
IM solution containing HP-β-CD and GG flowed. After that, purified water flowed on the surface of the gold sensor. Then f and D values did not recover until the first D value in Fig. 10(b). In other words, the f value decreased and D values increased from mucin. Therefore, GG was adsorbed onto the mucin layer. This suggests that mucin and GG interacted together.
f and D values did not decrease below the first D value in Fig. 10(b). Therefore, GG was adsorbed onto the mucin layer. This suggests that mucin and GG interacted together.
As shown in Fig. 10(c), when purified water flowed after adsorbing GG on the mucin layer, D and f values did not decrease below the initial D and f values. Based on these results, the presence of GG improves adhesion on the mucin surface.
GG is a polysaccharide. The structure consists of L-arabinose, D-galactose, D-mannose, xylose, D-glucuronic acid, and traces of methylpentose.14) Therefore, the structure has numerous hydroxy groups and carboxyl groups. On the other hand, mucoadhesive properties are considered to be macromolecular, based on the physical interactions and resistance between mucin chains.28) Mucin is composed of a polypeptide backbone and covalently linked oligosaccharide side-chains.28) The glycoprotein exhibits electrostatic, hydrophobic, and H- bonding interactions.29) When a polymer includes numerous hydroxy and carboxyl groups, it leads to mechanical resistance.30)
As GG has numerous hydroxy groups, mucin and the hydroxy groups of GG were considered to interact together. The use of a QCM-D sensor may be feasible for evaluating the adhesion properties of polymers and oral mucosa.
GG has mucoadhesive properties, as shown by the results of QCM-D sensor in Fig. 10(c). We expected greater efficacy when GG was added to IM solution due to the prolonged presence of IM in the oral cavity.
In this study, we prepared IM spray for stomatitis treatment. IM is widely used as a medicine for the treatment of gastric ulcer in Japan, South Korea, and China.
IM has poor solubility and bitter taste. Therefore, we used HP-β-CD to overcome these shortcomings.
The effects of IM against stomatitis has only been reported for oral administration as tablet. Therefore, the effects of IM being administered directly onto stomatitis were examined using acetic acid induced stomatitis model hamster. We found that stomatitis was cured by applying IM. Based on this result, when IM was administered directly onto stomatitis, healing of stomatitis is expected. However, the stomatitis model in this study was not induced by anticancer agents such as 5-FU; thus, further study is required in future study.
Furthermore, we examined the characteristics of atomization from spray containers. The amount of the IM formulation atomized was 60 µL. Thus, the concentration of IM was set at 8 mg/mL when mixed with HP-β-CD, meaning that a daily dose of IM of 4 mg/d could be administered with 9 sprays/d. Furthermore, we studied the mucoadhesiveness property of GG. We found that the mucoadhesion of 5 and 10% (w/v) GG solution was higher than that of 1% (w/v) CMC-Na, which is used as a basis of spray for the oral cavities.9,31) The interaction between mucin and GG was also examined by QCM-D. Mucin is a constituent part of the oral mucosa. Based on the results of QCM-D, GG was considered to adhere to the oral mucosa. Thus, by using GG as the basis for a spray formulation applying to the oral cavity, preparation of a medicine that remains in the oral cavity is possible.
We would like to thank San-ei gen F.F.I., Inc. for providing gum ghatti. We also would like to thank Aptar Pharm Japan for providing spray containers.
The authors declare no conflict of interest.