2016 年 64 巻 3 号 p. 193-206
2016 年 64 巻 3 号 p. 193-206
A chemical analysis of 30 samples of Ligularia virgaurea (Asteraceae) collected in Sichuan province and its adjacent territories in China was reviewed. These samples afforded 146 compounds, 73 of which were novel, and the chemical constituents were classified into 8 categories: (1) simple eremophilanes (without ring C) and eudesmanes including nor-derivatives, (2) furanoeremophilanes and lactones with a 1(10)-saturated bond, (3) furanoeremophilanes and lactones with a 1(10)-unsaturated bond, 1,10-epoxide, or 10-ol, (4) furanoeremophilanes and lactones with 1(10)-en-2-one, 1(10)-en-2-ol, or 1-en-3-one, (5) furanoeremophilanes and lactones with 1(10)-en-9-one, 1(10)-en-9-ol, or 1,10-epoxy-9-one, (6) cacalol and their derivatives, (7) bakkanes and their derivatives, and (8) others, as shown in Tables 1–7. In these studies, five chemotypes were identified in addition to three clades from the DNA sequences of L. virgaurea. The structural determination of some compounds was also discussed and a comment on how to express the real structure was proposed, particularly for spiro compounds.
Terpenoids constitute a wide variety of compounds biosynthesized from isoprene units. Their biosynthetic pathways are now being extensively studied,1–3) and the base sequences of plant genomes have also recently been attracting attention.4) A large number of terpenoid skeletons that include rearrangements and bond fission reactions have been reported.5) We have been investigating the chemical constituents of some Ligularia plants grown in China, particularly in Yunnan and Sichuan provinces.6,7) In this review, the diversity of L. virgaurea (MAXIM.) MATTF.8,9) is discussed based mainly on our recent findings on the isolation of terpenoids.10–13)
These studies were carried out in collaboration with many scientists, especially Dr. Xun Gong at the Kunming Institute of Botany, the Chinese Academy of Science, and Professors Chiaki Kuroda and Ryo Hanai at Rikkyo University, Japan. Plant samples were collected every year in summer from the end of July until mid-August because they bloom in this season, which makes it easy to determine their names, as performed by Dr. X. Gong. The expedition group visited a large number of locations and collected approximately 100 samples on average every year. We collected the roots of individual plants or 2 or 3 pieces of roots if they were small. They were washed and dried, and then cut into pieces, which were extracted with ethyl acetate. A small sample was cut into pieces and immediately extracted with ethanol for TLC analyses. Leaves were simultaneously collected for DNA studies. Organic extracts were subjected to silica gel column chromatography and each fraction was further separated by HPLC. Compounds were analyzed by one- and two-dimensional (1D and 2D) NMR, MS, high resolution (HR)-MS, IR, circular dichroism (CD), [α]D, and X-rays where possible. The places of collection of L. virgaurea in Sichuan province and its adjacent territories in China are shown in Fig. 1. Sample numbers and the types found in these studies are also displayed on this map. This work was initiated in 2000, with the first four digits of sample (20yy-xx) being the year of collection and the latter figures being the specimen numbers deposited in the Herbarium (Tables 1–7). Compounds in squares indicate the major constituents in Tables, while asterisks denote new compounds.
A total of 146 compounds were isolated from L. virgaurea samples, 73 of which were novel.10–13) They were divided into eight categories and listed in the following Tables (Fig. 2).
Simple eremophilanes and eudesmanes include those without ring C, a furan, or a lactone. Nor-type compounds are also included in this list. The chemical constituents of 30 samples were analyzed and listed in Tables 1–7. The column ‘chemotype’ means the chemotype found based on chemical constituents and the DNA study, which is discussed later.

Tables 1, 2 include six and five samples, respectively, assigned to the L-type of L. virgaurea (these types are discussed in 2.3), the major constituents of which were compound 7 or ligularol (21)14) and its derivatives (including lactones). Sample 1 (2003-62) produced nor-compounds 11 and 15, as well as lactone 70 (Table 1). Many compounds were isolated from sample 2 (2009-36). It is interesting to note that β-lactone 13 and other related nor-compounds are presumably closely related to each other biosynthetically. Compound 7 was separated into its isomers, 7a and b, the absolute configurations of which were determined using an X-ray analysis of their derivatives (vide infra). When they are separated in their pure form, the stereochemistry at C-11 may be identified based on the chemical shifts observed (vide infra). The NMR data of compound 86 were almost the same as those previously reported.15) However, we identified this compound as 8α-H lactone instead of 8β-H.16) Dimeric compounds 136 and 137 were also isolated.17) Sample 3 (2012-16) has not been finished yet; however, eight compounds were isolated. The guaiane type oxide, LB (139) was isolated (it was also detected in sample 9).18–21) LB (139) was first isolated from the Chinese drug ‘San Sion,’ imported from China, purchased through the Oriental drug store, the original plant of which was unclear at that time. Since then LB has not been isolated; at least no report has been found. LB was found in L. virgaurea, meaning that this plant may be one of the original plants of the Chinese drug ‘San Sion.’ Ligularol methyl ether (22) was isolated as a major product from sample 4 (2003-68). Since compound 77 is novel and rather labile, its separation and characterization was carried out in a short time to establish its structure. Although an enol-lactone structure was first reported by Bohlmann and colleagues,22) we identified many derivatives and established their structures (vide infra). The major compound of sample 5 (2003-79) was ligularol (21). Ligularol (21) is rather unstable and its methyl ether 22 is frequently isolated when methanol is used during the isolation procedure. The 6-methoxy derivatives 22, 72, and 93 appear to be artifacts. Ligularol (21), a major compound, and 3-acylated compounds, 23, 24, and 77, were isolated from sample 6 (2005-32).
| Sample No. | Specimen No. | Chemotype | (1) Simple eremophilane | (2) 1,10-Dihydro | (3) 1,10-Ene, 1,10-epoxide, 9-ene, 10-ol | (8) Other skeleton |
|---|---|---|---|---|---|---|
| 1 | 2003-62 | L | 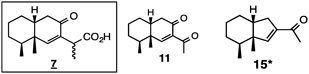 |  | ||
| 2 | 2009-36 | L |  |  |  |  |
| 3 | 2012-16 | L |  |  |  | |
| 4 | 2003-68 | L |  |  |  | |
| 5 | 2003-79 | L |  |  |  | |
| 6 | 2005-32 | L |  |  |  |
| Sample No. | Specimen No. | Chemotype | (1) Simple eremophilane | (2) 1,10-Dihydro | (3) 1,10-Ene, 1,10-epoxide, 9-ene, 10-ol | (8) Other skeleton |
|---|---|---|---|---|---|---|
| 7 | 2005-41 | L |  |  | ||
| 8 | 2005-52 | L |  |  |  |  |
| 9 | 2010-44 | L |  |  |  | |
| 10 | 2005-45 | L |  |  |  | |
| 11 | 2005-54 | L |  |  |  |
Ligularol (21), its methyl ether 22, carboxylic acid 7, and 3-acylated furan 23 were isolated from sample 7 (2005-41) (Table 2). Sample 8 (2005-52) had similar chemical constituents to those of sample 7. A large number of compounds were isolated from sample 9 (2010-44). Among them, β-lactone 13, ketone 6, and LB (139) were considered to be very interesting chemical constituents. Compound 13 is a relatively rare β-lactone biosynthesized from 11 and 13 is an intermediate to 14. Ketone 6 does not obey the normal octant rule because the hydroxy group at C-2 interferes with the back octant. Density functional theory (DFT) calculations have been used to determine absolute configurations (vide infra). Samples 10 and 11 (2005-45 and -54) produced the same constituents: 7, 21, 68, and 92, with a lactone with 10-ol being the major compound.
6-Hydroxyeuryopsin acetate (26) was the major constituent of sample 12 (2010-26), which was grouped as the H-type (vide infra) (Table 3). This sample is very interesting because it also produces the 1(10)-en-2-one derivatives 36, 38, and 99 (category 4), the 1(10)-en-9-one derivatives, 45 and 50 (category 5), and 26 (category 3). Sample 13 (2010-46) is also interesting. Although 6-hydroxyeuryopsin (25)25) was a major compound in sample 13, neoadenostylone (50)26) and its derivatives were also isolated. Cacalol (60)27,28) and its derivatives were also identified. Therefore, sample 13 was designated as a mixture of the H-, C-, and N-types. This sample produced two seco-derivatives, 125 and 128, both of which are novel compounds, the former being derived by bond fission between C-6 and C-7 of a bakkane precursor, and the latter between C-8 and C-9 of an eremophilane (may also be considered as being between C-7 and C-9 of a bakkane). A new dimer, 134, was isolated. Sample 14 (2010-51), the major compound of which was 6-hydroxyeuryopsin (25), belonged to a mixture of the H- and C-types because cacalol (60) was also included as a second major compound. The hybrid compound 135 was isolated, interestingly more bulky site of a phenol moiety attached to the C-8 position of an eremophilenolide.
| Sample No. | Specimen No. | Chemotype | (1) Simple eremophilane | (3) 1,10-Ene, 1,10-epoxide, 10-ol | (4) 1(10)-En-2-one, 1(10)-en-2-ol,1-en-3-one | (5) 1(10)-En-9-one, 1(10)-en-9-ol, 1,10-epoxy-9-one | (6) Cacalol | (7) Bakkane | (8) Other skeleton |
|---|---|---|---|---|---|---|---|---|---|
| 12 | 2010-26 | H |  |  |  |  |  | ||
| 13 | 2010-46 | H/C/N |  |  |  |  |  |  | |
| 14 | 2010-51 | H/C |  |  |  |  |  |
Samples 15–19 produced virgaurenone A (38) as a major constituent, and, thus, were assigned to the V-type. The TLC of the samples shown in Tables 1 and 2 displayed pink spot(s), while those in Table 4 displayed yellow spot(s), as visualized by Ehrlich’s reagent. The former mainly included ligularol (21) and its derivatives, while the latter contained virgaurenones and virgaurenolides as their major constituents. The characteristic 1(10)-en-2-one or 1(10),8-dien-2-one systems were the main partial structures for the compounds in Table 4. We identified differences between these types by TLC and assigned them to the L-type and V-types (chemotypes), respectively. The group including 6-hydroxyeuryopsin (25) as a major constituent was named the H-type, cacalol (60) C-type, and neoadenostylone (50) N-type (Table 3).
| Sample No. | Specimen No. | Chemotype | (4) 1(10)-En-2-one, 1(10)-en-2-ol,1-en-3-one | (8) Other skeleton |
|---|---|---|---|---|
| 15 | 2003-54 | V | 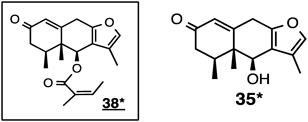 | |
| 16 | 2005-38 | V |  | |
| 17 | 2005-42 | V |  |  |
| 18 | 2005-58 | V |  | |
| 19 | 2009-62 | V |  |
Sample 15 (2003-54) afforded virgaurenones A (38) and C (35). Sample 16 (2005-38) produced virgaurenones A (38) and B (36) and virgaurenolides A (107) and B (104). Virgaurenones A (38) and C (35), along with virgaurenolide B (104) were obtained from sample 17 (2005-42). Virgaurenone D (39) was also isolated from sample 18 (2005-58). Sample 19 (2009-62) produced virgaurenone E (37).
Table 5 shows samples belonging to the V-type, with more derivatives than those in Table 4. Sample 20 (2009-60) produced virgaurenone A (38) as its major constituent. Lactols, the reduced type of virgaurenolides A and B, named virgaurenolidols A (111) and B (109), were isolated for the first time in our study; however, these types of compounds have already been reported.29,30) One bakkane type, compound 121 with the 1(10)-en-2-one system, was isolated. Sample 21 (2010-15) afforded epoxy-lactones 98, 99, 100, and 102 as well as virgaurenones, virgaurenolides, and virgaurenolidol B (109). Two bakkane-type enones, 119 and 120, and the 1(10)-en-type bakkane, 117, were also identified. The successful isolation of the anhydride-type bakkane derivative 123 was recorded for the first time. This compound arose through a rearrangement of the corresponding epoxide; however, the stereochemistry of this compound should be considered. If the epoxide present in compound 120 is rearranged, the methyl group is expected to be beta. However, it was alpha. Therefore, this methyl group may have been isomerized to the alpha position due to a bulky substituent at C-6 at a later stage of biosynthesis (vide infra). Samples 22 (2010-19) and 23 (2010-38) were similar to the samples described above. Virgaurenolidol A (111) was a major constituent in sample 24 (2010-68). The anhydride-type compound 122 was isolated from this sample. The stereochemistry of the spiro carbon atom differed from that of compound 123. In this case, the methyl group was beta and not isomerized.13)
| Sample No. | Specimen No. | Chemotype | (1) Simple eremophilane | (4) 1(10)-En-2-one, 1(10)-en-2-ol,1-en-3-one | (5) 1(10)-En-9-one, 1(10)-en-9-ol, 1,10-epoxy-9-one | (7) Bakkane | (8) Other skeleton |
|---|---|---|---|---|---|---|---|
| 20 | 2009-60 | V |  |  |  | ||
| 21 | 2010-15 | V |  |  | |||
| 22 | 2010-19 | V |  |  |  |  | |
| 23 | 2010-38 | V |  |  | |||
| 24 | 2010-68 | V |  |  |  |
Two samples shown in Table 6 belonged to the N-type. The major constituent of sample 25 (2010-50) was neoadenostylone (50). Three compounds, 52, 53, and 54, had the 1,10-epoxy-9-one structure, whereas three compounds, 57, 58, and 59, had 1,10-epoxy-9-ol, and were all derivatives of 50. The seco-compound 129 was also isolated. Sample 26 (2010-52) afforded similar compounds to sample 25; however, the major constituent was the 9-ol-type compound 59. Sample 27 (2010-63) produced a mixture of 6-hydroxyeuryopsin derivatives and neoadenostylone derivatives, with the major constituent being 50. Therefore, this sample was assigned as a mixture of the N- and H-types. The configuration of compound 90 was reported to be undetermined by Bohlmann et al.31) Our compound appeared to be the same as compound 90, which was unambiguously determined to be 8α-OH following the application of nuclear Overhauser effect spectroscopy (NOESY).16)
| Sample No. | Specimen No. | Chemotype | (3) 1,10-Ene, 1,10-epoxide, 10-ol | (5) 1(10)-En-9-one, 1(10)-en-9-ol, 1,10-epoxy-9-one | (7) Bakkane | (8) Other skeleton |
|---|---|---|---|---|---|---|
| 25 | 2010-50 | N |  |  |  | |
| 26 | 2010-52 | N |  |  |  | |
| 27 | 2010-63 | N/H |  |  |  |  |
Two samples shown in Table 7 were assigned to the C-type. The major constituent of sample 28 (2009-56) was cacalol (60). Many cacalol derivatives were isolated as well as the 1(10)-en-type compound 27 and 1(10)-en-9-one-type compounds, 47 and 49, as minor constituents. Sample 29 (2010-13) afforded six cacalol derivatives, with cacalol (60) being the major constituent. The final sample 30 (2010-21) produced cacalol (60) as a major constituent, along with 6-hydroxyeuryopsin (25) as a second major compound. Therefore, this sample was assigned as a mixture of the C- and H-types. The dimeric compounds, 132 and 134, both being related to cacalol (60), were obtained.
| Sample No. | Specimen No. | Chemotype | (3) 1,10-Ene, 1,10-epoxide, 10-ol | (5) 1(10)-En-9-one, 1(10)-en-9-ol, 1,10-epoxy-9-one | (6) Cacalol | (7) Bakkane | (8) Other skeleton |
|---|---|---|---|---|---|---|---|
| 28 | 2009-56 | C |  |  |  |  | |
| 29 | 2010-13 | C |  | ||||
| 30 | 2010-21 | C/H |  |  |  |  |
Samples may be grouped by comparing each constituent, as shown in Tables 1–7. For example, when compound 38 is a major constituent, it may be grouped as the V-type (Tables 4, 5). When compounds 21, 22, and 71 are major constituents, they may be grouped as the L-type (Tables 1, 2). By comparing these compounds, it is now possible to divide them into the following 5 groups: the L-, V-, H-, N-, and C-types.
The TLC patterns of these extracts show typical colors when visualized by Ehrlich’s reagent. The V-type gives yellow spots due to virgaurenone(s), the L-type pink spots due to ligularol (21) and its derivatives, the C-type blue spots (slowly coloring) due to cacalol (60), the H-type blue spots (rapidly coloring) due to 6-hydroxyeuryopsin (25), and the N-type pink to red spots due to neoadenostylone (50) and its epoxide. By comparing the patterns of TLC colors, isolated compounds, and DNA sequences, it is possible to identify the chemotypes of L. virgaurea. Mass spectra may also be used to identify these groups.32) Concerning intra-specific diversity of L. virgaurea, plants belonging to the V- and L-types were mainly and frequently encountered in the field, while those belonging to the C-, H-, and N-types were minor species. We previously proposed that ‘plants being able to produce furanoeremophilanes are more elaborate than those without these compounds.’6,7) This is also supported by the numbers of samples collected in the field, namely, the more samples encountered in the field, the more prosperous the species. We identified some hybrid-type samples in other Ligularia plants.33,34) Difficulties are associated with speculating on the evolution of these plants; however, the progression of evolution may be observed in the field in the Hengduan Mountain area.
2.4 Base SequencesInternal transcribed spacer (ITS) sequences were analyzed in order to compare similarities. Table 8 shows the base sequences of L. virgaurea, with different positions and bases from those of L. tongolensis, which was first analyzed by our group.35) It includes samples collected between 2007 and 2010, some of which had not been chemically analyzed until recently. A Blast search indicated that there were mainly 3 groups. It was interesting to note that clade A completely corresponded to the L-type, clade B to the V-type, and clade C to the C-type. Minor changes were observed in the H- and N-types because they are regarded as a branched or slightly changed type. Samples belonging to these three types were minor plants in the field. Plants belonging to the V- and L-types were frequently encountered species in the field.
| Sample | Chemotype | Clade | ITS1b) | 5.8Sb) | ITS2b) | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | |||||||||||||||||||||||
| 1 | 1 | 3 | 6 | 7 | 0 | 2 | 2 | 3 | 3 | 3 | 3 | 5 | 6 | 6 | 7 | 9 | 1 | 2 | 3 | 4 | 4 | 9 | 2 | 2 | 3 | 1 | 1 | 2 | 3 | 3 | 3 | 7 | 7 | 8 | 9 | 9 | 0 | 1 | 1 | 7 | 7 | 7 | 8 | 8 | 0 | 0 | 0 | 1 | 2 | ||||||
| 3 | 1 | 8 | 4 | 5 | 4 | 1 | 4 | 8 | 0 | 2 | 4 | 5 | 5 | 6 | 7 | 3 | 3 | 2 | 6 | 5 | 0 | 2 | 1 | 6 | 3 | 4 | 3 | 6 | 9 | 0 | 0 | 2 | 4 | 0 | 3 | 4 | 1 | 3 | 4 | 3 | 6 | 1 | 5 | 9 | 2 | 8 | 0 | 3 | 5 | 7 | 0 | ||||
| a) | 2007-58 | L | A | G | Y | G | T | A | Y | C | C | A | R | C | T | Y | C | G | C | C | R | T | G | G | Y | G | C | A | C | G | C | C | C | G | T | G | A | C | T | R | C | G | C | C | G | G | A | A | C | C | G | C | G | C | C |
| a) | 2007-72 | L | A | G | C | G | T | A | Y | C | C | A | R | C | T | Y | C | G | C | C | G | T | G | G | C | G | C | A | C | G | C | C | C | G | T | G | A | C | T | R | C | G | C | Y | G | G | A | A | C | C | G | C | G | C | C |
| a) | 2007-81 | L | A | G | C | G | T | A | Y | C | C | A | R | C | T | Y | C | G | C | C | G | T | G | G | Y | G | C | A | C | G | C | C | C | G | T | G | A | C | T | R | C | G | C | C | G | G | A | A | C | C | G | Y | G | C | C |
| a) | 2007-84 | L | A | G | C | G | T | A | Y | C | C | A | R | C | T | Y | C | G | C | C | G | T | G | G | Y | G | C | W | C | G | C | C | C | G | T | G | A | C | T | R | C | G | C | C | G | G | A | A | C | C | G | C | G | C | C |
| a) | 2007-91 | L | A | G | C | G | T | A | Y | C | C | A | R | C | T | Y | C | G | C | C | G | T | G | G | Y | G | C | A | C | G | Y | C | C | G | T | G | A | C | T | R | C | G | C | C | G | G | A | A | C | C | G | C | G | C | C |
| a) | 2007-107 | L | A | G | C | G | T | A | Y | C | C | A | R | C | T | Y | C | G | C | C | G | T | G | G | Y | S | C | A | C | G | C | C | C | G | T | G | A | C | T | R | C | G | C | C | G | G | A | A | C | C | G | C | G | C | C |
| a) | 2007-136 | L | A | G | C | R | T | A | Y | C | C | A | R | C | Y | Y | C | G | C | C | G | T | G | G | C | G | C | A | C | G | C | C | C | R | T | G | A | C | T | R | C | G | C | C | G | G | A | A | C | C | G | C | G | C | C |
| 2 | 2009-36 | L | A | G | C | G | T | A | Y | C | C | A | R | C | T | Y | C | G | C | C | G | T | G | G | Y | G | C | A | C | G | C | C | C | G | T | G | A | C | T | R | M | G | C | C | G | G | A | A | C | Y | G | C | G | C | C |
| a) | 2009-67 | L | A | G | C | G | T | A | C | C | C | A | R | Y | Y | Y | C | G | C | C | G | T | G | S | C | G | C | A | C | G | C | C | C | G | T | G | A | C | T | R | C | G | C | C | G | G | A | A | C | C | G | C | G | C | C |
| a) | 2009-77 | L | A | R | C | G | T | A | C | C | C | A | G | Y | Y | C | C | G | C | C | G | T | G | S | C | G | C | A | C | G | C | C | C | G | T | G | A | C | T | G | C | G | C | C | G | G | A | A | C | C | G | C | G | C | C |
| a) | 2010-29 | L | A | G | C | G | T | A | C | C | C | A | G | C | Y | C | C | G | C | C | G | T | G | G | C | K | C | A | C | G | C | C | C | G | T | G | A | C | T | G | C | G | C | C | G | G | A | A | C | C | G | C | G | C | C |
| a) | 2010-34 | L | A | G | C | G | T | A | Y | C | C | A | R | C | Y | Y | C | G | C | C | G | T | G | G | C | G | C | A | C | G | C | C | C | G | T | G | A | C | T | R | C | G | C | C | G | G | A | A | C | C | G | C | G | C | C |
| 9 | 2010-44 | L | A | G | C | G | T | A | C | C | C | A | G | Y | T | C | C | G | C | C | G | Y | G | S | Y | K | C | A | C | G | C | C | C | G | T | G | A | C | T | G | C | G | C | C | G | G | A | A | C | C | G | C | G | C | C |
| a) | 2010-64 | L | A | G | C | G | T | A | Y | C | C | A | R | C | T | Y | C | G | C | C | G | T | G | G | C | K | C | A | C | G | C | C | C | G | T | G | A | C | T | R | C | G | C | C | G | G | A | A | C | C | G | C | G | C | C |
| a) | 2010-72 | L | A | R | C | G | T | A | C | C | C | A | G | Y | Y | C | C | G | C | C | G | T | G | S | C | K | C | A | C | G | C | C | C | G | T | G | A | C | T | G | C | G | C | C | G | G | A | A | C | C | G | C | G | C | C |
| a) | 2007-96 | V | B | G | C | G | T | G | C | C | C | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | T | A | R | C | Y | G | C | G | C | C | G | G | G | C | C | C | G | C | R | Y | Y |
| a) | 2009-59 | V | B | G | C | G | T | G | C | C | Y | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | M | A | Y | G | C | C | C | G | T | A | R | C | T | G | C | G | C | C | G | G | G | C | C | C | G | C | R | T | C |
| 20 | 2009-60 | V | B | G | C | G | T | G | C | C | C | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | T | A | G | C | T | G | C | G | C | C | R | G | G | C | C | C | G | C | R | T | C |
| 19 | 2009-62 | V | B | G | C | G | T | G | C | C | Y | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | Y | A | G | C | T | G | C | G | C | C | G | G | G | C | C | C | G | C | R | T | C |
| a) | 2009-66 | V | B | G | C | G | T | G | C | C | Y | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | Y | A | G | C | T | G | C | G | C | C | G | G | G | C | C | C | G | C | R | T | C |
| a) | 2009-71 | V | B | G | C | G | T | G | C | C | Y | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | Y | A | G | C | T | G | C | G | C | C | G | G | G | C | C | C | G | C | R | T | C |
| a) | 2009-97 | V | B | G | C | G | T | G | C | C | C | A | G | C | T | C | C | R | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | T | A | R | C | Y | G | C | G | C | C | G | R | G | C | C | C | K | C | G | Y | C |
| 21 | 2010-15 | V | B | G | C | G | T | G | C | C | C | W | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | T | A | R | C | Y | G | C | G | Y | C | G | G | G | C | C | C | G | C | R | Y | Y |
| 22 | 2010-19 | V | B | G | C | G | T | G | C | C | C | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | Y | A | R | C | Y | G | C | G | C | C | R | G | G | C | C | C | G | C | R | Y | C |
| a) | 2010-36 | V | B | G | C | G | T | G | C | C | C | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | T | A | R | C | T | G | C | G | C | C | R | G | G | C | C | C | G | C | R | Y | C |
| 23 | 2010-38 | V | B | G | C | G | T | G | C | C | Y | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | T | A | G | C | Y | G | C | G | C | C | G | G | G | C | C | C | G | C | G | Y | C |
| a) | 2010-42 | V | B | G | C | G | T | G | C | C | C | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | T | A | R | C | Y | G | C | G | C | C | G | G | G | C | C | C | G | C | G | Y | C |
| a) | 2010-43 | V | B | G | C | G | T | G | C | C | Y | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | T | A | G | C | T | G | C | G | C | C | R | G | G | C | C | C | G | C | R | T | C |
| 24 | 2010-68 | V | B | G | C | G | T | G | C | C | C | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | T | A | G | C | T | G | C | G | C | C | G | G | G | C | C | C | G | C | R | T | C |
| 25 | 2010-50 | N | B | G | C | G | T | G | C | C | Y | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | T | A | G | C | T | G | C | G | C | C | G | G | G | C | C | C | G | C | R | T | C |
| 26 | 2010-52 | N | B | G | C | G | T | G | C | C | C | A | G | C | T | C | C | G | C | C | G | T | K | G | T | G | C | A | C | G | C | C | C | G | Y | A | R | C | Y | G | C | G | Y | C | G | G | G | C | C | C | G | C | R | Y | C |
| 27 | 2010-63 | N/H | B | G | C | G | T | G | C | C | C | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | Y | A | R | C | Y | G | C | G | C | C | G | G | G | C | C | C | G | C | R | Y | C |
| 12 | 2010-26 | H | B | G | C | G | T | G | C | C | C | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | T | A | R | C | Y | G | C | G | C | C | G | G | G | C | C | C | G | C | R | Y | C |
| 28 | 2009-56 | C | C | G | C | G | C | G | C | T | C | A | G | C | T | C | A | A | C | T | G | T | G | G | T | G | C | A | Y | G | C | M | T | G | T | G | G | S | T | G | C | G | Y | C | G | G | A | C | C | C | G | C | G | C | Y |
| 29 | 2010-13 | C | C | G | C | G | C | G | C | T | C | A | G | C | T | C | A | A | C | T | G | T | G | G | T | G | C | A | C | G | C | M | T | G | T | G | G | C | T | G | C | G | Y | C | G | G | A | C | C | C | G | C | G | C | Y |
| 30 | 2010-21 | H/C | C | G | C | G | C | G | C | T | C | A | G | C | T | C | A | A | C | T | G | T | G | G | T | G | C | A | C | G | C | M | T | G | T | G | G | S | T | G | C | G | Y | C | G | G | A | C | C | C | G | C | G | C | Y |
| 13 | 2010-46 | H/C/N | C | G | C | G | C | G | C | T | C | A | G | C | T | C | M | A | C | T | G | T | G | G | T | G | C | A | C | G | C | M | T | G | T | G | G | S | T | G | C | G | Y | C | G | G | A | C | M | C | G | C | G | C | Y |
| 14 | 2010-51 | H/C | C | G | C | G | Y | G | C | T | C | A | G | C | T | Y | M | R | Y | Y | G | T | G | G | T | G | C | A | C | S | Y | C | Y | G | T | G | G | C | T | G | C | R | C | C | R | G | A | C | M | C | G | C | G | C | C |
| refc) | L. tongolensis | G | C | G | T | G | C | C | C | A | G | C | T | C | C | G | C | C | G | T | G | G | T | G | C | A | C | G | C | C | C | G | T | A | R | C | Y | G | C | G | C | C | G | G | G | C | C | C | G | C | G | C | C | ||
a) Separation of the constituents was not carried out; b) Y=C+T, R=A+G, M=A+C, S=C+G, W=A+T, K=G+T; c) The base sequence was compared with that of Ligularia tongolensis, which we studied first (see the text).
Most compounds were analyzed by spectroscopic methods; 2D correlations were the key for structure determinations. Some structures were discussed here because some phenomena were encountered. There were two conformations in the cis decalin system: steroid and non-steroid36) (Fig. 3). These conformations depended on the substituents on the rings. Therefore, the conformation of the cis decalin system was initially determined. Especially for eremophilanolides, 8β-H, OH, and OCH3 lactones had steroid conformations, while 8α-H, OH, and OCH3 lactones had non-steroid conformations.37) This is typically determined by the nuclear Overhauser effects (NOEs) between H3-14 and H-1β and H-3β for steroid conformations, and between H3-14 and H-9β and between H3-15 and H-2β for non-steroid conformations.

Figure 4 shows the differences between the 7α,8α- and 7β,8β-epoxy lactones, 150 and 151. The epoxide oxygen between the C-7 and C-8 atoms and the methyl group at C-11 need to be in the same direction because of the biosynthetic pathway (vide infra). Difficulties are sometimes associated with detecting the NOE between H-6α and H3-13 or H-11. However, there has been no exception to this rule until recently. Structural revisions have been reported in the literature.38)

Other examples of anhydrides are shown in Fig. 5. They are derived from bakkane-type lactones by the rearrangement of the corresponding epoxides. Therefore, the methyl group at C-11 of compound 123 is expected to be isomerized at a later stage of biosynthesis by the influence of the bulky substituent at C-6. It is interesting to note that stereochemistry may only be determined by the sole NOE between H3-13 and H-9β or H-6α.

The configuration of the doublet methyl group at C-11 of compounds 7a and b was established by an X-ray crystallographic analysis of its p-bromophenacyl derivative 7c39) (Chart 1). Based on this finding, it was possible to distinguish two isomers based on chemical shifts of H-11. The proton H-11 of 7a resonated at δ 3.70, while that of 7b resonated at δ 3.59, a slightly higher field (both in C6D6). Compound 7a may be derived from enol-lactone 146 by an acid treatment through hydration and elimination, proving the absolute configuration at C-11.

The C-2′ configuration of the 2-methylbutyrate moiety was determined as follows. Diol 147 was treated with (S)-angelic anhydride to give the (2′S)-derivative 148, and with racemic anhydride to give the diastereoisomeric mixture 149.40) A comparison of the chemical shift of this doublet methyl group enabled us to determine the absolute configuration at C-2′ of 2-methylbutyrate.
CD spectra were used to determine absolute configurations.12) The back octant rule is applied to determine absolute configurations. However, in the case of compound 6, the Cotton effect was observed at 291 nm with a negative sign. Based on the back octant rule, it was predicted to have a positive sign (Fig. 6). The front view of this ketone, as shown in Fig. 6a, has a normal appearance; however, the top view in Fig. 6b indicates that the hydroxy group at the gamma position contributes to the front octant. Therefore, we calculated CD curves using DFT, compared them, and concluded that absolute configurations were expected to be the same as those shown in Fig. 6.12) The absolute configurations of eremophilanes were expected to have 4β,5β-dimethyl groups. All the compounds identified here had the same absolute configurations.

Difficulties are associated with drawing the structures of spiro compounds on paper because they have a tetra-substituted spiro carbon. They have a 90° skewed conformation, and thus, need to be shown in three-dimensional drawings. A simple drawing may result in an inaccurate conclusion. Our bakkane compound A represents a relevant example here (Fig. 7). From the front side, structure A1 may be easily imagined. However, from the back side of the molecule, it may be drawn as in A2. However, it is sometimes drawn as in A3, which is incorrect. The drawing A3 represents compound A4, which is a diastereoisomer of A.

An example has been described in the literature.41) Although the compound was elucidated by an X-ray analysis, an incorrect structure was drawn on paper. A drawing of the X-ray 3D structure in the SI on paper will be drawn as B (Chart 2). This is rotated 180° to give C, a back side view as explained above. This is then changed to D, a front side view. This is the enantiomeric structure of our eremophilanes. Therefore, all chiral centers are reversed (we sometimes forget to change the chirality of the epoxide). By this change, we get structure E. This is different from the reported structure F. Therefore, the incorrect structure may sometimes be drawn. We here propose how to draw the structures of these spiro type compounds: 1) view the molecule from the front side, 2) the β-bond (in this case an epoxide or a quaternary carbon at C-11) should be drawn upwards, and the α-bond downwards. This is a simple rule, and a drawing such as A1 is strongly recommended, while A2 and A3 need to be avoided.

Ligularia virgaurea is an interesting species that grows in the Hengduan Mountain area of China.42–51) We have identified five chemotypes to date: the V-, L-, C-, N-, and H-types, based on their chemical constituents, while three clades have been detected based on DNA sequences, particularly ITS. The V- and L-types are both abundant in the field of collection, whereas others are not collected as frequently, suggesting that these two types are ecologically advantageous. The blooming seasons of these types are not exactly the same, implying that hybridization is not possible and also that they do not depend on geological circumstances. The chemotypes based on chemical constituents are consistent with the DNA sequencing findings. We will be able to determine the evolutional progression of not only L. virgaurea, but also other Ligularia plants by studying their chemical constituents as well as DNA in the future. It is important to avoid making structural errors, particularly for compounds with complex structures, for example, a spiro compound may be correctly drawn based on the rules described herein.
The author thanks Professors Chiaki Kuroda and Ryo Hanai (Rikkyo University) and Dr. Xun Gong (the Kunming Institute of Botany, China) for their collaboration in this project. The collaboration, discussions, and advice of Professor Masakazu Sono, Dr. Katsuyuki Nakashima, Dr. Yoshinori Saito (present Nagasaki University), Dr. Yasuko Okamoto, graduate students, and undergraduate students (Department of Analytical Chemistry, Faculty of Pharmaceutical Sciences, Tokushima Bunri University) are highly appreciated. The author also wishes to thank Dr. Ayumi Ohsaki (Nihon University), Dr. Hiroshi Hirota (former Riken), Emeritus Professor Hajime Nagano (Ochanomizu University), Dr. Takayuki Kawahara (Forestry and Forest Products Research Institute), Dr. Yasunori Yaoita (Tohoku University of Pharmacy), Professor Yoshinori Asakawa (Tokushima Bunri University), and Professor Masami Ishibashi (Chiba University) for their help, discussions, information, guidance, and encouragement. This work was supported in part by a Grant-in-Aid for Scientific Research from JSPS (No. 25303010).
The author declares no conflict of interest.