2016 Volume 64 Issue 3 Pages 239-245
2016 Volume 64 Issue 3 Pages 239-245
Spray-freeze-drying (SFD) is a unique powderization technique to produce highly porous dry powders with a low density. The characteristic morphology can markedly contribute to the superior inhalation performances of SFD powders. Due to the increased specific surface area of the powders, however, moisture adsorption may readily occur, subsequently leading to losses of their inhalation potentials. In this study, hydrophobic amino acids were newly applied as pharmaceutical excipients to obtain SFD powders with both a favorable inhalation performance and antihygroscopic property. SFD powders composed of several hydrophobic amino acids were prepared. The morphology, particle size distribution, and crystallinity of the prepared powders were evaluated by scanning electron micrography, laser diffraction, and X-ray powder diffraction, respectively. The inhalation characteristics of the SFD powders were examined using a twin-stage liquid impinger equipped with an inspiratory pattern simulator and devices. To investigate their antihygroscopicity, moreover, the SFD powders were stored under a humidified condition to assess the morphology, crystallinity, and inhalation performance as described above. It was demonstrated that a SFD powder composed of L-leucine, L-isoleucine, or L-phenylalanine showed a superior inhalation performance, which was sufficiently maintained after storage under the humidified condition, strongly indicating their antihygroscopicity. These results indicated that the hygroscopicity of SFD powders can be effectively improved by the application of hydrophobic amino acids as excipients.
Inhalants that can deliver a drug deep into the lungs directly and noninvasively have attracted attention as dosage forms for the therapy of not only local diseases such as asthma, chronic obstructive pulmonary disease, and cystic fibrosis but also systemic diseases such as diabetes.1–3) There are three kinds of inhalant on the market: pressurized metered-dose inhalers (pMDIs), inhalation solutions, and dry powder inhalers (DPIs). DPIs have many advantages compared to the other two forms: (1) no propellant or battery is required, (2) the device is small, and (3) powders are dispersed on inhalation by a patient, minimizing the mismatch between the timing of inhalation and drug dispersal.4,5)
The powders for DPIs should have an appropriate mass median aerodynamic diameter (MMAD) suitable for inhalation and small adhesion cohesiveness. The commonly recognized optimum MMAD is in the range of 1–6 µm.4) However, the adhesion cohesiveness of such small particles is usually high, and they easily aggregate to form large particles that are not suitable for inhalation.6) To solve this problem, the small particles of an active ingredient are generally mixed with large inert carrier particles (50–100 µm) such as lactose monohydrate to prevent the small particles of an active ingredient from undergoing aggregation.7–9) These mixtures are expected to be broken down and release the active ingredient particles at the time of inhalation; however, it is often the case that a large part of the active ingredient remains on the surface of the carrier particle.10) As for the carrier method, improvement of the release of the active ingredient particles has been attempted by the surface treatment of carrier particles with ethanol to create a smooth surface11) and by the addition of tiny lactose particles to reduce the carrier surface roughness.12)
The second technique employed for market products is the granulation of an active ingredient without additives to produce large particles.13–15) However, it has been reported that the release ratio of the active ingredient depends on the inhalation rate.10)
The third technique to improve the dispersibility of the particles of an active ingredient is the use of low-density particles with a large geometric particle diameter that reduces the particle adhesion cohesiveness and a small MMAD suitable for inhalation.16,17) According to the simplest theory for spherical particles, the MMAD is the product of the geometric diameter and square root of the particle density,18) and the MMAD of low-density particles with a relatively large geometric diameter is still in the range suitable for inhalation.
In addition to the morphology of the particles mentioned above, chemical additives are also a useful tool to improve the dispersibility of tiny particles. Hydrophobic amino acids such as L-leucine (Leu) or L-phenylalanine have been reported to improve the dispersibility of small particles prepared with the spray-drying method.19,20)
The particle design described above is one of the critical factors to determine the effectiveness of inhalation therapy. Inhalation devices and inhalation patterns of patients are other important factors. There are reports that pulmonary drug delivery is affected by the inhalation pattern of the patient21,22) and the inhalation devices.23,24) Recently, we reported that mannitol powders with 5% Leu prepared by spray-freeze-drying (SFD) method showed a high inhalation performance regardless of inspiratory flow patterns and devices. The addition of Leu decreased the adhesion force and increased the surface potential of the powders.25) Chew et al. examined the effect of amino acids (5% in weight) on the dispersion of disodium cromoglycate (95% in weight) powders prepared by spray-drying technique. They also reported that Leu and Phe were the best two to maximize the inhalation performance of disodium cromoglycate.19) Li et al. prepared several admixtures of amino acids and lactose to enhance the aerosolization of spray-dried powders for pulmonary gene therapy.20) They did not try Leu but reported 1 : 10 admixture of arginine or 1 : 5 admixture of Phe with lactose showed the highest inhalation performance. These findings support that some hydrophilic amino acids are effective to improve inhalation performance of dry powders.
SFD method that combines an atomization process and a lyophilization process is one of the practical methods to prepare highly porous dry powders with a low density.26) The powders are expected to be applicable for clinical use as inhaled formulations since their characteristic morphology allows for high dispersibility and superior lung delivery. Due to the increased specific surface area of the powders, however, moisture absorption may readily occur, subsequently leading to a decrease in output efficiency and losses of their inhalation potential. Thus, the absorbed moisture would decrease the powders, the therapeutic effect by changing the deposition behavior of the fine active pharmaceutical ingredient (API) particles, and increase the side effects of fine API particles deposited in the oral cavity. Although amino acids have been studied as excipients of DPIs, the tolerance of them to moisture has not been reported yet to the best of our knowledge. Although 5% hydrophobic amino acids were effective to improve the inhalation performance of powders, our preliminary study showed that such a low concentration was not enough to improve antihygroscopicity of the powder.
In this study, we prepared highly porous dry powders composed of 99% hydrophobic amino acids, that have been reported as dispersing agents, by the SFD technique and investigated their physicochemical properties, inhalation performance, and antihygroscopic property.
Sodium fluorescein (FlNa) (Sigma-Aldrich Co., Ltd., St. Louis, MO, U.S.A.) was used as a model API and a label for quantification of the in-vitro inhalation performance. As a pharmaceutical excipient for DPIs, L-alanine (Ala), glycine (Gly) (Wako Pure Chemical Industries, Ltd., Osaka, Japan), L-isoleucine (Ile), L-leucine (Leu), L-phenylalanine (Phe), L-tryptophan (Trp), or L-valine (Val) (Sigma-Aldrich Co., Ltd.) was used. All the other reagents and solvents used were of analytical and HPLC grade.
Preparation of the SFD PowdersThe SFD powders were prepared by the following procedure: The sample solution (7.5 mL) was atomized into liquid nitrogen to generate frozen droplets with a 0.4 mm two-fluid nozzle placed approximately 15 cm above the surface of the liquid nitrogen at a flow rate of 5 mL/min and atomizing pressure of 150 kPa. The frozen droplets were rapidly transferred into a freeze dryer (EYELA DRC-1100 and FDC-2100, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) precooled at a shelf temperate of −40°C for lyophilization to obtain SFD powders. After evaporation of the liquid nitrogen, freeze-drying was conducted for 24 h at 10°C at a vacuum level ≦2 Pa. The obtained SFD powders were stored in glass vials in a desiccator with silica gel. We prepared seven types of SFD powder composed of one of the hydrophobic amino acids (Ala, Gly, Ile, Leu, Phe, Trp, or Val) and FlNa. Ninetynine-milligrams of amino acids and 1 mg of FlNa were dissolved in 7.5 mL of water to prepare all the formulations.
Morphological Analysis of the SFD Powders by Scanning Electron Microscopy (SEM)The morphology of the dry powders was examined using SEM (JSM-6060; JEOL, Tokyo, Japan). Before the observation, small amounts of powder samples were put in a disposable tip connected to a 1-mL disposable syringe with a three-way stopcock.27) The powders were manually dispersed on a specimen mount with double-sided tape by opening the three-way stopcock to release air compressed in the syringe, and coated with platinum by a sputter coater (JFC-1600, JOEL).
Measurement of Particle Size Distribution of the SFD Powders by Laser Micron SizerThe particle size distribution was measured with a laser diffraction scattering method using a diffractometer with a dry dispersing unit (LSM-2000e; Seishin Enterprise Co., Ltd., Tokyo, Japan). The dry dispersing unit was operated at a constant air pressure of 0.4 MPa to disperse the particles into the laser beam. The median diameter was used as the mean particle size of the samples.
Analysis of Crystallinity by X-Ray Powder Diffraction (XRPD)Powder crystallinity was determined by XRPD (Smart Lab; Rigaku Corporation, Tokyo, Japan). The operating conditions were as follows: a parallel beam at room temperature using CuKα radiation at 30 mA and 40 kV with an angular increment of 5°/min over a 2θ range of 5–45°.
Reproduction of Inspiratory Flow Patterns Using a SimulatorTo clarify the inter-individual variation in the inhalation characteristics of the SFD powders, we applied the inspiratory flow simulator described in our previous paper.28) In brief, this apparatus is composed of an evacuated container with a twin-stage liquid impinger (TSLI: Copley Scientific Ltd.; Nottingham, U.K.) connected by a valve and tube. We previously reported that the human inspiratory flow pattern was schematically characterized by three parameters: peak flow rate (PFR), area under the flow rate–time curve (AUC), and flow increase rate (FIR). These parameters were regulated by the diameter of the tube, volume of the container, and valve-opening speed, respectively. We previously determined that PFR was the most influential on the in-vitro inhalation characteristics of physically mixed dry powders among the three typical parameters mentioned above.28) In this study, we changed PFR from 15 to 120 L/min to determine the influence of PFR on the in-vitro inhalation characteristics of the prepared powders. The flow rate was monitored every 10 ms for 30 s by a hot-wire flow meter (Hitachi Automotive Systems, Co., Ltd., Takasaki, Japan) attached to the inhaler, and the flow pattern was visualized with a personal computer. After dispersion of the powders into the TSLI by the inspiratory flow simulator, the powders were collected and assayed as described below.
In-Vitro Inhalation Characteristics by TSLIInhalation performance of the powders was characterized using the TSLI equipped with the inspiratory flow simulator described above. Stages 1 and 2 of the TSLI contained 7 and 30 mL of phosphate buffer solution (PBS), respectively. Aliquots (1 mg) of the SFD powders were packed in a No. 2 HPMC capsule (Shionogi Qualicaps Co., Ltd., Tokyo, Japan). We employed three inhalers (Jethaler®, Hitachi Automotive Systems, Co., Ltd.) with different resistance levels (Single<Dual<Reverse). After one of the three inhalers was connected to the mouth piece of the TSLI, the capsule containing the SFD powders was placed in its holder with a pin to pierce it. Then, the inspiratory flow simulator was operated to disperse the powder in the capsule at several peak flow rates (PFRs). The AUC and FIR were fixed at 2.5 L and >100 L/min/s, respectively. After dispersion, the dry powders remaining in the capsule, device, throat, and each stage were collected by rinsing with PBS. The collected samples were diluted to 50 mL with PBS, and the concentration of FlNa in each sample was measured with a fluorescence microplate reader (excitation wavelength: 490 nm and emission wavelength: 515 nm) (Gemini EM Fluorescence Microplate Reader, Molecular Device Co., Ltd., Tokyo, Japan). All experiments were performed in triplicate. The in-vitro inhalation performance of the prepared SFD powders by TSLI was characterized by the output efficiency (OE) and fine particle fraction (FPF). OE stands for the amount ratio of drug particles emitted from a capsule and an inhalation device (Eq. 1) while FPF represents the amount ratio of API particles deposited on stage 2 of the TSLI (Eq. 2).
 | (1) |
 | (2) |
For the investigation of their antihygroscopicity, saturated sodium chloride solution was poured into an air-tight container to store the SFD powders under a humidified condition, reaching approximately 75% relative humidity (RH) at room temperature for 4 weeks. The morphology, crystallinity, and inhalation characteristics of the stored SFD powders were similarly examined as described above.
Statistical AnalysisStatistical comparisons were made with a one-way ANOVA. Comparisons of means were performed with the least significant difference test. The significance level was set at p<0.05.
All 7 powders had spherical and porous particles unique to the SFD powders (Fig. 1). No large aggregates were observed for the powders, suggesting that all of the hydrophobic amino acids were candidate dispersing agents.

(a), (b): Ala, (c), (d): Gly, (e), (f): Ile, (g), (h): Leu, (i), (j): Phe, (k), (l): Trp, and (m), (n): Val.
The particle size distribution is shown in Fig. 2. All 7 powders had a single peak and the geometric mean diameters (D50) were within 5–10 µm.

Inhalation performance levels of the 7 powders were evaluated with TSLI equipped with Reverse. Our previous study showed that a large part of the powders remained inside the device because Reverse had the most complex inner structure compared to Single and Dual.29) We employed Reverse for the first screening study of the powders to select the amino acid formulations that showed satisfactory dispersal from the device. The PFR that normal subjects can achieve with Reverse is 12–40 L/min.29) We adopted a PFR of 15 L/min to select the amino acid formulations that dispersed at a low flow rate. The AUC and FIR were fixed at 2.5 L and >100 L/min/s, respectively, since they are the default values we have employed so far in screening studies.
About 60% of Gly remained in the device, resulting in the lowest OE and FPF (Fig. 3). Among the other amino acids, Ile, Leu, and Phe showed high OE and FPF values. These results correlated with the particle size distribution data, i.e., Gly had the largest D50 while Ile, Leu, and Phe had smaller D50s, suggesting that the particle size is essential for the inhalation performance and Ile, Leu, and Phe produced smaller particles or acted as dispersing agents.

(a) Recovery rate and (b) OE and FPF values. The PFR, AUC, and FIR were fixed at 15 L/min, 2.5 L, and >100 L/min/s, respectively. Each value represents the mean±S.D. (n=3). The OE vale of Gly was significantly smaller than those of the other amino acids. The FPF value of Ala was significantly smaller than those of Ile, Leu, and Phe, that of Gly was significantly smaller than those of Ile, Leu, Phe, Trp, and Val, and that of Phe was significantly larger than those of Trp and Val.
According to the screening study with the TSLI, we further characterized the powders of Ile, Leu, and Phe as below.
In-Vitro Inhalation Characteristics of the SFD Powders with the PFRTo evaluate the effect of the PFR, we employed Single because its resistance is the lowest among the three devices and so the effect of the PFR may be the most clearly observed. The PFR that normal subjects can achieve with Single is 34–115 L/min.29) We examined the effect of the PFR at 30, 60, and 120 L/min with the AUC and FIR fixed at 2.5 L and >100 L/min/s, respectively.
The inhalation performance was high regardless of the amino acids and PFRs. The OE and FPF were 80–90 and 70–90%, respectively (Fig. 4).

(a) PFR 30 L/min (low flow rate), (b) PFR 60 L/min (middle flow rate), (c) PFR 120 L/min (high flow rate). The AUC and FIR were fixed at 2.5 L and >100 L/min/s, respectively. ■: Ile,  : Leu, and □: Phe. Cap, Dev, Thr, St1, and St2 stand for Capsule, Device, Throat, Stage 1, and Stage 2, respectively. Each value represents the mean±S.D. (n=3). No significant differences were observed among Ile, Leu, and Phe.
: Leu, and □: Phe. Cap, Dev, Thr, St1, and St2 stand for Capsule, Device, Throat, Stage 1, and Stage 2, respectively. Each value represents the mean±S.D. (n=3). No significant differences were observed among Ile, Leu, and Phe.
The pressure drop for Reverse at the PFR of 15 L/min was about 3 kPa, which was close to that of Single at the PFR of 45 L/min.29) Comparing data shown in Fig. 3 with Reverse and Fig. 4 with Single, the high inhalation performance of the three powders composed of Ile, Leu, and Phe did not depend on either the device or PFR. This suggests that Ile, Leu, and Phe exhibited high dispersibility and can be inhaled by patients with an impaired pulmonary function.
Comparison of the Inhalation Performance, Morphology, and Crystallinity before and after HumidificationThe powders of Ile, Leu, and Phe were stored at 75%RH for 4 weeks. The OE and FPF values were evaluated before placing them in the humidified chamber and after humidifying for 1 and 4 weeks. As a result, both values were maintained for 4 weeks at around 80% (Fig. 5). When we placed an SFD powder composed of 94% mannitol, 5% Leu, and 1% FlNa (Man-Leu) in the 75%RH chamber for a week, the powder melted. The addition of 5% Leu improved the inhalation performance; however, it was not sufficient to prevent the powder from being humidified (data not shown). This suggests that the powders with a high content of Ile, Leu, and Phe were effective not only to improve the inhalation performance but also increase the antihygroscopicity.

The PFR, AUC, and FIR were fixed at 30 L/min, 2.5 L, and >100 L/min/s, respectively. Each value represents the mean±S.D. (n=3). No significant differences were observed among Ile, Leu, and Phe.
The morphology of the particles composed of Ile, Leu, and Phe was not changed after storage at 75%RH for a week (Fig. 6). On the other hand, that of Man-Leu lost its porous characteristic to form a sphere with a smooth surface, providing evidence that keeping the porous morphology is essential for high inhalation performance.

(a), (b): Man-Leu, (c), (d): Ile, (e), (f): Leu, (g), (h): Phe.
The XRPD pattern of the bulk of Ile, Leu, and Phe showed several peaks, suggesting that they were crystalline forms. The hollow pattern observed for their SFD powders suggests that the particles became amorphous and the state was maintained for a week (Fig. 7). This finding also suggests that the powders of Ile, Leu, and Phe were highly antihygroscopic.
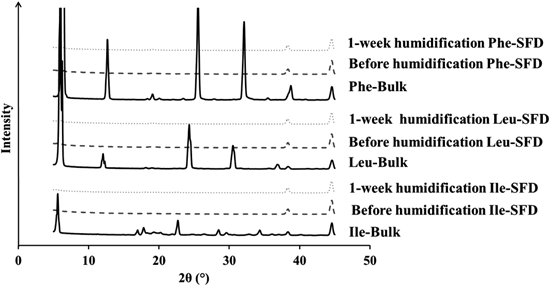
SFD is a unique method to prepare porous particles suitable for inhalation. However, such a porous particle with large surface area is apt to adsorb humidity, changing its morphology and impairing its inhalation performance. In this study, we prepared SFD powders with 7 different hydrophobic amino acids to investigate their physicochemical properties, inhalation performance, and antihygroscopic property when stored at 75%RH for 4 weeks. Among 7 porous powders with the 7 amino acids, Ile, Leu, and Phe powders were found to exhibit high inhalation performance as well as high antihygroscopicity. However, mannitol powder with 5% Leu, which was sufficient to act as a dispersing agent, was highly hygroscopic. These results suggest that several hydrophobic amino acids such as Ile, Leu, and Phe work as not only dispersing agents but also antihygroscopic agents at a high concentration, both actions of which are critical for porous SFD powders to maintain a high inhalation performance.
We thank Hitachi Automotive Systems, Co., Ltd., for supplying inspiratory flow meter and inhalation devices.
The authors declare no conflict of interest.