2016 Volume 64 Issue 2 Pages 119-127
2016 Volume 64 Issue 2 Pages 119-127
Tumor necrosis factor related apoptosis-inducing ligand (TRAIL) has emerged as a promising anticancer agent as it selectively kills cancer cells. However, TRAIL resistance limits its use as a therapeutic agent. An understanding the mechanisms responsible for TRAIL resistance and strategies to overcome it are important for its effective use as an anticancer agent. During our studies to screen natural products from medicinal plants, we identified a number of compounds with synergistic effects on TRAIL-induced apoptosis in tumor cells. This review describes our recent studies on the isolation of bioactive compounds with TRAIL-resistance overcoming activity.
Natural products serve as a vital source for new compounds with diversified structures possessing different biological activities. Several reports stated that natural products and natural-product based derivatives have played a significant role in the field of drug discovery studies.1,2) About 1073 new small molecule entities were approved between 1981–2010, 64% of which were natural products or related compounds.3) In recent years, there has been renewed interest in natural products research from the view point of the drug discovery and development process. As part of our extensive search for bioactive compounds that could overcome tumor necrosis factor related apoptosis-inducing ligand (TRAIL)-resistance, we explored natural resources such as actinomycetes and medicinal plants. Here, we describe some of our recent experimental results on the search for natural products to overcome TRAIL-resistance from the medicinal plants of Bangladesh and Thailand.
TRAIL or Apo 2 ligand, a member of the tumor necrosis factor (TNF) superfamily, can induce apoptosis in a variety of tumoral and transformed cells without affecting normal cells, and has thus emerged as a promising antitumor agent.4) Unlike other members of the superfamily, in vivo administration of TRAIL was confirmed to be safe. Because of its ability to induce significant tumor suppression and inhibit the progression of cancer in experimental animals without systemic toxicity, this naturally occurring cytokine is now under preclinical investigation. TRAIL is expressed on different immune cells and has a significant role in immune surveillance against malignant transformative diseases and viral infection.
TRAIL-induced apoptosis starts when it binds to its receptors (TRAIL-Rs). Four homologous human TRAIL-Rs have been reported so far, which are called TRAIL-R1 (also called DR4), TRAIL-R2 (DR5, Apo2, TRICK2 or KILLER), TRAIL-R3 (DcR1/TRID), and TRAIL-R4 (DcR2).5) Both death receptors (DRs), DR4 and DR5 comprise a conserved death domain (DD) motif and are responsible for apoptosis induction. Two other receptors (DcR1 and DcR2) are known as decoy receptors (DcRs), and are unable to induce apoptosis. DcR2 has a truncated, nonfunctional cytoplasmic death domain (DD), while DcR1 lacks a cytosolic region.5) It is well-known that apoptosis can be induced via an extrinsic pathway (DR pathway) and intrinsic pathway (mitochondrial pathway). TRAIL can induce apoptosis by activating either pathway, depending on the cells.4) The extrinsic (DR) pathway initiates with the binding of TRAIL to DRs. The binding of TRAIL (as a trimer) to DR4 and/or DR5 induces receptor trimerization, which is the prerequisite for construction of the death-inducing signaling complex (DISC). The adaptor protein Fas-associated death domain (FADD) is recruited to the DD of DRs via its own DD, and FADD recruits pro-caspase-8 to the DISC via the death effector domain (DED) interaction and induces proteolytic activation of caspase-8. After release from the DISC, the active caspase-8 homodimers then activate the effector caspase-3 to induce apoptosis5) (Fig. 1).

The intrinsic (mitochondrial) pathway triggers apoptosis in response to various types of cellular damage, including oxidative stress, misbalanced homeostasis, and DNA damage.6) The pathway involves the activation of the proapoptotic genes (Bak and Bax), which then stimulate the mitochondria to release some apoptogenic factors, such as cytochrome c and SMAC/DIABLO into the cytosol.7) In the cytosol, cytochrome c binds the adaptor protein APAF-1 and forms an “apoptosome” that activates caspase-9. Caspase-9 then activates “effector” caspase-3 and -7. SMAC/DIABLO promotes apoptosis by binding to the inhibitor of apoptosis proteins (IAPs) and preventing these factors8,9) (Fig. 1). Cross-talk occurs between the intrinsic and extrinsic apoptotic pathways. Caspase-8 activates Bid, which then translocates to the mitochondria and activates the mitochondrial apoptotic pathway.
However, it is problematic that a substantial number of tumor cells, especially some highly malignant cancers, are resistant to TRAIL-mediated apoptosis. In addition, some sensitive cancer cells can become resistant to TRAIL-mediated apoptosis after frequent exposure.10) Resistance to TRAIL may arise at various points in the TRAIL-induced apoptotic pathways. Dysfunction of the DR5 and DR4 DRs due to mutations can lead to the development of resistance. FADD and caspase-8 are essential for the formation of the DISC, and deficiencies in either of these molecules result in TRAIL resistance. The overexpression of the cellular FADD-like interleukin-1β-converting enzyme–inhibitory protein (cFLIP) correlates with TRAIL resistance in some cancers. The over expression of Bcl-2, Bcl-X(L) or IAPs, loss of Bak or Bax function, and reduced release of SMAC/DIABLO from the mitochondria to the cytosol were reported to lead to TRAIL resistance in mitochondria-dependent type II tumor cells. Overcoming TRAIL resistance and interpretation of the mechanisms responsible for such resistance are therefore very important for the successful use of TRAIL as an anticancer agent.11,12) However, several studies showed that TRAIL resistance can be abrogated by a combination of chemotherapeutic agents and irradiation.13) The search for agents that can overcome TRAIL resistance may contribute to developing effective antitumor lead drugs.
Amoora cucullata ROXB. (family: Meliaceae; synonym: Aglaia cucullata), locally known as amoor, natmi, or latmi, is a tall tree native to the coastal forests of Bengal, Burma, the Malay Peninsula, Borneo, and the Andaman Islands. Its leaves are used traditionally for the treatment of inflammation, skin diseases, dysentery, and cardiac disease.14,15) It was previously reported that the crude MeOH extracts of A. cucullata leaves showed antinociceptive, diuretic, antiinflammatory, and central nervous system-depressant activities.16,17) Bioassay-guided separation of its leaves resulted in the isolation of four new compounds (1–4)18) together with seven known compounds identified as ent-13-epi-mannol19) (5), kaur-15-en-17-ol20) (6), ent-2β-hydroxymanool21) (7), 1-O-formylrocagloic acid22) (8), 3′-hydroxyrocagloic acid22) (9), dasyclamide23) (10), and 2β,15-dihydroxy-ent-labda-8(17),13E-diene24) (11) (Fig. 2).

Among the isolated compounds, 1, 5, 8, and 9 exhibited activity to overcome TRAIL resistance (Fig. 3A). However, compound 8 showed the most potent activity and enhanced TRAIL-mediated apoptosis in TRAIL-resistant human gastric adenocarcinoma (AGS) cells at 1 and 2 nM without affecting the normal human renal epithelial (293T) cells at that dose (Fig. 3B). It was reported that TRAIL-induced apoptosis was enhanced by 8, involving the activation of caspase-3/7 and upregulation of DR4 and DR5.18)

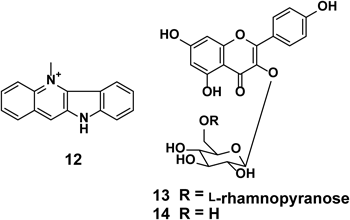
Sida acuta BURM. (Malvaceae) is a shrub found throughout Bangladesh where it is known as berela. Its leaves are traditionally used as a demulscent and diuretic; the roots are used as antipyretic, tonic, and diaphoretic agents.25) Previously it was described for its analgesic, antimicrobial, free radical scavenging, antiplasmodial, and apoptosis inducing activities. Bioassay-guided fractionation of S. acuta (whole plants) led to the isolation of an alkaloid, cryptolepine (12),26) together with two glycosides, kampferol-3-O-α-L-rhamnopyranosyl-β-D-glucopyranoside (13)27) and kampferol-3-O-β-D-glucopyranoside (14)28) (Fig. 4). Compound 12 showed strong TRAIL-resistance abrogating activity in AGS cells at 1.25, 2.5, and 5 µM (Fig. 5). It sensitized AGS cells to TRAIL-induced apoptosis by the activation of caspase-3/7.29)

Bioassay-guided fractionation of Thevetia peruviana (Apocynaceae) bark, collected from Thailand, resulted in the isolation of four cardenolide glycosides (15–18) identified as neriifolin (15),30) thevefolin (16),31) peruvoside (17),32) and (20S)-18,20-epoxydigitoxigenin α-L-thvetoside (18)33) (Fig. 6).

Compounds 15 (29 nM) and 16 (125 nM) produced 36 and 46% greater inhibition, respectively, of cell viability by cotreatment with TRAIL (100 ng/mL) compared with that produced by these compounds alone, indicating that compounds 15 and 16 have a synergistic effect on TRAIL-induced apoptosis in AGS cells (Fig. 7). Thevefolin (16) produced this synergistic effect by increasing DR4 and DR5 mRNA expression.34)

Erythrophleum succirubrum (Leguminosae), collected from Thailand, is a tall deciduous tree that grows in tropical areas. Bioassay-guided separation of E. succirubrum bark led to four new compounds, erythrophlesins A–D (19–22)35) (Fig. 8), were isolated. Erythrophlesins A–C (19–21) showed significant TRAIL resistance abrogation in AGS cells. In particular, compound 21 at 10 µM showed a 37% decrease in cell viability in combination with TRAIL (100 ng/mL) compared with that in the absence of TRAIL35) (Fig. 9).


Kandelia candel (Rhizophoraceae) is a mangrove found in coastal areas of South and Southeast Asia. From K. candel leaves, a new eudesmane sesquiterpenoid (23),36) three new guaiane sesquiterpene lactones, mehirugins A–C (24, 25, 27),36) notoserolide B37) (26), and matricarin38) (28) were isolated (Fig. 10). Co-treatment with compounds 23 and 25–28 with TRAIL showed TRAIL-resistance overcoming activity in AGS cells36) (Fig. 11).


Artocarpus champeden SPRENG. (Moraceae) is commonly distributed throughout Southeast Asia. The isolation of a number of prenylated flavonoids was previously described from its heartwood.39) Bioassay activity-guided separation of A. champeden roots resulted in the isolation of six compounds, pannokins A–C (29–31),40) artonin B (32),41,42) artoheterophyllin D (33),43) and heterophyllin (34)44) (Fig. 12).

When the isolates were tested for activity to overcome TRAIL resistance, relatively strong activity was found for 30, 33, and 34. Combined treatment with 30 (5 µM), 33 (5 µM), and 34 (4 µM) and TRAIL led to 34, 32, and 33% more inhibition of cell viability, respectively, than treatment with any of the three compounds alone (without TRAIL) (Fig. 13).

Heterophyllin (34), which had the strongest activity, was used for further study in AGS cells. Experimental evidence showed that the apoptotic cell death induced by the combined treatment of AGS cells with 34 and TRAIL was due to C/EBP-homologous protein (CHOP)-dependent upregulation of DR5 expression to induce apoptosis.40)
3.7. Flavonoids from the Fruit of Pongamia pinnataPongamia pinnata (Fabaceae) is a tall tree found in India, Taiwan, and Southeast Asian areas. Its sprouts and fruit are traditionally used for the treatment of cancer.45) Bioassay-guided separation of P. pinnata fruit collected from Bangladesh led to the isolation of a new compound (35)46) together with six known compounds (36–41), which were identified as 5-methoxy-(3″,4″-dihydro-3″,4″-diacetoxy)-2″,2″-dimethylpyrano-(7,8:5″,6″)-flavone47) (36), (−)-isolonchocarpin48) (37), pongamol49) (38), pongapin50) (39), [2″,3″:7,8]furanoflavone50) (40), and isopongaflavone51) (41), respectively (Fig. 14).

The isolated flavonoids and related compounds (35–41) were examined for their effects on TRAIL resistance in AGS cells. Compound 36 in combination with TRAIL (100 ng/mL) showed 39% and 44% more inhibition at 30 and 40 µM, respectively, than the compound alone, implying that 36 overcame TRAIL resistance significantly.46) Compound 41 at 30 and 40 µM caused a 30% and 25% decrease in cell viability, respectively, in the presence of TRAIL (100 ng/mL), indicating its moderate activity (Fig. 15). Experiments showed that the activity to overcome TRAIL resistance of 36 and 41 was related to the enhanced expression of DR5.46)

Artocarpus communis FORST. (Moraceae), known as “breadfruit,” is common in Southeast Asia. Bioassay activity-guided fractionation of A. communis roots led to the isolation of four new prenylated flavonoid and resveratrol derivatives (42–45),52) along with the eight known prenylflavonoids (46–53) artonin E (46),53) morusin (47),54) artobiloxanthone (48),55) heterophyllin (49),56) cycloartobiloxanthone (50),55) cudraflavone B (51),57) artorigidin A (52),56) and pannokin B58) (53) (Fig. 16).

The effects of each compound (42–53) on cell viability were examined at several concentrations, and they were found to be active in overcoming TRAIL resistance. However, compounds 42 (2 µM), 46 (3 µM), and 49 (2 µM) were more potent than the other compounds tested (Fig. 17). Artonin E (46), which exhibited the most potent activity, was selected for further examination using AGS cells. The experimental results showed that the combined treatment with 46 and TRAIL induced apoptosis in a caspase-dependent manner. Finally, it was found that the TRAIL resistance-abrogating activity of artonin E (46) was due to the induction of reactive oxygen species (ROS)- and p53-mediated upregulation of DR5.52)

Bioassay-guided isolation of various medicinal plants of Bangladesh and Thailand resulted in the isolation of a number of bioactive substances that are capable of abrogating TRAIL resistance in AGS cells. For example, 1-O-formyl rocagloic acid (8) from A. cucullata contributed to TRAIL-induced apoptosis by upregulating both DR5 and DR4 mRNA. Cryptolepine (12) from S. acuta, cardenolide glycosides (15 and 16) from T. peruviana, erythrophlesins A–C (19–21) from E. succirubrum, and the eudesmane-type sesquiterpenoid (23) and guaianolides (25–28) from K. candel showed strong activity to overcome TRAIL resistance. Heterophyllin (34) from A. champeden contributed to TRAIL-induced apoptosis by a CHOP-dependent increase in DR5 expression in AGS cells while flavonoids (36 and 41) from P. pinnata abrogated TRAIL-resistance by enhancing DR5 expression. On the other hand artonin E (46) from A. communis abrogated TRAIL resistance by inducing ROS- and p53-mediated increased levels in DR5.
This work was supported by KAKENHI Grant No. 23102008 on Innovative Areas “Chemical Biology of Natural Products” from the Ministry of Education, Culure, Sports, Science and Technology of Japan, KAKENHI Grant Nos. 26305001 and 25870128 from the Japan Society for the Promotion of Science, and by the Tokyo Biochemical Research Foundation, and the Uehara Memorial Foundation, Japan.
The authors declare no conflict of interest.