2016 年 64 巻 12 号 p. 1769-1780
2016 年 64 巻 12 号 p. 1769-1780
We report the preparation of new tripodal receptor-type C3- and CS-symmetrical molecules constructed on a tris(2-aminoethyl)amine (TAEA) template. Both the anti-herpes simplex virus type 1 (anti-HSV-1) activity and cytotoxic activity of synthesized receptor-type derivatives were evaluated in order to find a characteristic structural feature for these bioactivities of compounds. Among the compounds of synthesized symmetrical TAEA-related derivatives, compound 13k showed high anti-HSV-1 activity (50% effective concentration (EC50)=16.7 µM) and low cytotoxicity (50% cytotoxic concentration (CC50)=>200 µM). The presence of a hydrogen bond donor proton in the molecule is thought to be an important structural factor for expressing potential anti-HSV-1 activities.
It is well known that many types of receptors or functionalized proteins on membranes in a native state often have a high order of symmetrical interfaces.1) C3- or C2-symmetrical molecules have frequently been found in various synthetic biologically active compounds.2) Aiming to develop new synthetic bioactive compounds, we targeted such symmetric geometrical molecules constructed on a symmetrical template.3–5)
In our previous studies on bioactive compounds in the tripodal receptor-type tris(2-aminoethyl)amine (TAEA) series, we found that some of the new C3- and CS-symmetrical derivatives synthesized previously showed significant bioactivities against herpes simplex virus type 1 (HSV-1) or cytotoxic activities to Vero cells.6) Regarding amide-type TAEA derivatives, we have reported that some symmetrical molecules have selective carbohydrate recognition properties.7) These mid-size molecules that show lectin-like carbohydrate recognition properties might have interesting functions as new ligands or drug candidates. Considering the formation of strong drug-host interaction for host sugar recognition by multivalent molecules, attempts to synthesize such non-peptide mid-size molecules are also thought to be valid in the search for bioactive new seeds.5,8)
Since it is well known that many amide functionalities usually have the ability to form a strong hydrogen bond, and it has also often been reported that some molecules derived from TAEA behave as acceptors for an ion or a small molecule,11,12) we introduced a few amide functionalities into a tripodal receptor-type TAEA template. These molecular modifications may lead to new bioactive symmetrical molecules that have good properties for non-covalent interaction and bioactivities.
Here, we describe the synthesis of some new non-peptide molecules with amine or amide-relating functionalities constructed on a symmetrical tris(2-aminoethyl)amine (TAEA) template and on a spread tris(3-aminopropyl)amine (TAPA) template. The results of biological evaluations of the synthesized derivatives are also described (Fig. 1).

Our strategies for the synthesis of target TAEA and TAPA derivatives by using TAEA (1) or TAPA (17) as a starting material are summarized in Chart 1.

The pathway for the synthesis of C3-symmetrical tripodal receptor-type TAEA derivatives (3, 5, 7, 8) is shown in Chart 1. All reductive amination reactions with various aryl aldehydes (2) were performed as one-pot reactions, and the desired amine-type TAEA derivatives (3) were obtained in a manner similar to that for the preparation of compound 3a.13) Acetylation of compounds 3 with acid anhydride (4) gave new amide-type TAEA derivatives (5). Urea-type TAEA derivatives (7, 8) were also obtained by addition reactions of amine-type derivatives (3) or TAEA (1) to iso(thio)cyanate (6). All of these target C3-symmetrical TAEA derivatives (3, 5, 7, 8) were obtained in moderate to excellent yields (44–95%) (see Tables S1–S3 for Supplementary materials of the online version for details).
Synthesis of targeted CS-symmetrical TAEA derivatives (12, 13) was achieved from addition reactions of the starting TAEA (1) to isothiocyanate (6) with different ratios of two reagents (1 : 6=1 : 2 and 1 : 6=2 : 1), and the intermediate adducts (10, 11) that were formed were further used for reductive amination with an aldehyde (2d) and NaBH4 in a one-pot reaction to give amine-urea-type TAEA derivatives (12, 13) (see Table S4 for Supplementary materials). The yields of targeted derivatives (12, 13) were about 32–55%. The results indicated that control of the first step of the addition reaction is not easy. The high reactivity of isothiocyanate to amine is thought to be the reason for obtaining such experimental results. Amide-urea-type TAEA derivatives (14, 15) from the acylations of these isolated compounds (12, 13) with acetic anhydrate (4) were obtained in good to excellent yields (68–92%) (see Table S5 for Supplementary materials).
For the synthesis of some new additional C3-symmetrical tris(3-aminopropyl)amine (TAPA) derivatives, we used the same procedure as that described for the preparation of targeted C3-symmetrical TAEA derivatives. Thus, reductive amination of TAPA as a starting material with aldehyde (2a) afforded the corresponding amine-type TAPA derivative (18a) in 65% yield, and an amide-type C3-symmetrical TAPA derivative (21) was also obtained after acylation of TAPA with 3,5-ditrifluoromethylbenzoyl chloride (19) in 66% yield (see Experimental).
All of the structures of the synthesized symmetrical tripodal receptor-type compounds were confirmed by spectroscopic and analytical data. The geometries of the obtained symmetrical TAEA and TAPA derivatives described above were confirmed from 13C-NMR spectroscopic data14) (see Experimental for details).
In many synthetic receptor-type molecules mimicking the supramolecular interaction of biomolecules, it is expected that the ability to form multiple hydrogen bonds plays an important role in guest-molecule recognition. Considering the concept called molecular tectonics,15,16) it is also thought that a convergent orientation of incorporated functional groups is an effective array to produce a multiple hydrogen bonding interaction between host and guest molecules. From this point of view, we prepared title tripodal receptor-type C3- or CS-geometrical molecules (3, 5, 8, 18a, 21) that have donors or acceptors for hydrogen bonds on a symmetrical TAEA or TAPA template as new target molecules (Table 1).
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | n | R1 | R2 | EC50 (µM) | CC50 (µM) | Compound | n | R1 | R2 | EC50 (µM) | CC50 (µM) |
| 3a ·H2O | 2 | H | 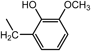 | >200 | >200 | 8l ·0.8H2O | 2 | H |  | >100 | >200 |
| 3b ·0.875CHCl3 | 2 | H | 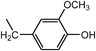 | >100 | >200 | 8m | 2 | H | 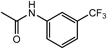 | >100 | >200 |
| 3d ·0.5NH3 | 2 | H |  | 20.5 | 35.9 | 8n | 2 | H |  | >100 | >200 |
| 5a ·0.9H2O | 2 | Ac | 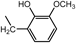 | >100 | >200 | 8p ·2-PrOH | 2 | H |  | >100 | 57.3 |
| 5b ·HCl ·2-PrOH·H2O | 2 | Ac | 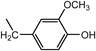 | >100 | >200 | 20a) | 2 | H |  | N.D. | 17.7 |
| 5c ·H2O | 2 | Ac |  | >100 | >200 | 18a ·1.2H2O | 3 | H |  | >200 | >200 |
| 5d ·0.7H2O | 2 | Ac |  | 90.7 | >200 | 21 | 3 | H |  | >200 | >200 |
| 5e ·0.5H2O | 2 | Ac | 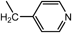 | >100 | >200 | ||||||
a) Data were taken from ref. 6.
Biological evaluations of anti-HSV-1 activities 50% effective concentration (EC50) by plaque reduction assays17) and cytotoxicities against Vero cells 50% cytotoxic concentration (CC50) of synthesized TAEA and TAPA derivatives (Table 1) showed that many C3-symmetrical amine-type or urea-type TAEA derivatives (3 or 8) had no significant anti-HSV-1 (EC50=>100 µM) and cytotoxic (CC50=>200 µM) activities. Among the tested amine-type C3-symmetrical derivatives (3) shown in Table 1, only compound 3d showed both anti-HSV-1 (EC50=20.5 µM) and cytotoxic (CC50=35.9 µM) activities. Compound 8p with a large log P value19) (9.94) showed moderate cytotoxic activity (CC50=57.3 µM). Among the C3-symmetrical compounds listed in Table 1 and also the CS-symmetrical derivatives listed in Table 2, there were few distinct correlations between log P values and anti-HSV-1 activities (EC50 values).
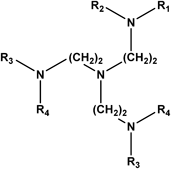 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | EC50 (µM) | CC50 (µM) | Compound | R1 | R2 | R3 | R4 | EC50 (µM) | CC50 (µM) |
| 9d ·2H2O | H |  | Ac | 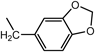 | >100 | 250 | 13o ·EtOH | H | 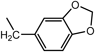 | H | 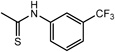 | 16.1 | 21.0 |
| 12k ·0.5H2O | H | 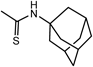 | H | 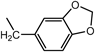 | 120.9 | >200 | 14k ·0.9H2O | H | 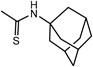 | Ac |  | >100 | >200 |
| 12l ·0.9H2O | H |  | H | 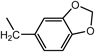 | >100 | >200 | 14l ·0.5H2O | H | 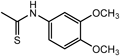 | Ac |  | >100 | >200 |
| 12o ·0.6H2O | H | 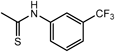 | H | 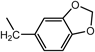 | >100 | 57.2 | 15k ·0.4H2O | Ac | 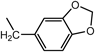 | H |  | >100 | >200 |
| 13k ·0.5H2O | H |  | H | 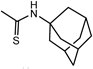 | 16.7 | >200 | 15l | Ac |  | H | 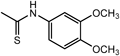 | >100 | 250 |
| 13l ·1.2H2O | H |  | H |  | >100 | >200 | Aciclovira) | 1.1 | >444 | ||||
a) Data were taken from ref. 18.
All of the C3-symmetrical amide-type TAEA derivatives (5) with loss of hydrogen bond donor protons by acylation reactions of an amine (–NH–) functionality, except for a very low level of anti-HSV-1 activity of compound 5d (EC50=90.7 µM), also showed no significant anti-HSV-1 (EC50=>100 µM) and cytotoxic (CC50=>200 µM) activities.14) Neither of the two prepared TAPA derivatives 18a and 21, which correspond to TAEA derivatives 3a and 20, respectively, showed significant anti-HSV-1 (EC50=>100 µM) and cytotoxic (CC50=>200 µM) activities.
The most important information from the above-described results is considered to be the fact that all of the N-acetylated cis–trans mixtures (compounds 5a–d) listed in Table 1 showed almost no anti-HSV-1 and cytotoxic activities at doses of <100 µM and <200 µM, respectively. Thus, the obtained biological results indicate at least that the loss of hydrogen atoms as hydrogen bond donors by N-acetylation of secondary amine moieties in amine-type molecules has a greater influence than the geometrical changes of these tripodal receptor-type TAEA derivatives on the expression of anti-HSV-1 activities.
On the other hand, regarding CS-symmetrical molecules (as shown in Table 2), many amine-urea-type TAEA derivatives (12k, l, o) that have one urea functionality showed no anti-HSV-1 activity (EC50=>100 µM); however, two CS-symmetrical amine-urea-type TAEA derivatives (13k, o) that have two urea functionalities showed considerably high levels of anti-HSV-1 activity (EC50=16.7, 16.1 µM, respectively). With regard to the molecules containing an adamantylthiourea group(s), we consider that the presence of two adamantylthiourea groups (13k) is a desirable structural feature in these amine-urea-type derivatives because of almost no anti-HSV-1 activity of the same geometric compound 12k. Since N-acetylated compounds 14k and 15k, which correspond to CS-symmetrical compounds 12k and 13k, respectively, showed no anti-HSV-1 activity, the presence of a benzylamino group as a hydrogen bond donor in the molecule 13k is also required. Compound 13o possessing a high level of cytotoxic activity (CC50=21.0 µM) had large calculated log P values19) over 6.0. We consider that both the structure and lipophilic property of the molecule contribute to its overall biological activity, especially its cytotoxic activity. In some C3-type molecules such as 8p and 20, a similar tendency for cytotoxic activity19) was observed (see Table 1). Regarding anti-HSV-1 activities (EC50) of these CS-symmetrical derivatives, amine-urea-type molecules with N-acetylation of secondary amine moieties (see compounds 14k, l, 15k, l) listed in Table 2 also showed almost no anti-HSV-1 activity at a dose of <100 µM, indicating that the loss of hydrogen atoms as hydrogen bond donors by N-acetylation of secondary amine moieties in amine-urea-type molecules has a greater influence than the geometrical features of these tripodal receptor-type TAEA molecules on expression of potential anti-HSV-1 activities.
As can be seen in the current geometrical and functional group modifications for the targeted TAEA derivatives [3d (C3)→12k (CS)→13k (CS)→8k (C3)6,20)→14k (CS)→15k (CS)], compound 13k (CS) revealed the maximum magnitude of anti-HSV-1 activity in these typical examples.
On the basis of the obtained structural information on biological activities in this TAEA series, we are investigating further molecular modifications by incorporating a few other new functional groups that work as hydrogen bonding donors into a TAEA framework in order to confirm the importance of the presence of hydrogen bonding donors and hopefully to find new promising bioactive seeds.
Melting points were determined using a micro melting point apparatus (Yanaco MP-S3) without correction. IR spectra were measured by a Shimadzu FTIR-8100 IR spectrophotometer. Low- and high-resolution mass spectra (LR-MS and HR-MS) were obtained by a JEOL JMS HX-110 double-focusing model equipped with an FAB ion source interfaced with a JEOL JMA-DA 7000 data system. lH- and 13C-NMR spectra were obtained by JEOL JNM A-500 and ECG600R (only for compounds 7cm, 9d and 16o). Chemical shifts were expressed in δ ppm downfield from an internal TMS signal for lH-NMR and the carbon signal of the corresponding solvent [CDCl3 (77.00 ppm), dimethyl sulfoxide (DMSO)-d6 (39.50 ppm)] for 13C-NMR. The abbreviations dd=double doublets, dt=double triplets, and dm=double multiplets are used for the multiplicity of lH-NMR data. The signal assignments were confirmed by two-dimensional (2D)-NMR analyses: lH–lH 2D correlation spectroscopy (COSY), lH–l3C heteronuclear multiple-quantum coherence (HMQC), and lH–l3C heteronuclear multiple-bond connectivity (HMBC). Microanalyses were performed with a Yanaco MT-6 CHN corder. Routine monitoring of reactions was carried out using precoated Kieselgel 60F254 plates (E. Merck). Open and flash column chromatography or centrifugal chromatography separations of the reaction products were performed on silica gel (Kanto 60N or Able-Biott) with a UV detector. Commercially available starting materials were used without further purification, and dry solvents were used in all reactions.
General Procedure for the Preparation of C3-Symmetrical Amine-Type TAEA Derivatives (3): Example: Synthesis of 4,4′,4″-[Nitrilotris(2,1-ethanediyliminomethylene)]tris(2-methoxyphenol) (3b) (Entry 2) (Step 1)To a solution of vanillin (2b, 2.28 g, 15.0 mmol) in methanol (MeOH, 6 mL) was added TAEA (1, 731 mg, 5.00 mmol) in MeOH (3 mL) at room temperature (r.t.) under an N2 atmosphere. After stirring for 1 h, the yellow solution changed to a yellow sticky suspension.
(Step 2)After dilution with MeOH (12 mL) and ice-cooling, compound 1 (1.84 g, 10.0 mmol) in dry benzene (20 mL) was added dropwise with stirring. After stirring for 20 min at r.t., the reaction mixture was refluxed for 1 h. After cooling to r.t., a small amount of NaBH4 (945 mg, 25.0 mmol) was added to the mixture and the mixture was stirred for 2.5 h at 0°C. It was then diluted with aqueous ammonium chloride (NH4Cl, 10%, 100 mL) and extracted with CH2Cl2 (3×200 mL). The combined organic layer was washed with brine (50 mL) and dried over anhydrous magnesium sulfate (MgSO4). Evaporation of the solvent gave crude 3b (2.43 g, 88%). An analytical sample of 3b was obtained by recrystallization from chloroform (CHCl3) as an off-white solid.
3b: mp 64–69°C (from CHCl3). IR (KBr) cm−1: 1279, 1034, 1125 (C–O). 1H-NMR (DMSO-d6) δ: 2.5–2.7 (12H, m, H1′, 2′), 3.21 (6H, br s, NH, OH), 3.54 (6H, br s, Hα), 3.71 (9H, br s, OCH3), 6.60–6.73 (6H, m, H5, 6), 6.84 (3H, d, J=1.5 Hz, H3), 8.30 (<1H, s, CHCl3). 13C-NMR (DMSO-d6) δ: 46.56 (C1′), 52.75 (Cα), 54.00 (C2′), 55.44 (OCH3), 79.08 (CHCl3), 112.07 (C3), 114.99 (C6), 120.14 (C5), 131.60 (C4), 145.09 (C1), 147.31 (C2). Positive-ion FAB-MS m/z: 555 (M+H+). HR-FAB-MS m/z: 555.3185 (Calcd for C30H43N4O6: 555.3183). Anal. Calcd for C30H42N4O6·0.875CHCl3: C, 56.26; H, 6.56; N, 8.50. Found: C, 56.29; H, 6.62; N, 8.45.
2,2′,2″-[Nitrilotris(2,1-ethanediyliminomethylene)]tris[6-methoxyphenol] (3a) (Entry 1)Compound 3a was prepared from the reaction of 1 with o-vanillin (2a) under the conditions shown in Table S1. Separation of the products by centrifugal chromatography (CH2Cl2 : 95% EtOH : 28% NH3=90 : 9.5 : 0.5→85 : 13 : 2) gave 3a (1.73 g, 62%) as a reddish purple solid.
3a: mp 35–38°C (mp 42–45°C).15) IR (KBr) cm−1: 3395 (OH), 3305 (NH), 1590, 1475 (C=C of Ar), 1235, 1075 (C–N and C–O). 1H-NMR (CDCl3) δ: 2.55 (6H, m, H2′), 2.68 (6H, m, H1′), 3.83 (9H, br s, CH3O–), 3.97 (6H, br s, Hα), 6.27 (6H, br s, NH, OH), 6.60 (3H, d, J=7.6 Hz, H3), 6.71 (3H, t, J=7.6 Hz, H4), 6.78 (3H, d, J=7.6 Hz, H5). 13C-NMR (CDCl3) δ: 45.78 (C1′), 51.76 (Cα), 53.90 (C2′), 55.76 (CH3O–), 110.75 (C5), 118.52 (C4), 120.69 (C3), 122.69 (C2), 147.10 (C1), 147.83 (C6). Positive-ion FAB-MS m/z: 555 (M+H)+. HR-FAB-MS m/z: 555.3202 (Calcd for C30H43N4O6: 555.3183). Anal. Calcd for C30H42N4O6·H2O: C, 62.92; H, 7.74; N, 9.78. Found: C, 62.93; H, 7.71; N, 9.57.
N2-[(3,4-Dimethoxyphenyl)methyl]-N1,N1-bis[2-[[(3,4-dimethoxyphenyl)methyl]amino]ethyl]-1,2-ethanediamine (3c) (Entry 3)Compound 3c was prepared from the reaction of 1 with 3,4-dimethoxybenzaldehyde (2c) under the conditions shown in Table S1. Separation of the products by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=93 : 6.6 : 0.4→85 : 14 : 1) gave 3c (5.00 g, 84%) as a sticky yellow oil.
3c: 1H-NMR (CDCl3) δ: 2.75–2.77 (12H, m, H1′, H2′), 3.84 (6H, s, Hα), 3.85 (9H, s, OCH3 on C3), 3.90 (9H, s, OCH3 on C4), 4.36 (3H, br s, NH), 6.79 (3H, d, J=8.2 Hz, H5′), 6.86 (3H, dd, J=8.2, 2.1 Hz, H6′), 7.13 (3H, d, J=2.1 Hz, H2′). 13C-NMR (CDCl3) δ: 45.21 (C1′), 52.24 (Cα), 53.02 (C2′), 55.92 (OCH3 on C4), 56.22 (OCH3 on C3), 111.16 (C5), 112.58 (C2), 121.45 (C6), 127.88 (C1), 149.02 (C3), 149.39 (C4). Positive-ion FAB-MS m/z: 597 (M+H+). HR-FAB-MS m/z: 597.3660 (Calcd for C33H49N4O6: 597.3652). Anal. Calcd for C33H48N4O6·2.4H2O: C, 61.93; H, 8.32; N, 8.45. Found: C, 61.99; H, 8.17; N, 8.72.
N2-(1,3-Benzodioxol-5-ylmethyl)-N1,N1-bis[2-[(1,3-benzodioxol-5-ylmethyl)amino]ethyl]-1,2-ethanediamine (3d) (Entry 4)Compound 3d was prepared from the reaction of 1 (1.46 g, 10.0 mmol) with piperonal (2d) under the conditions shown in Table S1. Separation of the products by centrifugal chromatography (CH2Cl2 : 95% EtOH : 28% NH3=85 : 14 : 1) gave 3d (0.760 g, 14%) as a pale yellow oil.
(Entry 5)This compound was also prepared as follows: To a solution of 2d (2.25 g, 15.0 mmol) in benzene (40 mL) were added compound 1 (731 mg, 5.0 mmol) and p-toluenesufonic acid (TsOH, 66 mg, 0.35 mmol) and the resulting mixture was refluxed. The resultant H2O was removed with a Dean–Stark distillation apparatus for 18 h under an N2 atmosphere. After evaporation of the solvent, the resulting mixture was diluted with MeOH (30 mL) and then a small amount of NaBH4 (1.57 mg, 41.5 mmol) was added and the mixture was stirred for 3 h at 0°C under an N2 atmosphere. After evaporation of the solvent, CHCl3 (20 mL), brine (5 mL), and 5% NaHCO3 (5 mL) were added to the obtained yellow solid, and then the resulting mixture was vigorously stirred for 1 h. After separation of the organic layer, the aqueous layer was re-extracted with CHCl3 (2×20 mL). The combined organic layer was dried over MgSO4, and evaporation of the solvent gave a brown oil. The product was purified by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=73 : 25 : 2→65 : 32 : 3) to give 3d (1.38 g, 50%) as a yellow oil. An analytical sample of 3d was obtained as a pale yellow solid by centrifugal chromatography (CH2Cl2 : 95% EtOH : 28% NH3=80 : 19.5 : 0.5).
3d: mp 215°C (dec.). IR (NaCl) cm−1: 3300 (NH of amine), 2825 (CH2 of methylenedioxy), 1245, 1040 (=C–O–C–), 930 (C–O). 1H-NMR (DMSO-d6) δ: 2.76 (6H, t, J=5.2 Hz, H2′), 3.06 (6H, t, J=5.2 Hz, H1′), 4.11 (6H, s, Hα), 6.04 (6H, s, H2), 6.93 (3H, d, J=7.9 Hz, H7), 7.07 (3H, dd, J=7.9, 1.5 Hz, H6), 7.27 (3H, d, J=1.5 Hz, H4), 9.48 (3H, br s, NH). 13C-NMR (DMSO-d6) δ: 43.28 (C1′), 49.54 (C2′ or Cα), 49.62 (Cα or C2′), 101.16 (C2), 108.13 (C7), 110.42 (C4), 124.24 (C6), 125.14 (C5), 147.22 (C3a), 147.58 (C7a). 1H-NMR (CDCl3) δ: 2.17 (3H, br s, NH), 2.56 (6H, t, J=5.8 Hz, H2′), 2.64 (6H, t, J=5.8 Hz, H1′), 3.64 (6H, s, Hα), 5.90 (6H, s, H2), 6.70 (6H, br s, H6, 7), 6.79 (3H, s, H4). 13C-NMR (CDCl3) δ: 46.88 (C1′), 53.57 (Cα), 54.29 (C2′), 100.76 (C2), 107.95 (C7), 108.55 (C4), 121.08 (C6), 134.14 (C5), 146.41 (C7a), 147.62 (C3a). Positive-ion FAB-MS m/z: 549 (M+H)+. HR-FAB-MS m/z: 549.2728 (Calcd for C30H37N4O6: 549.2713). Anal. Calcd for C30H36N4O6·0.5NH3: C, 64.67; H, 6.78; N, 11.31. Found: C, 64.42; H, 6.81; N, 11.04.
N1-(4-Pyridinylmethyl)-N2,N2-bis[2-[(4-pyridinylmethyl)amino]ethyl]-1,2-ethanediamine (3e) (Entry 6)Compound 3e was prepared from the reaction of 1 with isonicotinaldehyde (2e) under the conditions shown in Table S1. Separation of the products by centrifugal chromatography (CH2Cl2 : 95% EtOH : 28% NH3=73 : 15 : 2→65 : 32 : 3) gave 3e (3.97 g, 95%) as a yellow oil.
3e: 1H-NMR (CDCl3) δ: 2.00 (3H, br s, NH), 2.61 (6H, t, J=5.2 Hz, H2′), 2.67 (6H, t, J=5.2 Hz, H1′), 3.76 (6H, s, Hα), 7.20 (6H, dd, J=4.6, 1.5 Hz, H3, 5), 8.50 (6H, dd, J=4.6, 1.5 Hz, H2, 6). 13C-NMR (CDCl3) δ: 47.17 (C1′), 52.73 (Cα), 54.20 (C2′), 122.78 (C3, 5), 149.33 (C4), 149.80 (C2, 6). KM4-4-1 Positive-ion FAB-MS m/z: 420(M+H+). HR-FAB-MS m/z: 420.2873 (Calcd for C24H34N7: 420.2876).
General Procedure for the Preparation of C3-Symmetrical Amide-Type TAEA Derivatives (5): Example: Synthesis of N,N′,N″-(Nitrilotri-2,1-ethanediyl)tris[N-(3,4-dimethoxyphenylmethyl)acetamide] (5c) (Entry 9)To a solution of compound 3c (2.85 g, 5.00 mmol) in benzene (8 mL) was added dropwise a solution of acetic anhydride (Ac2O, 4, 1.55 g, 15.0 mmol) in benzene (2 mL) at r.t., and then the mixture was stirred for 2 h. After removal of the solvent by evaporation, 1 M NaOH solution (20 mL) was added to the resulting residue and then the mixture was extracted with EtOAc (3×40 mL). The organic layer was washed with brine (25 mL) and dried with MgSO4 and then evaporated to give a pale yellow oil, which was purified by flash chromatography (EtOH : EtOAc=4 : 6) to give 5c (2.88 g, 80%) as a colorless semisolid.
5c: mp 34–39°C. IR (KBr) cm−1: 1646 (C=O), 1261, 1233 (C–O of aromatic ether), 1137, 1025 (C–O of aliphatic ether). 1H-NMR (CDCl3) δ: 2.07, 2.09*, 2.11, 2.15 (9H, s, COCH3), 2.05, 2.52, 2.58*, 2.63 (6H, t, J=7.3 Hz, H2′), 3.17, 3.23, 3.34*, 3.37 (6H, t, J=7.3 Hz, H1′), 3.83, 3.84, 3.86, 3.86* (18H, s, OCH3), 4.39, 4.43*, 4.47, 4.50 (6H, s, Hα), 6.63–6.79 (6H, m, H2, 6), 6.80–6.86 (3H, m, H5). (The signals of the predominant conformer were asterisked.) 13C-NMR (CDCl3) δ: 21.64 (COCH3), 44.28 (C1′), 52.03 (C2′), 52.50 (Cα), 55.98 (OCH3), 109.76 (C2), 111.61 (C5), 118.51 (C6), 129.12 (C1), 148.74 (C3), 149.60 (C4), 170.87 (C=O). (Signals of only the predominant conformer were assigned.) Positive-ion FAB-MS m/z: 723 (M+H+). HR-FAB-MS m/z: 723.3967 (Calcd for C39H55N4O9: 723.3969). Anal. Calcd for C39H54N4O9·H2O: C, 63.22; H, 7.62; N, 7.56. Found: C, 63.18; H, 7.70; N, 7.61.
N,N′,N″-[2,2′,2″-Nitrilotris(ethane-2,1-diyl)]tris[N-(2-hydroxy-3-methoxybenzyl)acetamide] (5a) (Entry 7)Compound 5a was prepared by acylation of 3a with Ac2O (4) under the conditions shown in Table S2. After work-up, crude compound 5a (1.59 g, 75%) was obtained as a white solid. Recrystallization from AcOEt gave an analytically pure product 5a as colorless crystals.
5a: mp 107–109°C (from AcOEt). IR (KBr) cm−1: 3150 (OH), 1625 (C=O), 1070 (C–O of phenol, ether). 1H-NMR (DMSO-d6) δ: 1.98, 1.99, 2.02, 2.03* (9H, s, CH3CO–), 2.36–3.47 (3H, m, H2′), 2.50–2.58 (3H, m, H2′), 3.13–3.24 (6H, m, H1′), 3.74*, 3.78 (9H, s, CH3O–), 4.38, 4.40, 4.42* (6H, br s, Hα), 6.57–6.76 (6H, m, H3, 4), 6.83–6.88 (3H, m, H5), 8.76, 8.79, 8.82, 8.95, 8.96*, 8.98 (3H, br s, OH). (The signals of the predominant conformer were asterisked.) 13C-NMR (DMSO-d6) δ: 20.64, 20.86, 20.90*, 20.93, 21.28, 21.32* (CH3CO–), 43.05, 43.31, 43.37, 43.52, 43.77 (Cα, C1′), 46.16, 46.24*, 46.40 (C1′), 47.39, 47.51* (Cα), 51.07, 51.32*, 51.83, 52.07* (C2′), 55.70*, 55.79* (CH3O–), 110.98, 111.01, 111.10, 111.17*, 111.27 (C5), 118.61, 118.82*, 119.51 (C4), 119.61, 119.79, 121.25* (C3), 123.78, 123.86, 124.09* (C2), 143.99, 144.06, 144.50* (C1), 147.38*, 147.61, 147.64, 147.68 (C6), 169.78, 169.84, 170.43, 170.54*, 170.67 (C=O). (Signals of only the predominant conformer were asterisked.) Positive-ion FAB-MS m/z: 681 (M+H)+. HR-FAB-MS m/z : 681.3517 (Calcd for C36H49N4O9: 681.3500). Anal. Calcd for C36H48N4O9·0.9 H2O: C, 62.04; H, 7.20; N, 8.04. Found: C, 62.09; H, 7.15; N, 7.85.
N,N′,N″-(Nitrilotri-2,1-ethanediyl)tris[N-(4-hydroxy-3-methoxyphenylmethyl)acetamide] Hydrochloride (5b·HCl) and [[(Nitrilotri-2,1-Ethanediyl)tris(acetylazanediyl)]tris(methylene)]tris(2-methoxybenzene-4,1-diyl) Triacetate (5b′) (Entry 8)Compound 5b′ was prepared by acylation of compound 3b (0.943 g, 1.70 mmol) with Ac2O (4, 1.39 g, 13.6 mmol) under the conditions shown in Table S2. After work-up crude compound 5b′ (987 mg, 72%) was obtained as a white solid. After dilution of this material with EtOH (4 mL) and addition of 1 M HCl (in EtOH), evaporation of the solvent and azeotropic evaporation with dry benzene were repeated. Then the white semisolid material was recrystallized from 2-PrOH to give an analytically pure salt of the deacylated target compound 5b·HCl as colorless crystals.
5b·HCl: mp 69–73°C (from 2-PrOH). IR (KBr) cm−1: ca. 3200 (OH), ca. 2600 (NH+), 1636 (C=O), 1279, 1032, 1125 (C–O). 1H-NMR (DMSO-d6) δ: 1.04 (6H, d, J=6.1 Hz, CH3 of 2-PrOH), 2.10*, 2.11 (9H, s, COCH3), 3.17, 3.24*, 3.33 (6H, br s, H2′), 3.39 (4H, br s, OH) 3.66*, 3.75 (6H, br s, H1′), 3.75, 3.76, 3.77*, 3.78 (9H, s, OCH3), 3.80 (1H, q, J=6.1 Hz, CH of 2-PrOH), 4.38, 4.48* (9H, br s, Hα), 6.65* (0.8H, dd, J=7.9, 1.8 Hz, H5), 6.68–6.73 (0.4H, m, H5, 6), 6.79* (0.8H, d, J=7.9 Hz, H6), 6.82* (0.8H, br s, H3), 6.88–6.93 (0.2H, m, H3), 8.96*, 9.85, 10.88, 11.73 (3H, br s, NH, OH). (The signals of the predominant conformer were asterisked.) 13C-NMR (DMSO-d6) δ: 21.40 (COCH3), 25.39 (CH3 of 2-PrOH), ca. 40 (C1′), 50.33 (C2′), 51.29 (Cα), 55.64 (OCH3), 61.93 (CH of 2-PrOH), 111.52 (C3), 115.64 (C6), 119.23 (C4), 127.56 (C5), 146.01 (C1), 147.77 (C2), 171.38 (C=O). (Signals of only the predominant conformer were assigned.) Positive-ion FAB-MS m/z: 681 (M+H+). HR-FAB-MS m/z: 681.3491 (Calcd for C36H49N4O9: 681.3500). Anal. Calcd for C36H48N4O9·HCl·2-PrOH·H2O: C, 58.89; H, 7.48; N, 7.04. Found: C, 58.75; H, 7.70; N, 6.84.
5b′: Positive-ion FAB-MS m/z: 807 (M+H+). HR-FAB-MS m/z: 807.3812 (Calcd for C42H55N4O12: 807.3816). Both 1H- and 13C-NMR in CDCl3 were too complicated and we could not provide full assignments of the observed signals.
N,N',N″-(Nitrilotri-2,1-ethanediyl)tris[N-(1,3-benzodioxol-5-ylmethyl)acetamide] (5d) and N,N′-[[[2-[(1,3-Benzodioxol-5-ylmethyl)amino]ethyl]imino]di-2,1-ethanediyl]bis[N-(1,3-benzodioxol-5-ylmethyl)acetamide] (9d) (Entry 10)Compound 5d was prepared by acylation of 3d with Ac2O (4) under the conditions shown in Table S2. Separation of the products by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=95 : 4.7 : 0.3→93 : 6.6 : 0.4) gave 5d (2.67 g, 53%) as a colorless semisolid and 9d (0.94 g, 20%) as a colorless oil. Compound 9d was purified by centrifugal chromatography (EtOAc : EtOH=99.5 : 0.5) to give an analytically pure 9d as a hygroscopic white semisolid.
5d: mp 46–65°C. IR (KBr) cm−1: 1644 (C=O), 1248, 1037 (=C–O–C–), 923 (C–O of methylenedioxy). 1H-NMR (CDCl3) δ: 2.09*, 2.10, 2.11, 2.14 (9H, s, CH3), 2.40–2.70 [6H, 2.43 (br s), 2.49, 2.55* (t, J=7.0 Hz), 2.65 (br s), H2′], 3.10–3.45 [6H, 3.16, 3.22, 3.31* (t, J=7.0 Hz), 3.37 (br s), H1′], 4.36, 4.39*, 4.44, 4.46 (6H, s, Hα), 5.91, 5.91, 5.94*, 5.95, 5.96 (6H, s, H2), 6.57–6.80 (9H, m, ArH). (The signals of the predominant conformer were asterisked.) 13C-NMR (CDCl3) δ: 21.54 (CH3), 44.06 (C1′), 51.84 (C2′), 52.50 (Cα), 101.15 (C2), 106.79 (C4), 108.45 (C7), 119.57 (C6), 130.41 (C5), 147.14 (C3a or 7a), 148.27 (C7a or 3a), 170.71 (C=O). (Signals of only the predominant conformer were assigned.) Positive-ion FAB-MS m/z: 675 (M+H+). HR-FAB-MS m/z: 675.3027 (Calcd for C36H43N4O9: 675.3030). Anal. Calcd for C36H42N4O9·0.7H2O: C, 62.91; H, 6.36 ; N, 8.15. Found: C, 62.90; H, 6.57; N, 8.01.
9d: mp 76–81°C. IR (KBr) cm−1: 1638(C=O), 1251, 1037 (=C–O–C–), 924 (C–O of methylenedioxy). 1H-NMR (DMSO-d6) δ: 1.44 (1H, br s, NH), 1.98*, 1.99 (5H, s, CH3), 2.02 (0.6H, s, CH3), 2.06 (0.4H, s, CH3), 2.29–2.47 (4H, m, H2′), 2.50–2.57 (1H, m, H2″), 2.61–2.69 (1H, m, H2″), 3.05–3.31 (6H, m, H1′, 1‴), 3.45–5.57 (1H, m, Hα′), 3.83–3.89 (1H, m, Hα′), 4.32*, 4.36, 4.39, 4.44 (4H, s, Hα), 5.98*, 5.99 (4H, s, H2), 6.01*, 6.05 (2H, s, H2‴), 6.62–6.70 (2H, m, H6), 6.73–6.77 (2H, m, H4), 6.79–6.92 (4H, m, H7, 6‴, 7‴), 7.03–7.08 (1H, m, H4‴). 13C-NMR (DMSO-d6) δ: 21.50 (CH3), 43.30 (C1″), 45.57 (C1′), 47.55 (Cα), 49.99 (C2″), 51.56 (C2′), 58.48 (Cα′), 100.91 (C2‴), 101.09 (C2), 108.06 (C4), 108.14 (C7‴ or C7), 108.36 (C7 or C7‴), 110.18 (C4‴), 119.79 (C6), 123.82 (C6‴), 128.34 (C5‴), 132.05 (C5), 146.32 (C7a), 147.05 (C7a‴), 147.26 (C3a‴), 147.72 (C3a), 169.78 (C=O). (Signals of only the predominant conformer were assigned.) Positive-ion FAB-MS m/z: 633 (M+H+). HR-FAB-MS m/z: 633.2918 (Calcd for C34H41N4O8: 633.2924). Anal. Calcd for C34H40N4O8·2H2O: C, 61.07; H, 6.63 ;N, 8.38. Found: C, 61.01; H, 6.76; N, 8.13.
N,N',N″-(Nitrilotri-2,1-ethanediyl)tris[N-(pyridin-4-ylmethyl)acetamide] (5e) (Entry 11)Compound 5e was prepared by acylation of compound 3e with Ac2O (4) under the conditions shown in Table S2. Separation of the products by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=90 : 9.5 : 0.5→85 : 14 : 1) gave 5e (2.51 g, 61%) as a pale yellow sticky oil.
5e: IR (KBr) cm−1: 1636 (C=O), 796 (CH of pyridine). 1H-NMR (CDCl3) δ: 2.02, 2.04*, 2.07, 2.17, 2.21 (9H, s, CH3), 2.50–2.70 (6H, m, H2′), 3.18–3.45 (6H, m, H1′), 4.45, 4.50*, 4.55, 4.56, 4.58 (6H, s, Hα), 7.05–7.17 (6H, m, H3, 5), 8.52, 8.60* (6H, t, J=6.1 Hz, H2, 6). (The signals of the predominant conformer were asterisked.) 13C-NMR (CDCl3) δ: 21.46 (CH3), 44.55 (C1′), 51.59 (C2′), 51.78 (Cα), 121.15 (C3, 5), 145.92 (C4), 150.41 (C2, 6), 170.94 (C=O). (Signals of only the predominant conformer were assigned.) Positive-ion FAB-MS m/z: 546 (M+H+). HR-FAB-MS m/z: 546.3194 (Calcd for C30H40N7O3: 546.3193). Anal. Calcd for C30H39N7O3·0.5H2O: C, 64.96; H, 7.27; N, 17.68. Found: C, 65.00; H, 7.20; N, 17.46.
General Procedure for the Preparation of C3-Symmetrical Urea-Type TAEA Derivatives (7, 8): Example: Synthesis of N,N″,N-(Nitrilotri-2,1-ethanediyl)tris[N′-(3,4-dimethoxyphenyl)thiourea] (8l) (Entry 14)To a solution of 3,4-dimethoxyphenyl isothiocyanate (6l, 2.93 g, 15.0 mmol) in CH2Cl2 (20 mL) was added TAEA (1, 731 mg, 5.00 mmol) at r.t. After stirring for 1 h, the resulting white solid was filtrated to obtain crude compound 8l (3.35 g, 91%). Recrystallization from MeOH gave analytically pure compound 8l as a white solid.
8l: mp 161–163°C (from MeOH). IR (KBr) cm−1: 1513 (C=S), 1258, 1235, 1134, 1027 (C–O). 1H-NMR (CDCl3) δ: 2.67 (6H, t, J=6.9 Hz, H2′), 3.48–3.55 (6H, m, H1′), 3.72 (9H, s, –OCH3), 3.73 (9H, s, –OCH3), 6.79 (3H, dd, J=8.5, 2.1 Hz, H6), 6.90 (3H, d, J=8.5 Hz, H5), 6.96 (3H, d, J=2.1 Hz, H2), 7.36 (3H, br s, Hβ), 9.37 (3H, br s, Hα). 13C-NMR (CDCl3) δ: 41.97 (C1′), 52.19 (C2′), 55.45 (–OCH3), 55.70 (–OCH3), 109.23 (C2), 111.97 (C5), 116.28 (C6), 131.62 (C1), 146.27 (C4), 148.64 (C3), 180.28 (C=S). Positive-ion FAB-MS m/z: 732 (M+H+). HR-FAB-MS m/z: 723.2682 (Calcd for C33H46N7O6S3: 732.2672). Anal. Calcd for C33H45N7O6S3·0.8H2O: C, 53.10; H, 6.29 ; N, 13.14. Found: C, 53.08; H, 6.09; N, 13.22.
N,N″,N″″-(Nitrilotri-2,1-ethanediyl)tris[N-(3,4-dimethoxybenzyl)-N′-[3-(trifluoromethyl)phenyl]urea] (7cm) (Entry 12)Compound 7cm was prepared from 3c (282 mg, 0.473 mmol) and 3-(trifluoromethyl)phenyl isocyanate (6m, 266 mg, 1.41 mmol) under the conditions shown in Table S3. Separation of the product by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=97 : 2.7 : 0.3→95 : 4.7 : 0.3) gave 7cm (242 mg, 44%) as a colorless oil.
7cm: 1H-NMR (CDCl3) δ: 1.64 (3H, s, NH), 2.79 (6H, br t, J=6.6 Hz, H2′), 3.55 (6H, br s, H1′), 3.82 (9H, s, MeO on C3), 3.84 (9H, s, MeO on C4), 4.74 (6H, s, Hα), 6.75–6.80 (9H, m, H2, 5, 6), 7.19 (3H, br d, J=7.5 Hz, H4″), 7.23 (3H, t, J=7.5 Hz, H5″), 7.36 (3H, br d, J=7.5 Hz, H6″), 7.58 (3H, br s, H2″). 13C-NMR (CDCl3) δ: 45.44 (C1′), 51.03 (Cα), 52.68 (C2′), 55.89 (CH3O), 55.92 (CH3O), 110.24 (C2), 111.31 (C5), 116.40 (q, J=4.3 Hz, C2″), 119.28 (q, J=4.3 Hz, C4″), 122.83 (C6″), 123.89 (q, J=271.6 Hz, CF3), 129.07 (C1), 129.19 (C5″), 131.02 (q, J=31.8 Hz, C3″), 139.72 (C1″), 148.92 (C4), 149.66 (C3), 155.87 (C=O). Positive-ion FAB-MS m/z: 1158 (M+H+). HR-FAB-MS m/z: 1158.4369 (Calcd for C57H61F9N7O9: 1158.4387).
N,N″,N″″-(Nitrilotri-2,1-ethanediyl)tris[N-(pyridin-4-ylmethyl)-N′-[4-(trifluoromethyl)phenyl]thiourea] (7ep) (Entry 13)Compound 7ep was prepared from 3e (242 mg, 0.577 mmol) and 4-(trifluoromethyl)phenyl isothiocyanate (6p, 352 mg, 1.73 mmol) under the conditions shown in Table S3. After evaporation of the solvent, recrystallization of the resulting mixture from 2-PrOH gave 7ep (296 mg, 50%) as a white solid.
7ep: mp 122–127°C (from 2-PrOH). 1H-NMR (DMSO-d6) δ: 2.95 (6H, t, J=6.7 Hz, H2′), 3.88 (6H, br s, H1′), 5.10 (6H, br s, Hα), 7.18 (6H, d, J=5.6 Hz, H3, 5), 7.48 (6H, d, J=8.4 Hz, H2″, 6″), 7.59 (6H, d, J=8.4 Hz, H3″, 5″), 8.50 (6H, d, J=5.6 Hz, H2, 6), 9.84 (3H, br s, NH). 13C-NMR (DMSO-d6) δ: 49.65 (C1′), 51.25 (C2′), 53.10 (Cα), 121.69 (C3, 5), 124.40 (q, J=32.1 Hz, C4″), 124.74 (q, J=271.1 Hz, CF3), 124.94 (q, J=4.1 Hz, C3″, 5″), 125.23 (C2″, 6″), 144.35 (C1″), 145.99 (C4), 149.56 (C2, 6), 182.21 (C=S). Positive-ion FAB-MS m/z: 1029 (M+H+). HR-FAB-MS m/z: 1029.2927 (Calcd for C48H46F9N10S3: 1029.2925).
N,N″,N-(Nitrilotri-2,1-ethanediyl)tris[N′-[3-(trifluoromethyl)phenyl]urea] (8m) (Entry 15)Compound 8m was prepared from 1 (292 mg, 2.00 mmol) and 3-(trifluoromethyl)phenyl isocyanate (6m, 1.12 g, 6.00 mmol) under the conditions shown in Table S3. After filtration of the resulting precipitates, recrystallization from propionitrile (EtCN) gave 8m (1.19 g, 84%) as colorless crystals.
8m: mp 187–190°C (from EtCN). IR (KBr) cm−1: 3334 (NH), 1645 (CONH), 1340 (CF3). 1H-NMR (DMSO-d6) δ: 2.63 (6H, t, J=6.7 Hz, H2′), 3.17–3.25 (6H, m, H1′), 6.25 (3H, t, J=5.5 Hz, NHβ), 7.20 (3H, dd, J=7.6, 0.9 Hz, H4), 7.41 (3H, t, J=7.9 Hz, H5), 7.49 (3H, d, J=8.5, H6), 7.93 (3H, s, H2), 8.87 (3H, s, NHα). 13C-NMR (DMSO-d6) δ: 37.53 (C1′), 53.72 (C2′), 113.54 (q, J=4.1 Hz, C2), 117.08 (q, J=4.1 Hz, C4), 121.06 (C6), 124.16 (q, J=272.1 Hz, CF3), 129.34 (q, J=31.0, C3), 129.52 (C5), 141.24 (C1), 155.03 (C=O). Positive-ion FAB-MS m/z: 708 (M+H+). HR-FAB-MS m/z: 708.2352 (Calcd for C30H31F9N7O3: 708.2345). Anal. Calcd for C30H30F9N7O3: C, 50.92; H, 4.27; N, 13.86. Found: C, 50.84; H, 4.07; N, 13.89.
N,N″,N-(Nitrilotri-2,1-ethanediyl)tris[N′-[4-(trifluoromethyl)phenyl]urea (8n) (Entry 16)Compound 8n was prepared from 1 (292 mg, 2.00 mmol) and 4-(trifluoromethyl)phenyl isocyanate (6n, 1.12 g, 6.00 mmol) under the conditions shown in Table S3. After filtration of the resulting precipitates, recrystallization from EtOH gave 8n (1.24 g, 88%) as colorless crystals.
8n: mp 237–238°C (from EtOH). IR (KBr) cm−1: 1649 (CONH), 1329 (CF3). 1H-NMR (DMSO-d6) δ: 2.63 (6H, t, J=6.4 Hz, H2′), 3.22 (6H, dt, J=6.4, 6.1 Hz, H1′), 6.28 (3H, t, J=5.5 Hz, NHβ), 7.52 (6H, d, J=8.9 Hz, H3, 5), 7.56 (6H, d, J=8.9 Hz, H2, 6), 8.91 (3H, s, NHα). 13C-NMR (DMSO-d6) δ: 37.52 (C1′), 53.65 (C2′), 117.20 (C2, 6), 120.89 (q, J=32.1 Hz, C4), 124.54 (q, J=271.0 Hz, CF3), 125.74 (q, J=4.1 Hz, C3, 5), 144.09 (C1), 154.84 (C=O). Positive-ion FAB-MS m/z: 708 (M+H+). HR-FAB-MS m/z: 708.2352 (Calcd for C30H31F9N7O3: 708.2345). Anal. Calcd for C30H30F9N7O3: C, 50.92; H, 4.27; N, 13.86. Found: C, 50.88; H, 4.20; N, 13.92.
N,N″,N-(Nitrilotri-2,1-ethanediyl)tris[N′-[4-(trifluoromethyl)phenyl]-thiourea] (8p) (Entry 17)Compound 8p was prepared from 1 (292 mg, 2.00 mmol) and 4-(trifluoromethyl)phenyl isothiocyanate (6p, 1.12 g, 6.00 mmol) under the conditions shown in Table S3. Filtration of the resulting precipitates gave the crude product 8p (1.12 g, 74%). Recrystallization from 2-PrOH gave analytically pure 8p as colorless crystals.
8p: mp 198–200°C (from 2-PrOH). IR (KBr) cm−1: 3275 (NH), 1548, 1542 (S=C–N), 1329 (CF3), 1111, 1067 (C=S). 1H-NMR (DNSO-d6) δ: 2.80 (6H, t, J=6.7 Hz, H2′), 3.57–3.90 (6H, m, H1′), 7.62 (6H, d, J=8.4 Hz, H3, 5), 7.70 (6H, d, J=8.4 Hz, H2, 6), 7.93 (3H, br s, NHβ), 9.88 (3H, s, NHα). 13C-NMR (DMSO-d6) δ: 41.81 (C1′), 51.86 (C2′), 121.77 (C2, 6), 123.34 (q, J=32.1 Hz, C4), 124.28 (q, J=271.0 Hz, CF3), 125.50 (q, J=3.1 Hz, C3, 5), 143.19 (C1), 180.22 (C=S). Positive-ion FAB-MS m/z: 756 (M+H+). HR-FAB-MS m/z: 756.1658 (Calcd for C30H31F9N7S3: 756.1659). Anal. Calcd for C30H30F9N7S3·2-PrOH : C, 48.58; H, 4.69; N, 12.02. Found: C, 48.54; H, 4.59; N, 11.96.
General Procedure for the Preparation of CS-Symmetrical Amine-Urea-Type TAEA Derivatives (12, 13): Example: Synthesis of N-[2-[Bis[2-[(1,3-benzodioxol-5-ylmethyl)amino]ethyl]amino]ethyl]-N′-(tricyclo[3.3.1.13,7]dec-1-yl)thiourea (12k) (Entry 18) (Step 1)To a solution of TAEA (1, 1.46 g, 10.0 mmol) in CH2Cl2 (160 mL) was added 1-adamantyl isothiocyanate (6k, 2.93 g, 15.0 mmol) in CH2Cl2 (20 mL) and the mixture was stirred for 1 h at r.t. Evaporation of the solvent gave a pale yellow oil.
(Step 2)To a solution of the resulting oil in MeOH (5 mL) was added a solution of piperonal (2d, 3.00 g, 20.0 mmol) in MeOH (10 mL) at r.t. under an argon atmosphere and the mixture was stirred for 20 h.
(Step 3)To the resulting yellow solution was added MeOH (80 mL) and NaBH4 (2.04 g, 54.0 mmol) at 0°C under an argon atmosphere with stirring for 4 h, and then stirring was continued at r.t. for 15 h. After evaporation of the solvent, aqueous ammonium acetate (NH4OAc, 10%) was added to the resulting white solid and the mixture was extracted with CHCl3 (3×200 mL). The separated organic layer was dried over MgSO4 and filtrated, and evaporation of the solvent gave a pale yellow oil. Separation of the products by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=950 : 47 : 3→73 : 25 : 2) gave 16k (403 mg, 17%) as a white solid, 13k (365 mg, 6%) as a white solid, and 12k (2.03 g, 33%) as a colorless semisolid.
12k: mp 46–52°C. IR (KBr) cm−1: 3275 (NH of sec-amine), 1489 (C=S), 1247, 1038, 929 (C–O). 1H-NMR (CDCl3) δ: 1.58–1.67 (6H, m, Ha), 1.99 (6H, br s, Hb), 2.02 (3H, br s, Hc), 2.35 (3H, br s, NH), 2.61 (4H, br t, J=6.1 Hz, H2′), 2.66–2.71 (6H, m, H1′, 2″), 3.67 (4H, s, Hα), 5.92 (4H, s, H2), 6.09 (1H, br s, NH), 6.75 (4H, br s, H4, 6), 6.81 (2H, s, H7). 13C-NMR (CDCl3) δ: 29.44 (Cc), 36.11 (Ca), 42.03 (Cb), 43.06 (C1″), 46.63 (C1′), 53.43, 53.55, 53.62, 53.72 (C2′, 2″, α, d), 100.91 (C2), 108.14 (C4), 108.67 (C7), 121.37 (C6), 133.34 (C5), 146.72 (C3a), 147.78 (C7a), 180.73 (C=S). Positive-ion FAB-MS m/z: 608 (M+H+). HR-FAB-MS m/z: 608.3273 (Calcd for C33H46N5O4S: 608.3271). Anal. Calcd for C33H45N5O4S·0.5H2O: C, 64.26; H, 7.52; N, 11.35. Found: C, 64.32; H, 7.64; N, 11.25.
16k: 1H-NMR (CDCl3) δ: 1.69 (6H, br s, Ha), 2.13 (3H, br s, Hc), 2.15 (6H, br s, Hb), 2.70 (2H, dt, J=5.5, 0.3 Hz, H5), 3.62–3.68 (2H, m, H4), 6.49 (0.5H, br s, NH), 7.28 (0.5H, br s, NH). 13C-NMR (CDCl3) δ: 29.54 (Cc), 36.19 (Ca), 42.11 (Cb), 43.01 (C4), 53.53 (C5), 54.25 (Cd), 180.49 (C=S). Positive-ion FAB-MS m/z: 237 (32, M+H+). HR-FAB-MS m/z: 237.1417 (Calcd for C13H21N2S: 237.1425).
N,N″-[[[2-[(1,3-Benzodioxol-5-ylmethyl)amino]ethyl]imino]di-2,1-ethanediyl]bis[N′-(tricyclo[3.3.1.13,7]dec-1-yl)thiourea] (13k) (Entry 19)Compound 13k was prepared from 1 (1.46 g, 10.0 mmol) with 6k (3.87 g, 20.0 mmol) and 2d (1.50 g, 10.0 mmol) under the conditions shown in Table S4. Separation of the products by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=95 : 4.7 : 0.3→93 : 6.6 : 0.4) gave 13k (2.12 g, 32%) as a white solid and 12k (0.903 g, 15%) as a pale yellow oil.
13k: mp 48–50°C. IR (KBr) cm−1: 3276 (NH of sec-amine), 1539 (C=S), 1247, 1038, 933 (C–O). 1H-NMR (CDCl3) δ: 1.67 (12H, br s, Ha), 2.09 (19H, br s, Hb, Hc, NH), 2.63–2.68 (2H, m, H2′), 2.68–2.74 (6H, m, H1′, 2″), 3.56–3.64 (4H, m, H1″), 3.72 (2H, s, Hα), 5.93 (2H, s, H2), 6.08 (2H, br s, NH), 6.54 (2H, br s, NH), 6.73–6.79 (2H, m, H4, 6), 6.84 (1H, d, J=1.2 Hz, H7). 13C-NMR (CDCl3) δ: 29.51 (Cc), 36.16 (Ca), 42.12 (Cb), 42.95 (C1″), 46.72 (C1′), 53.11 (C2″), 53.62 (Cα), 53.80 (C2′), 54.07 (Cd), 100.88 (C2), 108.16 (C4), 108.73 (C7), 121.41 (C6), 133.61 (C5), 146.65 (C3a), 147.74 (C7a), 180.77 (C=S). Positive-ion FAB-MS m/z: 667 (5, M+H+). HR-FAB-MS m/z: 667.3824 (Calcd for C36H55N6O2S2: 667.3828). (YO2-6) Anal. Calcd for C36H54N6O2S2·0.5H2O: C, 63.96; H, 8.20; N, 12.43. Found: C, 63.99; H, 8.19; N, 12.16.
N-[2-[Bis[2-[(1,3-benzodioxol-5-ylmethyl)amino]ethyl]amino]ethyl]-N′-(3,4-dimethoxyphenyl)thiourea (12l) and 1-(3,4-Dimethoxyphenyl)-2-imidazolidinethione (16l) (Entry 20)Compound 12l was prepared from 1 (1.46 g, 10.0 mmol) with 6l (1.95 g, 10.0 mmol) and 2d (3.00 g, 20.0 mmol) under the conditions shown in Table S4. Separation of the products by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=93 : 6.6 : 0.4→85 : 14 : 1) gave 16l (trace) as a white solid, 13l (1.50 g, 22%) as a white solid, and 12l (2.77 g, 45%) as a white oil.
12l: IR (KBr) cm−1: 3290 (NH of sec-amine), 1513 (C=S), 1242, 1034, 928 (C–O). 1H-NMR (CDCl3) δ: 2.76 (6H, t, J=5.8 Hz, H2″, 2′), 2.82 (4H, t, J=5.8 Hz, H1′), 3.75 (2H, t, J=5.8 Hz, H1″), 3.82 (3H, s, OCH3 on C4‴), 3.82 (2H, s, Hα), 3.83 (3H, s, OCH3 on C3‴), 4.48 (3H, br s, NH), 5.92 (4H, s, H2), 6.73 (2H, d, J=7.9 Hz, H7), 6.76 (1H, d, J=8.5 Hz, H5‴), 6.85 (2H, dd, J=7.9, 1.5 Hz, H6), 6.91 (2H, d, J=1.5 Hz, H4), 6.93 (1H, dd, J=8.5, 2.4 Hz, H6‴), 7.26 (1H, br s, H2‴), 9.06 (1H, br s, NH). 13C-NMR (CDCl3) δ: 42.12 (C1″), 45.25 (C1′), 51.92 (Cα), 52.28 (C2′), 54.39 (C2″), 55.99, 56.08 (OCH3), 101.31 (C2), 108.55 (C7), 109.10 (C2‴), 109.60 (C4), 111.19 (C5‴), 116.53 (C6‴), 123.14 (C6), 127.58 (C5), 132.14 (C1‴), 146.79 (C3‴), 147.93 (C7a), 148.14 (C3a), 148.90 (C4‴), 181.62 (C=S). Positive-ion FAB-MS m/z: 610 (M+H+). HR-FAB-MS m/z: 610.2714 (Calcd for C31H40N5O6S: 610.2699). Anal. Calcd for C31H39N5O6S·0.9H2O: C, 59.48; H, 6.57; N, 11.19. Found: C, 59.51; H, 6.50; N, 11.09.
16l: 13C-NMR (CDCl3) δ: 42.98 (C4), 53.24 (C5), 56.02, 56.09 (OCH3), 109.47 (C2′), 111.70 (C5′), 117.53 (C6′), 130.34 (C1′), 147.69 (C4′), 149.41 (C3′), 181.30 (C=S). (Signals of only the predominant conformer were assigned.) Positive-ion FAB-MS m/z: 239 (M+H+). HR-FAB-MS m/z: 239.0850 (Calcd for C11H15N2O2S: 239.0854).
N,N″-[[[2-[(1,3-Benzodioxol-5-ylmethyl)amino]ethyl]imino]di-2,1-ethanediyl]bis[N′-(3,4-dimethoxyphenyl)thiourea] (13l) (Entry 21)Compound 13l was prepared from 1 (731 mg, 5.00 mmol) with 6l (1.95 g, 10.0 mmol) and 2d (751 mg, 5.00 mmol) under the conditions shown in Table S4. Separation of the products by flash chromatography (CH2Cl2: 95% EtOH : 28% NH3=95 : 4.7 : 0.3→93 : 6.6 : 0.4) gave 8l (1.11 g, 30%) as a white solid and 13l (1.15 g, 34%) as a colorless semisolid.
13l: mp 65–69°C. IR (KBr) cm−1: 3264 (NH of sec-amine), 1512 (C=S), 1236, 1026, 927 (C–O). 1H-NMR (CDCl3) δ: 1.81 (2H, br s, NH), 2.59 (4H, br s, H1′, 2′), 2.66 (4H, t, J=6.1 Hz, H2″), 3.56 (2H, s, Hα), 3.63 (2H, br s, H1″), 3.82 (6H, s, CH3O on C3‴), 3.84 (6H, s, CH3O on C4‴), 5.87 (2H, s, H2), 6.66–6.71 (2H, m, H7, 6), ca. 6.7 (1H, br s, NH), 6.73 (1H, br s, H4), 6.76 (2H, dd, J=8.2, 2.1 Hz, H6‴), 6.82 (2H, d, J=8.2 Hz, H5‴), 6.83 (2H, br s, H2‴), 7.88 (2H, br s, NH). 13C-NMR (CDCl3) δ: 43.48 (C1″), 46.77 (C1′), 53.36 (Cα), 53.43 (C2′ or 2″), 53.62 (C2″ or 2′), 56.09, 56.12 (OCH3), 100.89 (C2), 108.11 (C7 or 4), 108.59 (C4 or 7), 109.83 (C2‴), 111.65 (C5‴), 118.04 (C6‴), 121.25 (C6), 129.69 (C1‴), 133.50 (C5), 146.61 (C3a or 7a), 147.72 (C4‴), 148.14 (C7a or 3a), 149.67 (C3‴), 181.27 (C=S). Positive-ion FAB-MS m/z: 671 (M+H+). HR-FAB-MS m/z: 671.2684 (Calcd for C32H43N6O6S2: 671.2686). Anal. Calcd for C32H42N6O6S2·1.2H2O: C, 55.50; H, 6.46; N, 12.14. Found: C, 55.54; H, 6.24; N, 11.86.
N-[2-[Bis[2-[(1,3-benzodioxol-5-ylmethyl)amino]ethyl]amino]ethyl]-N′-[3-(trifluoromethyl)phenyl]thiourea (12o), N,N′-[[[2-[(1,3-Benzodioxol-5-ylmethyl)amino]ethyl]imino]di-2,1-ethanediyl]bis[N′-[3-(trifluoromethyl)phenyl]thiourea] (13o) and 1-[3-(Trifluoromethyl)phenyl]-2-imidazolidinethione (16o) (Entry 22)Compounds 12o and 13o were prepared from 1 (1.46 g, 10.0 mmol) with 6o (2.03 g, 10.0 mmol) and 2d (3.00 g, 20.0 mmol) under the conditions shown in Table S4. Separation of the products by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=93 : 6.6 : 0.4→85 : 14 : 1) gave 16o (466 mg, 19%) as a pale yellow semi-solid, 13o (1.51 g, 22%) as a pale yellow semi-solid, and 12o (3.42 g, 55%) as a pale yellow oil.
12o: IR (KBr) cm−1: 3260 (NH of sec-amine), 1490 (C=S), 1332 (ArCF3), 1249, 1038, 930 (C–O). 1H-NMR (CDCl3) δ: ca. 2.3 (4H, br s, NH), 2.58 (4H, t, J=5.5 Hz, H2′), 2.65–2.70 (6H, m, H1′, 2″), 3.60 (4H, s, Hα), 3.60 (2H, br s, H1″), 5.87 (4H, s, H2), 6.67–6.71 (4H, m, H6, 7), 6.74 (2H, s, H4), 7.34–7.37 (2H, m, H4‴, 5‴), 7.56 (2H, br s, H2‴, 6‴). 13C-NMR (CDCl3) δ: 43.24 (C1″), 46.66 (C1′), 52.57 (C2″), 53.46 (Cα), 53.95 (C2′), 100.95 (C2), 108.19 (C7), 108.57 (C4), 120.36 (q, J=4.1 Hz, C2‴), 121.30 (C6), 121.49 (q, J=4.1 Hz, C4‴), 125.96 (q, J=272.1 Hz, CF3), 127.05 (C6‴), 129.22 (C5‴), 131.21 (q, J=33.1 Hz, C3‴), 133.20 (C5), 139.29 (C1‴), 146.77 (C3a), 147.83 (C7a), 181.05 (C=S). Positive-ion FAB-MS m/z: 135 (100), 247 (14), 618 (6, M+H+). HR-FAB-MS m/z: 618.2363 (Calcd for C30H35F3N5O4S: 618.2362). Anal. Calcd for C30H34F3N5O4S·0.6H2O: C, 57.33; H, 5.65; N, 11.14. Found: C, 57.35; H, 5.65; N, 10.94.
13o: IR (KBr) cm−1: 3256 (NH of sec-amine), 1490 (C=S), 1332 (ArCF3), 1251, 1038, 930 (C–O). 1H-NMR (CDCl3) δ: 1.23 (3H, t, J=7.0 Hz, CH3 of EtOH), ca. 2.15 (3H, br s, NH and/or OH), 2.66–2.71 (2H, m, H2′), ca. 2.7 (1H, br s, NH or OH), 2.71–2.78 (6H, m, H1′, 2″), 3.57 (2H, s, Hα), 3.69 (4H, br s, H1″), 3.70 (2H, q, J=7.0 Hz, CH2 of EtOH), ca. 3.7 (1H, br s, NH), 5.78 (2H, s, H2), 6.57–6.68 (3H, m, H6, 7, 4), ca. 6.65 (1H, br s, NH), 7.22–7.31 (4H, m, H4‴, 5‴), 7.36 (2H, br d, J=7.6 Hz, H6‴), 7.59 (2H, s, H2‴). 13C-NMR (CDCl3) δ: 18.40 (CH3 of EtOH), 43.36 (C1″), 46.82 (C1′), 53.29 (C2′), 53.59 (Cα), 53.66 (C2″), 58.34 (CH2 of EtOH), 100.99 (C2), 108.33 (C7), 108.61 (C4), 120.45 (q, J=4.1 Hz, C2‴), 121.56 (C6), 121.86 (q, J=4.1 Hz, C4‴), 123.68 (q, J=272.1 Hz, CF3), 127.07 (C6‴), 129.25 (C5‴), 131.07 (q, J=30.0 Hz, C3‴), 132.27 (C5), 138.90 (C1‴), 146.93 (C3a), 147.85 (C7a), 181.20 (C=S). Positive-ion FAB-MS m/z: 135 (100), 247 (61), 687 (22, M+H+). HR-FAB-MS m/z: 687.2006 (Calcd for C30H33F6N6O2S2: 687.2011). Anal. Calcd for C30H32F6N6O2S2·EtOH: C, 52.45; H, 5.23; N, 11.47. Found: C, 52.44; H, 5.00; N, 11.46.
16o: Positive-ion FAB-MS m/z: 135 (57), 247 (100, M+H+). HR-FAB-MS m/z: 247.0515 (Calcd for C10H10F3N2S: 247.0517). 1H-NMR (CDCl3) δ: 2.70 (2H, t, J=4.8 Hz, H5), 3.80 (2H, br d, J=4.8 Hz, H4), 7.20–7.24 (1H, m, H5′), 7.26 (1H, d, J=6.8 Hz, H4′), 7.50 (1H, br d, J=6.9 Hz, H6′), 7.91 (1H, br s, NH), 7.97 (1H, br s, H2′). 13C-NMR (CDCl3) δ: 41.97 (C4), 52.57 (C5), 119.65 (br s, C2′), 121.11 (C4′), 123.79 (q, J=273.1 Hz, CF3), 126.27 (C6′), 129.11 (C5′), 130.66 (q, J=33.2 Hz, C3′), 139.58 (C1′), 180.88 (C=S).
General Procedure for the Preparation of CS-Symmetrical Amide-Urea-Type TAEA Derivatives (14, 15): Example: Synthesis of N-(1,3-Benzodioxol-5-ylmethyl)-N-[2-[Bis[2-[[thioxo(tricyclo[3.3.1.13,7]dec-1-ylamino)methyl]amino]ethyl]amino]ethyl]acetamide (15k) (Entry 24) (Step 1)To a pale yellow suspension of compound 13k (1.88 g, 2.82 mmol) in tetrahydrofuran (THF) (5 mL) was added Ac2O (4, 288 mg, 2.82 mmol) and the mixture was stirred for 30 min at r.t. The crude precipitated solid was obtained as a white substance 15k (1.83 g, 92%). Recrystallization from THF gave an analytically pure sample as a white powder.
15k: mp 188–192°C (from THF). IR (KBr) cm−1: 1627 (C=O), 1540 (C=S), 1357, 1238, 1038 (C–O). 1H-NMR (CDCl3) δ: 1.68 (12H, br s, Ha), 2.11 (6H, br s, Hc), 2.14 (12H, br s, Hb), 2.19 (3H, s, CH3), 2.63–2.68 (6H, m, H2′, 2″), 3.43 (2H, t, J=5.5 Hz, H1′), 3.55 (4H, q, J=5.2 Hz, H1″), 4.53 (2H, br s, Hα), 5.96 (2H, s, H2), 6.32 (2H, br s, NH), 6.62 (2H, br s, NH), 6.63 (1H, d, J=7.9 Hz, H6), 6.64 (1H, br s, H4), 6.7–6.8 (1H, d, J=7.9 Hz, H7). 13C-NMR (CDCl3) δ: 22.26 (CH3), 29.60 (Cc), 36.31 (Ca), 42.17 (Cb), 42.86 (C1″), 43.89 (C1′), 51.53 (Cα), 52.53 (C2′), 53.19 (C2″), 53.94 (Cd), 101.27 (C2), 106.75 (C4), 108.65 (C7), 119.63 (C6), 129.72 (C5), 147.30 (C3a), 148.42 (C7a), 172.52 (C=O), 181.19 (C=S). Positive-ion FAB-MS m/z: 709 (M+H+). HR-FAB-MS m/z: 709.3948 (Calcd for C38H57N6O3S2: 709.3934). Anal. Calcd for C38H56N6O3S2·0.4H2O: C, 63.72; H, 7.99; N, 11.73. Found: C, 63.69; H, 8.13; N, 11.47.
N,N′-[[[2-[[Thioxo(tricyclo[3.3.1.13,7]dec-1-ylamino)methyl]amino]ethyl]imino]di-2,1-ethanediyl]bis[N-(1,3-benzodioxol-5-ylmethyl)acetamide] (14k) (Entry 23)Compound 14k was prepared from 12k (1.22 g, 2.00 mmol) and Ac2O (4, 408 mg, 4.00 mmol) under the conditions shown in Table S5. Separation of the product by flash chromatography (EtOAc : EtOH=99 : 1) gave 14k (934 mg, 68%) as a white solid.
14k: mp 66–68°C. IR (KBr) cm−1: 3324 (NH), 1637 (C=O), 1490 (C=S), 1248, 1038, 924 (C–O). 1H-NMR (CDCl3) δ: 1.66 (6H, br s, Ha), 2.06 (3H, br s, Hc), 2.12*, 2.16 (6H, s, CH3), 2.08, 2.20* (6H, br s, Hb), 2.6–2.55 (4H, m, H2′), 2.6–2.7 (2H, m, H2″), 3.2–3.25, 3.3–3.4* (4H, m, H1′), 3.4–3.5, 3.5–3.6* (2H, m, H1″), 4.36, 4.45*, 4.48 (4H, s, Hα), 5.92, 5.96*, 5.97 (4H, s, H2), 6.34 (1H, br s, NH), 6.6–6.7 (4H, m, H4, 6), 6.7–6.8 (2H, m, H7), 7.18 (1H, br s, NH). (The signals of the predominant conformer were asterisked.) 13C-NMR (CDCl3) δ: 21.84 (CH3), 29.51 (Cc), 36.30 (Ca), 41.88 (Cb), 42.41 (C1″), 43.38 (C1′), 51.34 (Cα), 51.99 (C2′), 52.69 (C2″), 53.27 (Cd), 101.09 (C2), 106.66 (C4), 108.50 (C7), 119.45 (C6), 130.07 (C5), 147.05 (C3a), 148.23 (C7a), 171.50 (C=O), 180.82 (C=S). (Signals of only the predominant conformer were assigned.) Positive-ion FAB-MS m/z: 692 (M+H+). HR-FAB-MS m/z: 692.3488 (Calcd for C37H50N5O6S: 692.3482). Anal. Calcd for C37H49N5O6S·0.9H2O: C, 62.76; H, 7.23; N, 9.89. Found: C, 62.87; H, 7.33; N, 9.64.
N,N′-[[[2-[[Thioxo(3,4-dimethoxyphenylamino)methyl]amino]ethyl]imino]di-2,1-ethanediyl]bis[N-(1,3-benzodioxol-5-ylmethyl)acetamide] (14l) (Entry 25)Compound 14l was prepared from 12l (1.53 g, 2.51 mmol) and 4 (0.512 g, 5.02 mmol) under the conditions shown in Table S5. Separation of the product by centrifugal chromatography (CH2Cl2 : 95% EtOH : 28% NH3=95 : 4.7 : 0.3→93 : 6.6 : 0.4) gave 14l (1.39 g, 80%) as a white solid.
14l: mp 54–58°C. IR (KBr) cm−1: 1639 (C=O), 1512, 1490 (C=S), 1237, 1036, 923 (C–O). 1H-NMR (CDCl3) δ: 2.03, 2.08, 2.10*, 2.11 (6H, s, CH3CO), 2.45–2.6 (4H, m, H2′), 2.6–2.70 (2H, m, H2″), ca. 2.65 (1H, br s, OH), 3.05–3.15, 3.24–3.3, 3.3–3.35* (4H, m, H1′), 3.57 (2H, br s, H1″), 3.80, 3.82*, 3.83 (6H, s, OCH3), 4.33, 4.42*, 4.44 (4H, s, Hα), 5.91, 5.95*, 5.95 (4H, s, H2), 6.55–6.95, 7.17–7.23 (9H, m, H6, 4, 7, 5‴, 6‴, 2‴), 7.49, 8.30, 8.63* (2H, br s, NH). (The signals of the predominant conformer were asterisked.) 13C-NMR (CDCl3) δ: 21.64 (CH3CO), 42.46 (C1″), 43.61 (C1′), 51.53 (Cα), 51.88 (C2′), 52.74 (C2″), 55.71, 55.84 (OCH3), 100.99 (C2), 106.58 (C4), 108.32 (C7), 108.91 (C2‴), 111.19 (C5‴), 116.22 (C6‴), 119.41 (C6), 129.92 (C5), 131.31 (C1‴), 146.96 (C3a or C7a), 147.72 (C4‴), 148.09 (C7a or C3a), 148.84 (C3‴), 171.32 (C=O), 181.02 (C=S). (Signals of only the predominant conformer were assigned.) Positive-ion FAB-MS m/z: 694 (M+H+). HR-FAB-MS m/z: 694.2928 (Calcd for C35H44N5O8S: 694.2911). Anal. Calcd for C35H43N5O8S·0.5H2O; C, 59.81; H, 6.31; N, 9.96. Found: C, 59.74; H, 6.31; N, 10.04.
N-(1,3-Benzodioxol-5-ylmethyl)-N-[2-[Bis[2-[[thioxo(3,4-dimethoxyphenylamino)methyl]amino] Ethyl]amino]ethyl]acetamide (15l) (Entry 26)Compound 15l was prepared from 13l (394 mg, 587 µmol) and 4 (59.9 mg, 587 µmol) under the conditions shown in Table S5. Separation of the product by centrifugal chromatography (CH2Cl2 : EtOH=95 : 5) gave 15l (368 mg, 88%) as a white solid. An analytical sample of 15l was obtained by recrystallization from dichloromethane (CH2Cl2) as an off-white solid.
15l: mp 193–194°C (from CH2Cl2). IR (KBr) cm−1: 1616 (C=O), 1511, 1453 (C=S), 1239, 1034, 922 (C–O). 1H-NMR (DMSO-d6) δ: 1.99, 2.08* (3H, s, CH3CO), 2.54–2.66 (6H, m, H2′, 2″), 3.18–3.27 (2H, m, H1′), 3.49 (4H, br t, J=5.5 Hz, H1″), 3.71 (6H, s, CH3 on C3‴), 3.72 (6H, s, CH3 on C4‴), 4.39*, 4.41 (2H, br s, Hα), 5.70*, 6.00 (2H, s, H2), 6.64–6.98 (9H, m, H6, 7, 4, 6‴, 5‴, 2‴), 7.31 (2H, br s, NHβ), 9.39 (2H, s, NHγ). (The signals of the predominant conformer were asterisked.) 13C-NMR (DMSO-d6) δ: 21.15 (CH3CO), 41.94 (C1″), 45.93 (C1′), 47.64 (Cα), 51.71 (C2′), 52.40 (C2″), 55.44, 55.68 (OCH3), 100.76 (C2), 107.93 (C7), 108.08 (C4), 109.30 (C2‴), 111.98 (C5‴), 116.39 (C6‴), 120.96 (C6), 131.42 (C1‴ or C5), 132.12 (C5 or C1‴), 146.27 (C3a or C7a), 146.38 (C4‴), 147.29 (C7a or C3a), 148.70 (C3‴), 169.64 (C=O), 180.26 (C=S). (Signals of only the predominant conformer were assigned.) Positive-ion FAB-MS m/z: 713 (M+H+). HR-FAB-MS m/z: 713.2786 (Calcd for C34H45N6O7S2: 713.2791). Anal. Calcd for C34H44N6O7S2: C, 57.29; H, 6.22; N, 11.79. Found: C, 57.10; H, 6.17; N, 11.75.
Preparation of C3-Symmetrical TAPA Derivatives (18a, 21) 2,2′,2″-[Nitrilotris(3,1-propanediyliminomethylene)]tris[6-methoxyphenol] (18a)Compound 18a was prepared starting from the reaction of TAPA (17, 942 mg, 5.0 mmol) and 2a (3.04 g, 20.0 mmol) in a manner similar to that for the preparation of compound 3a. The reactions in both steps were conducted in EtOH instead of MeOH with the ratio of TAPA : 2a : NaBH4=1 : 4 : 8.2. Separation of the product by centrifugal chromatography (CHCl3 : 2-PrOH : 28% NH3=84 : 14 : 2) gave 18a (1.93 g, 65%) as a light-brown semisolid.
18a: mp 31–35°C. IR (KBr) cm−1: 3420 (OH), 3310 (NH), 1590, 1480 (C=C of Ar), 1235, 1075 (C–O). 1H-NMR (CDCl3) δ: 1.63 (6H, qu, J=6.7 Hz, H2′), 2.41 (6H, t, J=6.7 Hz, H3′), 2.64 (6H, t, J=6.7 Hz, H1′), 3.84 (9H, s, CH3O), 3.93 (6H, s, Hα), 6.26 (6H, br s, NH, OH), 6.61 (3H, d, J=7.6 Hz, H3), 6.71 (3H, dd, J=7.9, 7.6 Hz, H4), 6.79 (3H, d, J=7.9 Hz, H5). 13C-NMR (CDCl3) δ: 26.74 (C2′), 47.35 (C1′), 52.21 (C3′ or Cα), 52.24 (Cα or C3′), 55.83 (CH3O), 110.83 (C5), 118.48 (C4), 120.55 (C3), 122.69 (C2), 147.40 (C1), 147.96 (C6). Positive-ion FAB-MS m/z: 597 (M+H+). HR-FAB-MS m/z: 597.3644 (Calcd for C33H49N4O6: 597.3652). Anal. Calcd for C33H48N4O6·1.2 H2O: C, 64.10; H, 8.22; N, 9.06. Found: C, 64.15; H, 8.31; N, 8.91.
N,N′,N″-(Nitrilotri-3,1-propanediyl)tris[3,5-bis(trifluoromethyl)benzamide] (21)To a solution of 17 (942 mg, 5.0 mmol) and triethylamine (TEA, 1.77 g, 17.5 mmol) in CH2Cl2 (50 mL) was added a solution of 3,5-bis(trifluoromethyl)benzoyl chloride (19, 4.78 g, 17.3 mmol) in CH2Cl2 (25 mL) at 0°C under an N2 atmosphere. After stirring for 30 min, the reaction mixture was stirred at r.t. for another 1 h. The resulting mixture was poured into water (100 mL), and then the aqueous layer was extracted with CH2Cl2 (2×100 mL). The combined organic layer was washed with brine (50 mL) and dried over MgSO4, and the solvent was evaporated to give a yellow oil. Separation of the product by flash chromatography (CH2Cl2 : 95% EtOH : 28% NH3=97 : 2.7 : 0.3) and then recrystallization from MeOH gave compound 21 (2.99 g, 66%) as colorless crystals.
21: mp 205–206°C (from MeOH). IR (KBr) cm−1: 3265 (NH), 1645, 1550, 1280 (C=O), 1135 (CF3). 1H-NMR (DMSO-d6) δ: 1.71(6H, t, J=7.0 Hz, H2′), 2.49 (6H, m, H3′), 3.35 (6H, m, H1′), 8.23 (3H, s, H4), 8.41 (6H, s, H2, H6), 8.94 (3H, t, J=5.3 Hz, NH). 13C-NMR (DMSO-d6) δ: 26.39 (C2′), 38.03 (C1′), 50.93 (C3′), 123.08 (CF3, q, J=273.1 Hz), 124.58 (C4), 127.86 (C2, C6), 130.41 (C3, C5, q, J=33.1 Hz), 136.68 (C1), 163.13 (C=0). Positive-ion FAB-MS m/z: 909 (M+H+). HR-FAB-MS m/z: 909.2101 (Calcd for C36H31F18N4O3: 909.2109). Anal. Calcd for C36H30F18N4O3: C, 47.59; H, 3.33; N, 6.17. Found: C, 47.58; H, 3.34; N, 6.22.
Antiviral Activity Assay and Cytotoxicity of Synthesized Trisubstituted Tris(aminoalkyl)amine DerivativesThe anti-HSV-1 activities (EC50) of the synthesized tris(aminoalkyl)amine derivatives were assessed by using a plaque reduction assay17) and their cytotoxicity against Vero cells (CC50) was also evaluated. The results are summarized in Tables 1, 2 together with data for aciclovir.18) Log P values for all of the compounds for which biological activities were evaluated were calculated by using CAChe v.6.1.12. There were few distinct correlations between log P values and anti-HSV-1 activities (EC50 values) among the compounds listed in Tables 1, 2.
The authors would like to thank Ms. Ai Yuzuriha, Ms. Izumi Sakai, Ms. Marina Sano, Ms. Yuuna Kawaguchi, Ms. Saki Sawai, Mr. Ryo Sato, Mr. Kenta Morimoto, and Mr. Shunsuke Shimomura for their valuable technical assistance.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.
Table S1. Synthesis of C3-Symmetrical Amine-Type TAEA Derivatives (3)
Table S2. Synthesis of C3-Symmetrical Amide-Type TAEA Derivatives (5)
Table S3. Synthesis of C3-Symmetrical Urea-Type TAEA Derivatives (7, 8)
Table S4. Synthesis of CS-Symmetrical Amine-Urea-Type TAEA Derivatives (12, 13)
Table S5. Synthesis of CS-Symmetrical Amide-Urea-Type TAEA Derivatives (14, 15)