2016 年 64 巻 12 号 p. 1720-1725
2016 年 64 巻 12 号 p. 1720-1725
The aim of this study is to develop a novel milling system using supercritical carbon dioxide (SC-CO2) for the improvement of dissolution characteristics of water-poorly soluble drugs. SC-CO2 possesses high potential in the application of nanotechnology, due to the attractive properties of SC-CO2 fluid such as cheap, inert and non-polluting. In addition, SC-CO2 has density comparable to a liquid, viscosity similar to a gas, and high diffusion capacity. Most of all, carbon dioxide exists as gas in room temperature and pressure, which enables the removal of fluid instantaneously. In this study, a novel method of milling using SC-CO2 was proposed to produce fine-drug particles. SC-CO2 milling was conducted and its performance was compared with the ones by various milling methods such as jet milling, dry milling and wet milling. A comparison on the effect of each milling medium on its milling performance, drug size distribution, and particle morphology was conducted. Operating variables of the SC-CO2 milling system were also investigated to clarify the factors affecting the milling properties and to improve drug release characteristics of water-poorly soluble drugs.
Currently, almost half of new active pharmaceutical ingredients (API) are reported as being either insoluble or poorly soluble in water.1–3) These drug products are categorized as class II of the Biopharmaceutics Classification System (BCS), which have high permeability but low aqueous solubility.4) For the BCS class II drugs, the dissolution step is the rate limiting factor in drug absorption. Improving the dissolution rate of poorly water-soluble drugs is thus critical for enhancing drug bioavailability and it has become a major challenge for the pharmaceutical industry.5) One of the strategies to improve the drug dissolution is to use a milling.6,7) Milling, also known as micronization, grinding and pulverization, is defined as an operation in which mechanical energy is applied to coarse particles to physically break down them into fine particles.8) Through milling, drug particles can be broken down into smaller size, leading to the increase in surface area.9) It is also expected to produce drugs in its amorphous state, which improves drug bioavailability.10,11) Milling equipment has improved drastically over the years to produce finer particles down to sub-micron size. Generally, the method of milling includes dry milling and wet milling.12) Dry milling is conducted with the drug in its dry state whereas wet milling is conducted in a liquid medium. In the dry milling, the ground products require no drying process. However, due to its dry state, the product prepared by the dry milling has higher surface free energy (mostly due to the van der Waals force or hydrogen bonding), which leads to undesirable aggregation, and thus reducing milling efficiency.13) By contrast, aggregation of drug particles can be prevented by using a wet milling system, with liquid medium (water or organic solvents). Surfactants and polymers are often added to minimize the aggregation and improve the drug flowability.14) This allows the production of finer drug particles around sub-micron size, thus effectively improving the solubility of drugs.15) However, as stated previously, it is necessary to extract liquid medium from the drug solute, which also leads to the aggregation of drug particles. Furthermore, the use and disposal of organic solvents cost an enormous expenditure, and it may cause damage to the human body and the environment since it is toxic.16–18) For these reasons, the use of supercritical carbon dioxide (SC-CO2) as a medium for wet milling gathers special interest.
SC-CO2 is a fluid state of carbon dioxide where it is held at or above its critical temperature and pressure.19,20) The supercritical temperature of carbon dioxide is 304 K, and the supercritical pressure is 7.4 MPa. The supercritical points are relatively low as compared to other fluids, making SC-CO2 suitable as a medium for the milling of drugs which are typically weak to heat. The SC-CO2 has density similar to a liquid, allowing the molecules to move freely and collide with the drug particles more frequently. At the same time, SC-CO2 possesses viscosity similar to a gas, which allows the molecules to penetrate into the drug particles.21–23) Most of all, carbon dioxide exists as gas in room temperature and pressure, producing drugs in its dry state once it is released to the atmosphere. Various studies have been conducted relating to the use of SC-CO2 as solvent, solutes, anti-solvents and reaction media in the bottom-up approach.24,25) For example, saturation of SC-CO2 with a solid substrate produces nanoparticles in the rapid expansion of supercritical solutions (RESS) process.26–28) Formation or “synthesis” of nano-sized materials from the molecular scale effectively produces nanoparticles with a uniform size distribution.29) However, limiting factors such as solubility of drug particles in SC-CO2 causes restriction in the compatibility usage in the pharmaceutical industry.30) In addition, poor yield of the bottom-up process causes difficulty in scaling-up of these methods.31,32) Thus, a method of using SC-CO2 in the top-down approach has been proposed. Milling using SC-CO2 was used as a method to produce fine-drug particles with a larger yield and at the same time producing fine-drug particles with a uniform size distribution.
The aim of this research is to develop a novel milling system using SC-CO2 for the improvement of dissolution characteristics of water-poorly soluble drugs. A study was conducted to test the capability of SC-CO2 as a milling medium of drugs by making comparison with dry milling, wet milling with water and SC-CO2 milling with under the same condition. A study was also conducted to investigate the effect of mechanical parameters of SC-CO2 milling system, and the properties of SC-CO2 on its milling performance, drug size distribution, X-ray diffraction (XRD) patterns and improvement of drug release characteristics.
A non-steroidal anti-inflammatory Indomethacin (IDM, Mw=357.79 g/mol) obtained from Wako Pure Chemical Industries, Ltd., Japan, was used as a model drug in this experiment. IDM is known as a class II drug of the BCS that exhibits poor solubility to water. The original IDM has a median diameter of 56.72 µm, melting point of 433 K, and solubility of 0.937 mg/L (at 298 K) in water.
Milling SystemFigure 1 indicates an experimental set-up used. The equipment consists of a carbon dioxide tank, cooling system, pump, heater and a motor with an agitating blade. A 0.8 L milling vessel was used, in which zirconia beads (Nikkato Co., Japan) of 0.5 mm were filled with 15 vol% of the vessel.
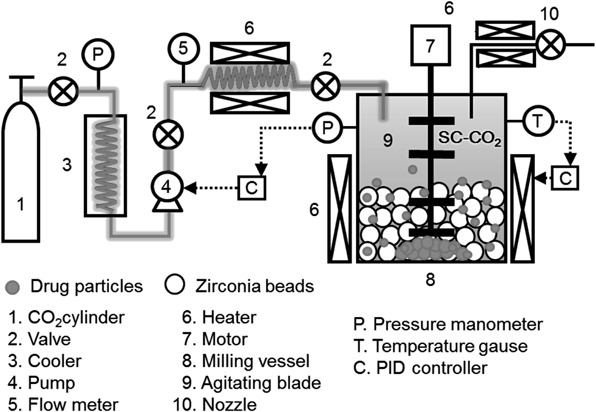
Five grams of IDM was charged into the milling vessel filled with 15 vol% of 0.5 mm zirconia beads. 257 K carbon dioxide liquid was heated to 313 K before being supplied into the milling vessel. The temperature and pressure inside the milling vessel were set at to 305 K and 10 MPa respectively to achieve its critical condition. The agitating blade was rotated and the drugs were milled for a pre-determined time. The rotational speed of the milling vessel is set to a maximum speed of 1600 rpm. Based on the preliminary experiments, setting the grinding time to 10 min produces the finest drug particles compared to a longer grinding time, due to the fact that excess input of energy causes unnecessary aggregation in particles. After the milling operation, the pressure was released back to atmospheric pressure while the temperature inside the vessel was kept constant. This procedure also produced dried IDM powders. In this study, performance of SC-CO2 milling system was compared with the ones of different kinds of milling system; dry milling was conducted under no medium condition and wet milling was conducted by adding deionized water were conducted using the same milling system. All milling operations were conducted with the same operating conditions to compare the compatibility and feature of each medium for the milling of drug particles without the effect of operating parameters. In order to collect IDM powders stuck on the beads, beads collected in each experiment were collected and immersed in 200 mL deionized water and an ultrasonic treatment was conducted for 5 min. The dispersion was separated from the beads and IDM powder was extracted with a rotary vapor for evaluation. Experiments were also conducted to study the effect of various pressure and temperatures of supercritical fluid on the dissolution rate of drugs. Pressure was varied at 10–14 MPa, while temperature was varied at 305–325 K.
AnalysisParticle size distribution of IDM was measured using a laser diffraction particle size analyzer (SALD-2100, Shimadzu Co., Japan). Volume based median size was calculated based on the obtained results. The same measurement was conducted 3 times for each product. Particle morphology of IDM was analyzed by a high resolution scanning field emission electron microscopy (SEM; JSM-6700F, JEOL, Japan) operated at 15 kV. The samples were sputter coated with Pt–Pd for 3 min by a sputter coater (Hitachi Ion Sputter E-10, Hitachi, Japan) before the analysis. The crystallinity of IDM was measured by an XRD device (RINT 1500, Rigaku, Tokyo, Japan). X-Rays are produced with Cu radiation (40 kV, 80 mA), diffraction intensity was measured between 2θ=5–40° every 0.1°.
In Vitro Drug Release StudiesDissolution profile of IDM was measured using a dissolution tester (NTR-3000, Toyama Sangyo Co., Ltd., Japan) with a flow cell, online sampling system and an UV-Vis analysis system (UV-1200, Shimadzu Co.). Four milligrams of IDM was inserted into a gelatin capsule (Matsuya Co., Ltd., Japan) and then fed into the 900 mL deionized water at 310 K and dissolution test was performed under a paddle speed at 100 rpm. The UV reading was conducted at wavelength of 320 nm. In these evaluations, an ultra-micro filter with pore size of 0.2 µm (F-216, Toyama Sangyo Co., Ltd.) was used to filter the dissolution liquid before feeding to the UV-Vis. Dissolution test was repeated 3 times for each condition.
Dry milling, wet milling with water and SC-CO2 milling were conducted using the same beads milling system to break down the IDM particles. On the other hand, IDM particles from jet milled were supplied by Kongo Chemical Co., Japan as a control data for dry milling to show the limit of milling capability of a dry milling system. Figure 2 shows particle size distribution of IDM particles obtained from each milling process. Figures 3 and 4 indicate the SEM images and the dissolution profile of IDM obtained by various milling operations, respectively. Figure 5 shows the results of X-ray powder diffraction of the original powder and milled powder obtained from each milling process. From Fig. 2, each milling operations decreased IDM size drastically. Median diameter of the original particles change from 56.7 µm (geometric standard deviation, σ=3.17) to 3.51 µm (σ=2.20) for dry milling, 2.25 µm (σ=1.80) for jet milling, 2.13 µm (σ=1.93) for wet milling with water and 1.68 µm (σ=1.81) for SC-CO2 milling. It is clear that the milling performance of wet milling is better than the one of dry milling. IDM drugs primitively possess high surface free energy, mainly due to the fact that IDM is highly polarized with strong hydrogen bond and dipole forces. Dry milling thus induced strong intermolecular forces between IDM particles, resulting in aggregation between particles. As a result, the product in dry milling showed less satisfying milling performance. On the other hand, wet milling was conducted in a liquid medium to decrease the surface free energy of IDM drugs. In this case, water helped stabilize IDM drugs in the suspension, allowing better performance in the milling process. However, it could be said that milling IDM with water alone was not effective due to the fact that drug particles supplied to the system was unable to disperse uniformly into the water medium. IDM is known for its water insoluble properties, where the molecular structure of IDM exhibits hydrophilic properties. A lot of studies including the addition of polymers or surfactants help in the dispersion of IDM in water for the wet milling operation.33–35) Finally, the use of SC-CO2 as a medium showed better results as compared to water. This is due to the fact that SC-CO2 has high density, low viscosity and high permeability. The high density in SC-CO2 allowed carbon dioxide and drug particles to collide with each other more frequently, while low viscosity allowed zirconia beads to move freely in the liquid medium thus providing higher collision frequency. The high permeability of SC-CO2 contributed to milling operation by allowing carbon dioxide molecules to penetrate into the drug particles. The release of SC-CO2 back to atmospheric state was also a major factor in which fine particles were formed. When the temperature inside the milling vessel was maintained at a constant value while pressure was returned to atmospheric state, sublimation of SC-CO2 occurs. Carbon dioxide gas was formed rapidly from the supercritical state, and this helps prevent the aggregation of drug particles inside the milling vessel. Subsequently, drug particles were produced at its dry state. However, as seen in Fig. 2, two sharp peaks can be seen from the particle size distribution of drug particles obtained from SC-CO2 milling. It can be assumed that drug particles aggregated upon contact with water molecules in the air during the collection and analyzation of milled drug particles. Humidity control may be necessary in the future experiments. As a conclusion, SC-CO2 milling shows the best milling performance among the other milling methods. In Fig. 3, SEM images show that IDM particles were broken down into smaller size after each milling operations. However, the state of breakage by jet mill, dry milling and wet milling with water suggest that IDM particles aggregate with one another, due to the strong intermolecular force of particles. Breakage occurred only on the surface of particles, producing a product with a mixture of large and fine particles. On the other hand, the state of breakage by SC-CO2 milling shows that, particles were broken down into smaller consistent size. This indicates that particles were able to disperse uniformly in the fluid. As a result, aggregation was minimized and breakage energy was transferred equally on the entire suspension, producing fine, uniform sized particles. In Fig. 4, dissolution rate of IDM improved in the order of dry milling, jet milling, wet milling with water and SC-CO2 milling. This order happens to be the same in the decreasing order of the particle size of IDM. As described in the Noyes Whitney equation,36) the dissolution rate is directly proportional to the specific surface area of a dissolving solid. A decrease in particle size leads to an increase in the specific surface area, and subsequently increases the dissolution rate of drug. In this case, dissolution rate is the highest for IDM milled with SC-CO2, which has the smallest particle size. As a conclusion, the particle size of IDM particles plays an important factor in the enhancement of drug dissolution, where the smaller the particle size of drugs, the higher the dissolution rate. Results from Fig. 5 show that crystallinity of IDM showed slight decrease in intensity for jet milling, dry milling, wet milling and SC-CO2 milling. In addition, the crystallinity of wet milling and SC-CO2 milling decreased more compared to the crystallinity of jet milling and SC-CO2. This is due to the fact that the presence of medium inside the fluid helps increase the energy transfer from beads to the drug particles. Collision of beads and drug particles only is insufficient compared to the collision of beads and drug particles and secondary collision of drug particles and water/CO2 molecules. With the presence of medium molecules inside the fluid, secondary collision increases the collision frequency, and this helps increase the energy transfer to the drug particles. However, crystallinity changes of milled particles were not drastic and amorphous of drugs was not confirmed. The reason for this is the size of the beads used was too small, and the collision energy was insufficient to cause irregularity on the arrangement of IDM particles.
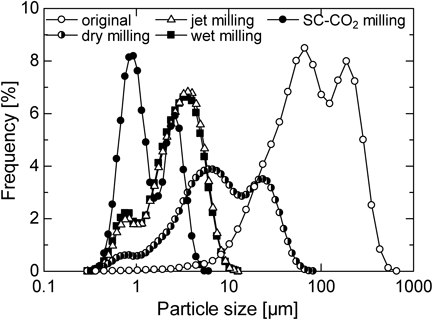
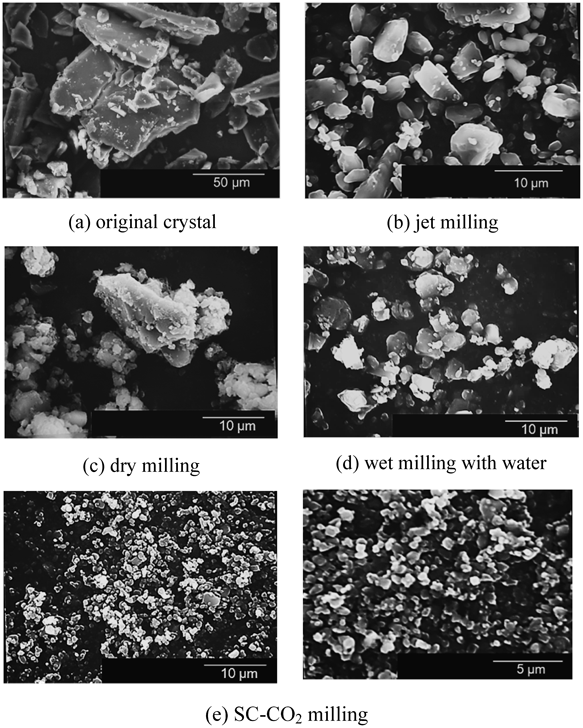

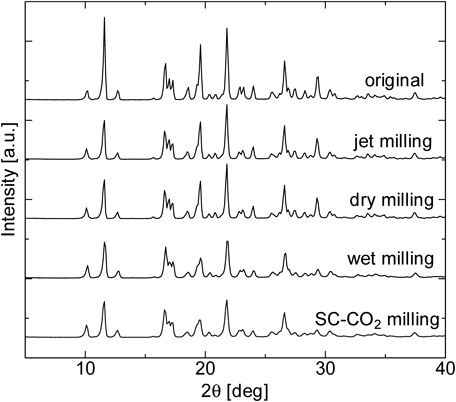
Figure 6 indicates the effect of SC-CO2 density on the median diameter of IDM, whereas Fig. 7 shows the effect of SC-CO2 density on the dissolution rate of IDM at 120 min. Density is calculated using a modified equation of state proposed by Heidaryan and Jarrahian.37) In Fig. 6, particle median diameter is linearly decreased with the density of SC-CO2 whereas Fig. 7 shows that the dissolution rate of IDM is proportional to the density. SC-CO2 has the properties in which a tiny change in either pressure or temperature leads to a remarkable change in density, particularly around the critical point of carbon dioxide. High density of SC-CO2 allowed drug particles to disperse uniformly in the fluid, and this helped minimize the aggregation of particles. The higher the density of SC-CO2, the stronger the intermolecular force between carbon dioxide molecules and drug particles. During the milling process, the high surface energy of drug particles was diminished. As a result, aggregation of particles was inhibited to produce fine drug particles. On the other hand, specific surface area increases with the decrease in particle size. This leads to an increase in dissolution rate of drug particles. As a conclusion, the higher the density, the higher the milling performance and dissolution rate of IDM.
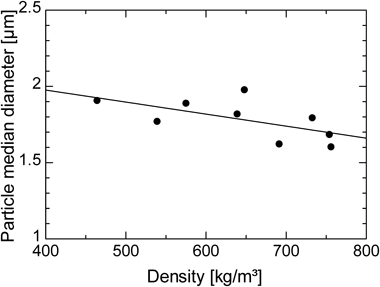
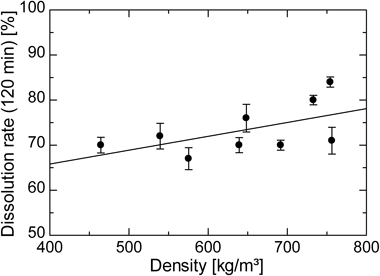
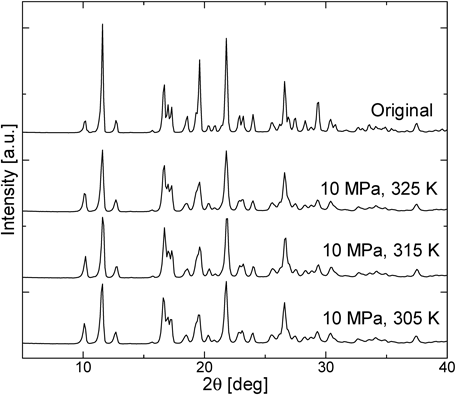
Figure 8 shows the result of X-ray powder diffraction of the original powder and milled powder with 0.5 mm beads for 10 min at 10 MPa for different temperatures. Results show that crystallinity of milled IDM showed slight decrease in intensity compared to the original crystal, but it was confirmed that there were no changes in crystallinity when temperature was varied. Furthermore, changes were not drastic and amorphous of drugs was not confirmed, due to the fact that the size of the beads used was too small, and collision energy was insufficient to cause irregularity on the arrangement of IDM particles.
In this study, a novel SC-CO2 milling system has been developed and its milling performance was investigated. This process was also applied to the milling of water-poorly soluble IDM and the improvement of its dissolution property was attempted. It was found that wet milling exhibited better milling performance than dry milling, where size distribution of IDM decreased whereas drug dissolution increased in the order of dry milling, jet milling, wet milling and SC-CO2, respectively. The results thus suggested that SC-CO2 milling was proven to be the most effective for the production of fine drug particles. Also, particle size of drug was found to play a major factor in the improvement of drug dissolution. Due to the high dispersion properties of drug particles in the supercritical fluid, SC-CO2 milling contributes to improving dissolution rate up to 50%. Temperature and pressure of SC-CO2 significantly changes its density and viscosity. It was found that the larger density of SC-CO2 indicated the better milling performance. This is due to the fact that a larger density helps provide a uniform medium for the dispersion of drug particles, and this provides better surface exposure for collision from zirconia beads. Finally, lower temperature of the supercritical fluid results in low viscosity which helps beads to move more vigorously in the fluid thus producing finer particles with lower crystallinity are produced. However, changes in the crystallinity of IDM particles were not drastic and amorphous of drugs was not confirmed. As a conclusion, the novel SC-CO2 milling process is a promising technology for the improvement of dissolution rate of water poorly soluble drugs.
The authors declare no conflict of interest.