2016 Volume 64 Issue 4 Pages 326-339
2016 Volume 64 Issue 4 Pages 326-339
A novel therapy that stimulates endogenous glucagon-like peptide-1 (GLP-1) secretion by Takeda G-protein-coupled receptor 5 (TGR5) agonists might be a superior alternative for the treatment of type 2 diabetes mellitus. A series of 4-phenoxythiazol-5-carboxamides were developed as highly potent TGR5 agonists using a bioisosteric replacement strategy based on the scaffold of 4-phenoxynicotinamides. The structure–activity relationship on the bottom phenyl ring and the thiazole ring was extensively studied, and the 2-methyl-thiazole derivatives 30c and e displayed the best in vitro potency toward human TGR5, with EC50 values of approximately 1 nM. While endowed with excellent in vitro potency, the 2-methyl-thiazoles were flawed with high microsomal clearance.
Takeda G-protein-coupled receptor 5 (TGR5), also known as G-protein coupled bile acid receptor 1 (GPBAR1), is a cell surface receptor responsive to bile acids. It has been found to be ubiquitously expressed in animal and human tissues, including the liver, intestine, heart, spleen, skeletal muscle, brown adipose tissue, gallbladder and brain.1–4) It is thought to play an important role in the regulation of energy homeostasis and glucose metabolism. Activation of the TGR5 receptor by bile acids triggers an increase in energy expenditure in brown adipose tissue and skeletal muscle.5) More importantly, TGR5 activation also promotes glucagon-like peptide-1 (GLP-1) secretion in the intestine.6) As a member of the incretin family, GLP-1 acts as an important physiological regulator in glucose control via several mechanisms of action.7) But endogenous GLP-1 is significantly limited as a direct drug for the treatment of type 2 diabetes because of its rapid inactivation in plasma by dipeptidyl peptidase-4 (DPP-4). There are two main classes of alternative GLP-1-based drugs being widely used in a clinical setting: DPP-4 inhibitors and DPP-4-resistant GLP-1 receptor (GLP-1R) agonists.8) However, these drugs may not be able to halt the progression of type 2 diabetes because they all exert their actions through increasing the plasma concentration of “GLP-1R agonists,” and they thus may lack some of the local actions that endogenous GLP-1 is likely to have.9) Therefore, a novel therapy that stimulates endogenous GLP-1 secretion by TGR5 agonists is considered as a superior alternative for the treatment of type 2 diabetes mellitus.
In recent years, many different kinds of TGR5 agonists have been reported (Fig. 1). Structurally, TGR5 agonists can fall into two categories. The first category is bile acid derivatives,10–12) such as lithocholic acid (LCA) and 6α-ethyl-23(S)-methylcholic acid (INT-777). The second category is non-bile acid derivatives, including naturally occurring non-bile acid agonists such as oleanolic acid and betulinic acid,13,14) and synthetic small molecular TGR5 agonists.15–22) However, only one compound (SB-756050) has ever been advanced into the clinical trial phase, and most are still in the discovery phase, because of a lack of appropriate Pharmacokinetic/Pharmacodynamic (PK/PD) profiles, or having side effects such as stimulating filling of the gallbladder,22,23) or causing changes in heart rate and blood pressure.18) Therefore, the search for a greater variety of promising TGR5 agonists with potent in vitro activity to expand the chemical library of TGR5 agonists still represents a very urgent need, and further optimizations based on such agonists could increase the possibility of producing drug candidates with good in vivo potency and low side-effects.

In our initial efforts to find novel TGR5 agonists, a scaffold of 4-phenoxynicotinamide (Fig. 2, scaffold A) was developed.22) Using this scaffold as a basis, most of our previous structural explorations were focused on the upper tetrahydroquinoxaline region and the bottom phenyl ring,22,24) whereas few explorations were carried out at the central core part, which was previously thought of as a limited region to alter. For this work, we investigated whether these alterations at the central core part would generate additional potent TGR5 agonists for further studies. First, we compared scaffold A with the scaffolds of other reported small molecular TGR5 agonists, and observed that scaffold A was very similar to the scaffolds of 5–8, with a five or six-membered heteroaromatic ring as a core part, one phenyl ring or heteroaromatic ring linked to the core via an amide bond, and another phenyl ring linked to the core directly or via oxygen. We therefore hypothesized that replacing the pyridine of scaffold A with five-membered heteroaromatic rings using a bioisosteric replacement strategy was likely to yield other TGR5 agonists with equivalent or even better potency (Fig. 2). To explore other scaffold that met our requirement, pyrazoles, 1,2,3-triazoles and 2-methyl-thiazoles were designed and synthesized. Herein, we describe our efforts in the synthesis, biological evaluations and structure–activity relationship (SAR) studies of this series of TGR5 agonists.

The synthesis of pyrazole derivatives 13a–d and 18a–c are elucidated in Chart 1. Nucleophilic substitution on 3-chloropyrazole (10) with commercially available phenols afforded the aldehydes 11a–d, which were subsequent oxidized with sodium chlorite to give the key intermediates 12a–d. In contrast, 3-aminopyrazole (14) was transformed into a chlorine derivative (15) through diazotization in the presence of cupric chloride. Nucleophilic substitution with commercially available phenols and hydrolysis with sodium hydroxide generated the key intermediates 17a–c. Finally, the acids 12a–d and 17a–c were condensed with 1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline25) to give the title compounds 13a–d and 18a–c.

Reagents and conditions: (a) various substituted or unsubstituted phenols, KOH, DMF, 100°C; (b) NaClO2, NaH2PO4, tBuOH, THF, H2O; (c) (COCl)2, DMF, DCM, reflux; (d) 1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline, Et3N, DCM, r.t.; (e) CuCl2, tBuONO, MeCN, 60°C; (f) various substituted or unsubstituted phenols, K2CO3, DMF, 120°C; (g) NaOH, 1,4-dioxane, H2O, r.t.
The synthesis of 1,2,3-triazole derivatives is outlined in Chart 2. First, compound 19 was synthesized using a method described in the literature,26) followed by treatment with phosphorus pentachloride, which yielded compound 20.27) Next, the chlorine derivative 20 reacted with substituted phenols in the presence of sodium hydride to provide the diaryl ethers 21a-b. Through hydrolysis with sodium hydroxide and condensation with 1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline or 1-methyl-1,2,3,4-tetrahydroquinoxaline, the key intermediates 23a and b and 24a and b were obtained. In addition, deprotection of compounds 24a and b gave the target compounds 25a and b, whereas deprotection of compounds 23a and b was not completed because of the unstable N-cyclopropyl group.

Reagents and conditions: (a) PCl5, toluene, 0°C to r.t.; (b) various substituted phenols, NaH, DMF, 0 to 85°C; (c) NaOH, 1,4-dioxane, H2O, r.t.; (d) (COCl)2, DMF, DCM, reflux; (e) 1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline or 1-methyl-1,2,3,4-tetrahydroquinoxaline, Et3N, DCM, r.t.; (f) TFA, 65°C.
The synthesis of 2-methyl-thiazole derivatives is shown in Chart 3. This started with compound 26, which was prepared according to the procedure described in a patent,28) followed by formation of a sodium salt and immediate treatment with various o-fluoronitrobenzenes under microwave assistance; this gave the key intermediates 27a–e. Next, reduction of the nitros 27a–e to aminos 28a–e, and subsequent diazotization with tert-butyl nitrite in the presence of cupric chloride, cupric bromide or direct reflux, produced the intermediates 29a–j. Finally, via hydrolysis with sodium hydroxide and condensation as described above, the desired compounds 30a–n were obtained. Analogs 31a–c were prepared from 30e, g and n via the Negishi cross-coupling reaction.29) Analog 31d was prepared through a PdCl2(dppf)·CH2Cl2-catalyzed reaction with bis(pinacolato)diboron and subsequent oxidation with hydrogen peroxide. In addition, direct hydrolysis of the intermediates 27a and b and subsequent condensation with 1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline yielded 32a and b.

Reagents and conditions: (a) EtONa, EtOH; (b) substituted or unsubstituted o-fluoronitrobenzene, NMP, Microwave 125°C; (c) Fe, NH4Cl, THF, H2O; (d) tBuONO, CuCl2, MeCN, r.t.–60°C; (e) tBuONO, CuBr2, MeCN, r.t.–60°C; (f) tBuONO, MeCN, reflux; (g) NaOH, 1,4-dioxane, H2O, r.t.; (h) (COCl)2, DMF, DCM, reflux; (i) 1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline or 1-methyl-1,2,3,4-tetrahydroquinoxaline, Et3N, DCM, r.t.; (j) Zn(CH3)2, PdCl2(dppf)·CH2Cl2, 1,4-dioxane, 80°C; (k) PdCl2(dppf)·CH2Cl2, bis(pinacolato)diboron, KOAc, 1,4-dioxane, 130°C; (l) H2O2, THF, 0°C–r.t.
The synthesis of other thiazoles 37a–h that were substituted with ethyl, isopropyl, phenyl or 2-pyridyl groups on the thiazole ring is show in Chart 4, which is similar to the protocols for preparation of 2-methyl-thiazole derivatives 30c and i.

Reagents and conditions: (a) EtOH, reflux; (b) EtONa, EtOH; (c) 4-chloro-2-fluoro-1-nitrobenzene, NMP, Microwave 125°C; (d) Fe, NH4Cl, THF, H2O; (e) tBuONO, CuCl2, MeCN, r.t.–60°C; (f) tBuONO, MeCN, reflux; (g) NaOH, 1,4-dioxane, H2O, r.t.; (h) (COCl)2, DMF, DCM, reflux; (i) 1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline, Et3N, DCM, r.t.
The newly-synthesized pyrazoles, 1,2,3-triazoles and 2-methyl-thiazoles were evaluated for their activity as agonists against human TGR5 (hTGR5) using a cAMP-responsive element (CRE)-driven luciferase assay in HEK293 cells.22,24,30,31) Compounds with EC50 values against hTGR5 of less than 100 nM were then selected for the mouse TGR5 (mTGR5) activity test. The results are shown in Tables 1–3.
 | |||
|---|---|---|---|
| Compound | R1 | R2 | hTGR5 EC50 (nM)a) |
| 13a | H | Me | NA |
| 13b | 2,5-diCl | Me | NA |
| 13c | 2,5-diMe | Me | NA |
| 13d | 3-OMe | Me | NA |
| 18a | H | H | NA |
| 18b | 2,5-diMe | H | NA |
| 18c | 3-OMe | H | 129 nM |
a) hTGR5 EC50 values given are the means of at least two independent experiments; NA: no measurable activity at a concentration of 0.1 µM with two independent experiments.
 | ||||
|---|---|---|---|---|
| Compound | R1 | R2 | R3 | hTGR5 effecta) |
| 23a | 2,5-diCl | cPr | PMB | NA |
| 23b | 3-OMe | cPr | PMB | NA |
| 24a | 2,5-diCl | Me | PMB | NA |
| 24b | 3-OMe | Me | PMB | NA |
| 25a | 2,5-diCl | Me | H | NA |
| 25b | 3-OMe | Me | H | NA |
a) NA: no measurable activity at a concentration of 0.1 µM with two independent experiments.
 | ||||
|---|---|---|---|---|
| Compound | R1 | R2 | hTGR5 EC50 (nM)a) | mTGR5 EC50 (nM)b) |
| INT-777 | — | — | 1226±357 | 358±77 |
| 30a | 2-Cl-5-Me | cPr | 1.4±0.22 | 65±3.7 |
| 30b | 2-Cl-5-Me | Me | 9.1±2.0 | 484±87 |
| 30c | 2,5-diCl | cPr | 1.1±0.07 | 71±3.3 |
| 30d | 2,5-diCl | Me | 8.2±1.5 | 563±29 |
| 30e | 2-Br-5-Cl | cPr | 0.97±0.15 | 72±15 |
| 30f | 2-Br-5-Cl | Me | 6.0±1.0 | 499±35 |
| 30g | 2-Br-5-Me | cPr | 1.2±0.27 | 67±4.8 |
| 30h | 2-Br-5-Me | Me | 11±1.8 | 479±80 |
| 31a | 5-Cl-2-Me | cPr | 4.4±2.1 | 141±0.28 |
| 31b | 2,5-diMe | cPr | 3.6±0.47 | 100±50 |
| 32a | 5-Cl-2-NO2 | cPr | 7.6±0.16 | 408±39 |
| 32b | 5-Me-2-NO2 | cPr | 7.5±2.0 | 238±28 |
| 30m | 2-Cl | cPr | 15±2.8 | 227±31 |
| 30i | 3-Cl | cPr | 31±5.4 | 931±90 |
| 30l | 4-Cl | cPr | 235±21 | — |
| 30j | 3-Me | cPr | 26±6.1 | 435±34 |
| 30k | 2,4-diCl | cPr | 11±0.22 | 331±68 |
| 30n | 4-Br-2,5-diCl | cPr | 2.8±0.42 | 200±59 |
| 31c | 2,5-diCl-4-Me | cPr | 1.5±0.40 | 106±40 |
| 31d | 2,5-diCl-4-OH | cPr | 1.4±0.31 | 85±6.3 |
a) EC50 values of hTGR5 are expressed as the mean±S.D. of at least three independent experiments. b) EC50 values of mTGR5 are expressed as the mean±S.D. of at least two independent experiments.
As shown in Table 1, the 5-methylpyrazoles were designed and prepared first. Compound 13a (with no substituent on the bottom phenyl ring) showed no measurable activity of hTGR5 at a concentration of 0.1 µM. Introduction of electron-withdrawing 2,5-dichloro (13b), electron-donating 2,5-dimethyl (13c) or 3-methoxyl (13d) group, also did not result in any promising activity. We hypothesized that the repulsive interaction between the methyl substituent on position 5 of pyrazole (compounds 13a–d) and the upper phenyl ring might cause a relatively great change of the spatial configuration between the upper phenyl ring and the middle core ring compared with scaffold A. Following removal of the 5-methyl substituent from the pyrazole ring, a series of 5-hydropyrazole derivatives (18a–c) with similar substituents on the bottom phenyl ring were synthesized and evaluated in vitro. As anticipated, a great improvement in the in vitro potency was observed. Compound 18c with a 3-methoxyl substituent on the bottom phenyl ring exhibited moderate agonist activity, with a hEC50 value of 129 nM. However, the in vitro potency of compound 18c was not sufficient for further studies, and as a result we discontinued exploration of the pyrazole scaffold.
The in vitro activity of 1,2,3-triazoles is shown in Table 2. It was disappointing that the intermediates 23a and b and 24a and b did not show any measureable activity at a concentration of 0.1 µM; neither did compounds 25a and b. We speculated that the presence of a hydrogen bond donor at the triazole ring of compounds 25a and b might be extremely unfavorable for binding with the receptor, and the p-methoxybenzyl group on the triazole ring of compounds 23a and b and 24a and b was so large that it might result in a big change of the spatial configuration between the bottom phenyl ring and the middle triazole ring compared with the pyrazole derivative 18c.
As shown in Table 3, the 2-methyl-thiazole series exhibited a dramatic improvement, especially for hTGR5 activity. To carry out preliminary screening of the 2-methyl-thiazole scaffold, the introductions of two substituents selected from a methyl, chloro or bromo moiety to the 2,5-di-positions of the bottom phenyl ring and a cyclopropyl or methyl group to the R2 region gave the desired compounds 30a–h. Of these compounds, 30a, c, e and g were significantly more potent than the corresponding compounds with a methyl group in the R2 region, and all displayed extremely high potency toward hTGR5 in vitro, with EC50 values of approximately 1 nM.
Next, we selected the R2 region with a cyclopropyl group into a subsequent library to further explore the SAR of the bottom phenyl region. Replacement of the 2-chloro of compounds 30a and c with a weak electron-donating methyl group (analogs 31a, b) led to a 3−4-fold decrease of the hEC50 and a slight decrease of the mEC50, and resulted in more reduction with an electron-withdrawing nitro group (compounds 32a, b). In addition, removal of the 2-chloro of compound 30a provided analog 30j, which exhibited only moderate activity on hTGR5. Another key finding was that the in vitro activity of compounds 30c, i, k–m, which were substituted with a single chloro group or two chloro groups at different positions, followed the order 4-Cl≪3-Cl<2-Cl<2,4-diCl≪2,5-diCl, indicating that the 2,5-di-substitution was the best favored. Additionally, while keeping the 2,5-diCl groups intact, position 4 of compound 30c was also explored. It could be noted that various substitutions at position 4 of compound 30c were well tolerated, such as bromo (30n), methyl (31c) and hydroxyl (31d) substituents, which suggested that other groups might also be well tolerated.32) As a general rule, the 2-methyl-thiazole scaffold successfully maintained excellent in vitro potency.
To further explore the SAR of the thiazole ring, a series of thiazole derivatives that were substituted with ethyl, isopropyl, phenyl or 2-pyridyl groups on the thiazole ring were designed and synthesized (Table 4). Consistent with the observed SAR around the 2-methyl-thiazoles, the 2,5-dichloro analogs of these series were much better than the corresponding 3-dichloro analogs. Among the 2,5-dichloro analogs, compound 30c still displayed the best in vitro hTGR5 and mTGR5 potency. Interestingly, replacement of the methyl of compound 30c with ethyl (compound 37a) or isopropyl (compound 37c) resulted in a sharp decrease of potency on hTGR5, but only caused a slight loss with phenyl (compound 37e, hEC50=6.9 nM) or 2-pyridyl (compound 37g, hEC50=4.4 nM). To explain this result, the preferred conformations of compounds 30c, 37a, c, e and g were predicted in the software of ChemBio3D Ultra 14.0 using the MM2 algorithm. The methyl group of compound 30c, the phenyl group of compound 37e and the 2-pyridyl group of compound 37g were observed at the plane of thiazole ring, whereas part of the ethyl group of compound 37a and the isopropyl group of compound 37c were observed outside of the plane of thiazole ring. This observation suggested that the substitutions outside of the plane of the thiazole ring were not tolerated for high binding affinity, and which might disturb the postulated π–π stacking interaction between the thiazole ring and the receptor of hTGR5. Due to the fact that the human TGR5 receptor shares only 83% amino acid identity with mouse TGR5 receptor,10) the in vitro activity of all thiazole derivatives on mTGR5 was significantly weaker than that on hTGR5. In general, in vitro activity of the 2-substituted-thiazole derivatives follow the order that methyl>2-pyridyl>phenyl>ethyl>isopropyl.
 | ||||
|---|---|---|---|---|
| Compound | R1 | R2 | hTGR5 EC50 (nM)a) | mTGR5 EC50 (nM)b) |
| 30c | Methyl | Cl | 1.1±0.07 | 71±3.3 |
| 37a | Ethyl | Cl | 32±6.2 | 2137±317 |
| 37b | Ethyl | H | 185±74 | — |
| 37c | Isopropyl | Cl | 242±71 | — |
| 37d | Isopropyl | H | 2817±110 | — |
| 37e | Phenyl | Cl | 6.9±2.3 | 1865±252 |
| 37f | Phenyl | H | 57±19 | >5000 |
| 37g | 2-Pyridyl | Cl | 4.4±1.2 | 746±7.0 |
| 37h | 2-Pyridyl | H | 61±23 | >5000 |
a) EC50 values of hTGR5 and mTGR5 are expressed as the mean±S.D. of at least three independent experiments. b) EC50 values of hTGR5 and mTGR5 are expressed as the mean±S.D. of at least three independent experiments.
Finally, given the fact that many reported small molecule TGR5 agonists were found with poor metabolic stability profile, which severely limited their further development as orally available agents, then some representative thiazoles were also selected to determine their metabolic clearance in human and mouse liver microsomes (MLM). Unfortunately, the select thiazoles all exhibited extremely high intrinsic clearance in both human liver microsome (HLM) and MLM (Table 5), especially for compound 31b (HLM CLint=2027 mL/min/g, T1/2=1.0 min; MLM CLint=1341 mL/min/g, T1/2=1.6 min), which indicated that these compounds were deficient as tool compounds to investigate the therapeutic potential of systemic TGR5 agonists for the treatment of type 2 diabetes mellitus due to their high metabolic clearance.
 | ||||
|---|---|---|---|---|
| Compound | R | Species | T1/2 (min) | CLint in vitro (mL/min/g) |
| 30a | 2-Cl-5-Me | Human | 1.1 | 1893 |
| Mouse | 1.6 | 1297 | ||
| 30c | 2,5-diCl | Human | 1.2 | 1698 |
| Mouse | 2.7 | 780 | ||
| 30e | 2-Br-5-Cl | Human | 1.4 | 1453 |
| Mouse | 2.8 | 762 | ||
| 30g | 2-Br-5-Me | Human | 1.3 | 1678 |
| Mouse | 2.2 | 959 | ||
| 31b | 2,5-diMe | Human | 1.0 | 2027 |
| Mouse | 1.6 | 1341 | ||
| 32b | 5-Me-2-NO2 | Human | 1.2 | 1783 |
| Mouse | 3.7 | 569 | ||
In summary, a series of 4-phenoxythiazol-5-carboxamides were developed as highly potent TGR5 agonists using a bioisosteric replacement strategy based on scaffold A, and extensive SAR explorations were described. Due to extremely high metabolic clearance, this series were insufficient as systemically targeted tool compounds. Considering that systemic TGR5 agonists that exposed to the gallbladder and heart were thought likely to result unfavorable side effects, and that making TGR5 agonists with low systemic exposure had the potential to minimize the side effects, our additional efforts are ongoing to determine if they can be used for intestinal targeting with limited systemic exposure combined with other medicinal chemistry strategies, such as introducing polar, hydrophilic groups to the position 4 of the bottom phenyl ring to reduce their absorption.
hTGR5/CRE/HEK293 or mTGR5/CRE/HEK293 stable cell line was obtained by transfection of HEK293 cells with human or mouse TGR5 expression plasmid (hTGR5-pcDNA3.1 or mTGR5-pcDNA3.1) and CRE-driven luciferase reporter plasmid (pGL4.29; Promega, Madison, WI, U.S.A.), and employed to assess the activity of test compounds by reporter gene assay. Briefly, cells were seeded into 96-well plates and incubated overnight in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) in 5% CO2 at 37°C. Then, cells were incubated with fresh medium containing different concentrations of test compounds or 20 µM INT-777 as a positive control for 5.5 h. Luciferase activity in cell lysate was determined using the Steady-Glo Luciferase Assay System (Promega) according to the manufacturer’s instructions. Compounds with EC50 values on human TGR5 (hTGR5) less than 100 nM were selected for the mouse TGR5 (mTGR5) activity test.
In Vitro Microsomal Assay for Metabolic StabilityThe test compound was dissolved in dimethyl sulfoxide (DMSO) and diluted to the desired concentration with DMSO and 0.1% aqueous bovine serum albumin (BSA). Human or mouse liver microsomes were incubated in a 96-well plate containing 0.1 M Tris buffer (pH 7.4), 0.33 mg/mL microsomal protein, 0.1 µM test compound, 5.0 mM MgCl2, 0.005% BSA, and 1.0 mM reduced nicotinamide adenine dinucleotide phosphate (NADPH). Incubations were conducted at 37°C for 60 min. An aliquot was removed at each time point (0, 7, 17, 30, 60 min) and the enzymatic reaction was stopped by protein precipitation in methanol. The loss of the test compound was determined by LC-MS/MS. The intrinsic clearance (CLint, mL/min/g microsomal protein) was calculated from the following equations.
 |
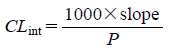 |
Unless otherwise noted, all materials were purchased from commercial suppliers and used without further purification. All reaction yields were not optimized. Analytical TLC (0.15–0.2 mm) and preparative TLC (0.4–0.5 mm) were performed on HSGF254 (Yantai Jiangyou Company, Yantai, Shandong, China), compounds were visualized by UV light (254 nm). Column chromatography was carried out on silica gel (200−300 mesh) or with pre-packed silica cartridges (4–40 g) from Bonna-Agela Technologies Inc. (Tianjin, China) and eluted with system of the Teledyne Isco CombiFlash Rf. Microwave reactions were performed in a Biatage Inititor. Melting point of target compounds was measured by SGWX-4 melting point apparatus (Shanghai Precision and Scientific Instrument Corporation, Shanghai, China). 1H- and 13C-NMR spectra were recorded on a Bruker AC300 or a Bruker AC400 or a Bruker AC500 NMR spectrometer, using tetramethylsilane (TMS) as an internal reference. 1H-NMR date are reported as multiplicity (s singlet, d doublet, t triplet, q quarter, dd doublet of doublets, dt doublet of triplets, br s broad singlet, m multiplet), number of protons, and coupling constant in hertz (Hz). Spectra were carried in CDCl3, DMSO-d6, and CD3OD. IR spectra were recorded on IS5 FT-IR (Thermo). Low-resolution mass spectra were determined on an Agilent LC-MS systems that consisted of an Agilent 1260 infinity LC coupled to an Agilent 6120 Quadrupole mass spectrometer (electrospray ionization (ESI)+) using an Agilent (Symmetry® C18 Colum, 3.5 µm, 2.1×50 mm) with aqueous CH3CN (0.4 mL/min, 0–5 min, 30–90%; 5–9 min, 90–90%) containing 0.1% formic acid and monitored at λ 240 nm. High-resolution (HR) mass spectra were recorded on a Q-TOF Ultima Globe mass spectrameter (Micromass, Manchester, U.K.).
5-(2,5-Dimethylphenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde (11c)Compound 10 (500 mg, 3.15 mmol) was dissolved in N,N-dimethylformamide (DMF) and treated with excess 2,5-dimethylphenol (1.15 g, 9.43 mmol) and potassium hydroxide (KOH) (270 mg, 4,82 mmol), then the reaction mixture was stirred at 120°C for 2 h. After consumption of 10 as monitored by TLC, the mixture was cooled to room temperature, then poured into water and extracted three times with EtOAc. The combined organic phases were washed with brine (3×), dried over MgSO4, filtered, and concentrated to dryness. The residue was purified by flash column chromatography to give 11c (661 mg, 86%). 1H-NMR (300 MHz, CDCl3) δ: 9.33 (1H, s), 7.13 (1H, d, J=5.7 Hz), 6.89 (1H, d, J=5.7 Hz,), 6.53 (1H, s), 3.64 (3H, s), 2.45 (3H, s), 2.32 (3H, s), 2.24 (3H, s).
5-(2,5-Dimethylphenoxy)-1,3-dimethyl-1H-pyrazole-4-carboxylic Acid (12c)To a solution of 11c (0.62 g, 2.54 mmol) in mixed of tertiary butanol (9 mL), tetrahydrofuran (THF) (9 mL) and H2O (3 mL), was added NaH2PO4 (1.83 g, 15.2 mmol), NaClO2 (1.38 g, 15.2 mmol) and 2-methylbut-2-ene (4 mL), and then allowed to stir at room temperature (r.t.) for 3 h. After completion of the reaction, the solvent was removed by rotary evaporation, and the crude was suspended in water. The precipitate was collected via filtration to yield 12c (0.60 g, 92%). 1H-NMR (300 MHz, DMSO) δ: 12.01 (1H, br), 7.14 (1H, d, J=7.8 Hz), 6.80 (1H, d, J=7.8 Hz), 6.25 (1H, s), 3.52 (3H, s), 2.32 (3H, s), 2.28 (3H, s), 2.16 (3H, s).
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(5-(2,5-dimethylphenoxy)-1,3-dimethyl-1H-pyrazol-4-yl)methanone (13c)To a solution of 12c (0.5 g, 1.92 mmol) in dry dichloromethane (DCM) was added oxalyl chloride (491 µL, 5.76 mmol) and three drops DMF, and held at reflux for 2 h. Then the reaction mixture was cooled to room temperature and concentrated. The residue was re-dissolved in dry DCM, 1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline (341 mg, 1.92 mmol) and Et3N (797 µL, 5.76 mmol) were added, and the reaction was stirred at r.t. overnight. After completion, the reaction was diluted with water and extracted with DCM (3×). The combined organic phases were collected, washed with brine, dried over MgSO4, concentrated and purified by flash column chromatography to afford the title compound 13c (624 mg) as an off-white solid in 78% yield. mp 124°C. 1H-NMR (300 MHz, CDCl3) δ: 7.07 (1H, dd, J=0.9 Hz, 6.3 Hz), 6.99–6.94 (2H, m), 6.75 (2H, d, J=5.7 Hz), 6.49 (1H, dt, J=0.9, 6.0 Hz), 6.40 (1H, s), 3.73 (2H, m), 3.49 (3H, s), 2.91 (2H, m), 2.30 (1H, m), 2.26 (3H, s), 2.20 (3H, s), 2.01 (3H, s), 0.74 (2H, m), 0.45 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 162.07, 154.12, 148.23, 146.18, 139.16, 137.04, 131.01, 125.41, 125.27, 123.82, 123.07, 122.55, 116.28, 113.34, 112.97, 103.61, 48.36, 40.66, 34.15, 31.27, 21.12, 15.55, 13.62, 7.89. IR (KBr) cm−1: 1626.27, 1504.57, 1419.43, 1404.60, 1361.74, 738.51. HPLC-MS: 5.66 min, 417.3 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C25H29O2N4: 417.2285. Found: 417.2275.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(1,3-dimethyl-5-phenoxy-1H-pyrazol-4-yl)methanone (13a)The title compound 13a was prepared from 10 and phenol according to a procedure similar to that described for the preparation of the title compound 13c. mp 145°C. 1H-NMR (300 MHz, CDCl3) δ: 7.24 (2H, t, J=6.0 Hz), 7.13 (1H, d, J=5.7 Hz), 7.05 (1H, d, J=5.7 Hz), 7.00 (1H, d, J=6.3 Hz), 6.73 (2H, d, J=6.0 Hz), 6.70 (1H, d, J=6.3 Hz), 6.48 (1H, t, J=5.7 Hz), 3.72 (2H, m), 3.53 (3H, s), 2.85 (2H, m), 2.30 (1H, m), 2.28 (3H, s), 0.78 (2H, m), 0.51 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 161.89, 156.36, 148.53, 145.96, 139.18, 129.77, 125.41, 125.24, 123.45, 122.64, 116.24, 115.04, 112.76, 103.29, 48.25, 40.50, 34.20, 31.25, 13.61, 7.99. IR (KBr) cm−1: 1621.84, 1502.90, 1489.58, 1411.74, 1400.72, 1320.10, 756.37. HPLC-MS: 4.93 min, 389.3 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C23H25O2N4: 389.1972. Found: 389.1965.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(5-(2,5-dichlorophenoxy)-1,3-dimethyl-1H-pyrazol-4-yl)methanone (13b)The title compound 13b was obtained as a white solid from 10 and 2,5-dichlorophenol according to the general procedure. mp 139°C. 1H-NMR (300 MHz, CDCl3) δ: 7.15 (1H, d, J=7.8 Hz), 7.00 (1H, dt, J=1.2, 7.8 Hz), 6.72 (1H, d, J=7.8 Hz), 6.62 (1H, d, J=8.7 Hz), 6.49 (1H, t, J=7.8 Hz), 6.30 (1H, d, J=2.4 Hz), 6.18 (1H, dd, J=2.4, 8.7 Hz), 3.76 (2H, m), 3.52 (3H, s), 3.02 (2H, m), 2.35 (1H, m), 2.22 (3H, s), 0.80 (2H, m), 0.55 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 165.04, 161.57, 156.72, 151.78, 148.00, 145.43, 139.52, 133.40, 131.21, 125.81, 124.49, 122.84, 120.85, 116.53, 116.00, 113.19, 48.91, 41.19, 34.49, 31.26, 13.44, 7.98. HPLC-MS: 5.76 min, 457.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C23H23O2N4Cl2: 457.1193. Found: 457.1186.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(5-(3-methoxyphenoxy)-1,3-dimethyl-1H-pyrazol-4-yl)methanone (13d)The title compound 13d was prepared from 10 and 3-methoxyphenol following the general procedure. mp 109°C. 1H-NMR (300 MHz, CDCl3) δ: 7.15 (2H, m), 6.99 (1H, t, J=8.1 Hz), 6.71 (1H, d, J=7.5 Hz), 6.61 (1H, m), 6.48 (1H, m), 6.31 (2H, m), 3.77 (2H, m), 3.74 (3H, s), 3.52 (3H, s), 2.90 (2H, m), 2.30 (1H, m), 2.25 (3H, s), 0.78 (2H, m), 0.50 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 160.84, 157.34, 151.14, 148.44, 139.24, 130.30, 130.20, 125.40, 122.65, 116.24, 112.80, 109.02, 108.96, 107.13, 106.96, 101.78, 55.44, 48.26, 40.98, 34.22, 31.20, 22.70, 13.60, 7.99. HPLC-MS: 4.99 min, 419.3 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C24H27O3N4: 419.2078. Found: 419.2072.
Ethyl-5-chloro-1-methyl-1H-pyrazole-4-carboxylate (15)To a solution of tBuONO (2.95 mL, 2.46 mmol) in acetonitrile (ACN) was added CuCl (1.76 g, 1.77 mmol), and then added 14 (2.5 g, 1.48 mmol) to the mixture in portions. The reaction was stirred at r.t. for 2 h, and then heated at 60°C for 1 h. After completion as TLC monitored, the reaction mixture was cooled to r.t. and diluted with 2 N aq. HCl, then extracted with DCM (3×). The combined phases were washed with brine, dried over MgSO4, filtered, concentrated and purified by flash column chromatography to give 15 (2.08 g) in 75% yield. 1H-NMR (300 MHz, CDCl3) δ: 7.90 (1H, s), 4.30 (2H, q, J=5.4 Hz), 3.86 (3H, s), 1.34 (3H, t, J=5.4 Hz).
Ethyl-1-methyl-5-phenoxy-1H-pyrazole-4-carboxylate (16a)In a microwave reaction vessel was combined 15 (300 mg, 1.59 mmol), phenol (300 mg, 3.19 mmol) and Cs2CO3 (1.52 g, 4.66 mmol) in DMF. The vessel was sealed and the resulting mixture was heated at 120°C by microwave for 30 min. Then the reaction mixture was cooled to r.t., quenched with water and extracted with EtOAc three times. The organic layer was collected, washed with brine, dried over MgSO4 and filtered. After the solvent was removed, the crude was purified by flash column chromatography to yield 16a (280 mg, 72%). 1H-NMR (300 MHz, CDCl3) δ: 7.92 (1H, s), 7.3 (2H, dt, J=2.1, 7.2 Hz), 7.10 (1H, dt, J=1.2, 7.2 Hz), 6.89 (2H, m), 4.07 (2H, q, J=7.2 Hz), 3.71 (3H, s), 1.03 (3H, t, J=7.2 Hz).
1-Methyl-5-phenoxy-1H-pyrazole-4-carboxylic Acid (17a)16a (270 mg, 1.10 mmol) was dissolved in a solution of 1,4-dioxane–H2O=2 : 1, and NaOH (88 mg, 2.20 mmol) was added. The mixture was stirred at r.t. until consumption of 16a as monitored by TLC, and then the solvent was removed. The residue was re-dissolved in water, acidified with 2 N aqueous HCl to pH 3 and then the product precipitated out. The precipitate was collected by filtration to afford 17a (227 mg, 95%) and was carried forward without further purification. 1H-NMR (300 MHz, DMSO) δ: 12.25 (1H, s), 7.85 (1H, s), 7.36 (2H, dd, J=1.5, 7.5 Hz), 7.12 (1H, dt, J=0.9 Hz, 7.5 Hz), 6.92 (2H, m), 3.61 (3H, s).
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(1-methyl-5-phenoxy-1H-pyrazol-4-yl)methanone (18a)The title compound 18a was prepared as an off-white solid from 17a and 1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline according to a procedure similar to the preparation of 13c from 12c. mp 104°C. 1H-NMR (300 MHz, CDCl3) δ: 7.54 (1H, s), 7.26 (2H, m), 7.15 (1H, d, J=8.1 Hz), 7.08 (1H, d, J=7.5 Hz), 7.02 (1H, d, J=8.4 Hz), 6.79 (1H, d, J=7.8 Hz), 6.75 (2H, d, J=8.1 Hz), 6.52 (1H, d, J=7.5 Hz), 3.74 (2H, t, J=5.4 Hz), 3.60 (3H, s), 2.94 (2H, t, J=5.4 Hz), 2.31 (1H, m), 0.77 (2H, m), 0.52 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 160.92, 156.47, 146.70, 140.20, 139.36, 129.78, 125.67, 125.26, 123.50, 123.21, 116.29, 115.03, 112.86, 105.00, 48.34, 40.60, 34.61, 31.25, 7.97. HPLC-MS: 4.84 min, 375.3 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C22H23O2N4: 375.1816. Found: 375.1810.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(5-(2,5-dimethylphenoxy)-1-methyl-1H-pyrazol-4-yl)methanone (18b)The title compound 18b was obtained as a white solid from 15 according to the general procedure that preparation of title compound 18a. mp 131°C. 1H-NMR (300 MHz, CDCl3) δ: 7.52 (1H, s), 7.10 (1H, d, J=8.1 Hz), 6.99 (2H, m), 6.81 (1H, d, J=8.1 Hz), 6.76 (1H, d, J=7.5 Hz), 6.51 (1H, d, J=7.5 Hz), 6.37 (1H, s), 3.76 (2H, t, J=5.4 Hz), 3.57 (3H, s), 3.00 (2H, t, J=5.4 Hz), 2.26 (1H, m), 2.20 (3H, s), 2.06 (3H, s), 0.74 (2H, m), 0.47 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 161.14, 154.32, 146.95, 140.07, 139.32, 136.95, 131.10, 125.57, 125.45, 123.90, 123.25, 123.17, 116.32, 113.14, 113.02, 105.15, 48.47, 40.72, 34.53, 31.27, 21.13, 15.53, 7.88. HPLC-MS: 5.57 min, 403.3 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C24H27O2N4: 403.2129. Found: 403.2122.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(5-(3-methoxyphenoxy)-1-methyl-1H-pyrazol-4-yl)methanone (18c)The title compound 18c was obtained from 15 according to the general procedure that preparation of title compound 18a. mp 58°C. 1H-NMR (300 MHz, CDCl3) δ: 7.53 (1H, s), 7.14 (2H, m), 7.03 (1H, dt, J=0.9, 6.0 Hz), 6.80 (1H, d, J=6.0 Hz), 6.61 (1H, m), 6.51 (1H, dt, J=1.2, 5.7, 6.0 Hz), 6.32 (2H, m), 3.76 (2H, t, J=4.2 Hz), 3.75 (3H, s), 3.60 (3H, s), 2.97 (2H, t, J=4.2 Hz), 2.31 (1H, m), 0.78 (2H, m), 0.53 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 160.94, 160.85, 157.45, 146.55, 140.16, 139.44, 130.20, 125.69, 125.25, 123.24, 116.30, 112.91, 109.08, 106.88, 105.09, 101.70, 55.41, 48.35, 40.64, 34.62, 31.21, 7.96. IR (KBr) cm−1: 2925.24, 1637.36, 1607.05, 1501.83, 1489.73, 1430.37, 1130.12, 743.44. HPLC-MS: 4.90 min, 405.3 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C23H25O3N4: 405.1921. Found: 405.1913.
Ethyl-5-hydroxy-1-(4-methoxybenzyl)-1H-1,2,3-triazole-4-carboxylate (19)Compound 19 was prepared as the literature described.26) 1H-NMR (300 MHz, DMSO) δ: 7.20 (2H, d, J=6.3 Hz), 6.91 (2H, d, J=6.3 Hz), 5.27 (2H, s), 4.24 (2H, q, J=5.4 Hz), 3.72 (1H, s), 1.27 (3H, t, J=5.4 Hz).
Ethyl-5-chloro-1-(4-methoxybenzyl)-1H-1,2,3-triazole-4-carboxylate (20)Compound 20 was synthesized from 19 with the method described in literature.27) 1H-NMR (300 MHz, CDCl3) δ: 7.26 (2H, d, J=8.7 Hz), 6.87 (2H, d, J=8.7 Hz), 5.50 (2H, s), 4.42 (2H, q, J=4.2 Hz), 3.79 (1H, s), 1.40 (3H, t, J=4.2 Hz).
Ethyl-1-(4-methoxybenzyl)-5-(3-methoxyphenoxy)-1H-1,2,3-triazole-4-carboxylate (21b)3-Methoxyphenol (334 µL, 3.04 mmol) was dissolved in dry DMF, then cooled to 0°C and added NaH (60%, 90 mg, 2.25 mmol). The reaction mixture was warmed to r.t. and stirred for 0.5 h. The residue was treated with 20 (600 mg, 2.03 mmol) and held at 85°C. After completion as monitored by TLC, the reaction was cooled to r.t. and slowly quenched with water. The resulting mixture was extracted twice with EtOAc, dried over MgSO4 and concentrated. The crude was purified by flash column chromatography to give 21b (576 mg) in 74% yield. 1H-NMR (300 MHz, CDCl3) δ: 7.20 (2H, d, J=8.7 Hz), 7.14 (1H, t, J=8.7 Hz), 6.78 (2H, d, J=8.7 Hz), 6.64 (1H, dd, J=2.1, 8.7 Hz), 6.32 (2H, m), 5.33 (2H, s), 4.19 (2H, q, J=4.2 Hz), 3.75 (3H, s), 3.71 (3H, s), 1.12 (3H, t, J=4.2 Hz).
1-(4-Methoxybenzyl)-5-(3-methoxyphenoxy)-1H-1,2,3-triazole-4-carboxylic Acid (22b)Compound 22b (502 mg) was prepared from 21b (570 mg, 1.49 mmol) according to the procedure described that preparation of 17a from 16a in excellent yield (95%). 1H-NMR (300 MHz, DMSO) δ: 12.99 (1H, br), 7.19 (1H, t, J=8.4 Hz), 7.14 (2H, t, J=8.7 Hz), 6.83 (2H, d, J=8.7 Hz), 6.69 (1H, ddd, J=0.6, 2.4, 8.7 Hz), 6.42 (1H, t, J=2.4 Hz), 6.36 (1H, ddd, J=0.6, 2.4, 8.7 Hz) 5.35 (2H, s), 3.69 (3H, s), 3.68 (3H, s).
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(1-(4-methoxybenzyl)-5-(3-methoxyphenoxy)-1H-1,2,3-triazol-4-yl)methanone (23b)The title compound 23b was obtained as a white solid from 22b following a similar procedure that preparation of 13c from 12c. mp 111°C. 1H-NMR (300 MHz, CDCl3) δ: 7.26–6.97 (6H, m), 6.76 (2H, d, J=7.8 Hz), 6.61 (1H, d, J=7.8 Hz), 6.40 (1H, m), 6.19 (2H, m), 5.23 (2H, s), 3.88 (2H, m), 3.76 (3H, s), 3.69 (3H, s), 2.91 (2H, m), 2.28 (1H, m), 0.76 (2H, m), 0.51 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 160.78, 159.70, 159.01, 156.67, 139.41, 130.20, 129.66, 129.52, 125.99, 125.90, 124.10, 116.22, 114.12, 112.94, 109.91, 107.24, 101.81, 55.43, 55.31, 50.48, 48.10, 41.44, 31.20, 7.98. HPLC-MS: 5.72 min, 512.3 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C29H30O4N5: 512.2292. Found: 512.2285.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(5-(2,5-dichlorophenoxy)-1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methanone (23a)The title compound 23a was obtained as a white solid with a similar method that preparation of the title compound 23b from 20. mp 119°C. 1H-NMR (300 MHz, CDCl3) δ: 7.26 (2H, m), 7.11 (3H, m), 7.01 (1H, dt, J=1.2, 2.0 Hz), 6.95 (1H, dd, J=2.1, 6.3 Hz), 6.72 (2H, d, J=6.3 Hz), 6.54 (1H, m), 6.36 (1H, m), 5.31 (2H, s), 4.06 (2H, m), 3.75 (3H, s), 3.28 (2H, m), 2.34 (1H, m), 0.77 (2H, m), 0.54 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 159.84, 158.52, 151.23, 139.65, 133.15, 131.12, 130.27, 129.72, 126.15, 125.20, 124.98, 124.30, 123.64, 121.14, 116.47, 114.20, 113.31, 55.29, 51.14, 48.86, 31.34, 7.97. IR (KBr) cm−1: 1642.80, 1515.46, 1497.38, 1473.21, 1254.21. HPLC-MS: 6.37 min, 550.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C28H26O3N5Cl2: 550.1407. Found: 550.1403. HR-MS (ESI): m/z (M+H+) Calcd for C28H26O3N5Cl2: 550.1407. Found: 550.1403.
(5-(2,5-Dichlorophenoxy)-1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)(4-methyl-3,4-dihydro-quinoxalin-1(2H)-yl)methanone (24a)In addition to replaced1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline with 1-methyl-1,2,3,4-tetrahydroquinoxaline, the title compound 24a was obtained as a white solid with a similar method that preparation of the title compound 23b from 20. mp 116°C. 1H-NMR (300 MHz, CDCl3) δ: 7.26 (2H, m), 7.12 (2H, m), 7.02–6.94 (2H, m), 6.73 (2H, d, J=8.4 Hz), 6.58 (1H, d, J=8.1 Hz), 6.45 (1H, m), 6.37 (1H, m), 5.30 (2H, s), 4.05 (2H, m), 3.75 (3H, s), 3.19 (2H, m), 2.83 (3H, s). 13C-NMR (126 MHz, CDCl3) δ: 159.83, 158.52, 151.17, 139.43, 133.16, 131.07, 130.09, 129.73, 126.62, 125.24, 124.96, 123.90, 121.17, 116.23, 115.72, 114.20, 111.52, 55.29, 51.14, 50.83, 37.93. IR (KBr) cm−1: 1644.47, 1514.06, 1474.96, 1248.25, 748.35. HPLC-MS: 5.88 min, 524.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C26H24O3N5: 524.1251. Found: 524.1251.
(1-(4-Methoxybenzyl)-5-(3-methoxyphenoxy)-1H-1,2,3-triazol-4-yl)(4-methyl-3,4-dihydro-quinoxalin-1(2H)-yl)methanone (24b)In addition to replaced 1-cyclopropyl-1,2,3,4-tetrahydroquinoxaline with 1-methyl-1,2,3,4-tetrahydroquinoxaline, the title compound 24b was obtained as a white solid with a similar method that preparation of the title compound 23b from 20. mp 122°C. 1H-NMR (300 MHz, CDCl3) δ: 7.12–7.07 (4H, m), 7.00 (1H, dt, J=1.2, 6.0 Hz), 6.76 (2H, d, J=6.3 Hz), 6.62 (1H, dd, J=1.2, 6.3 Hz), 6.57 (1H, d, J=6.3 Hz), 6.33 (1H, m), 6.19 (2H, m), 5.23 (2H, s), 3.88 (2H, m), 3.76 (3H, s), 3.70 (3H, s), 2.77 (5H, m). 13C-NMR (126 MHz, CDCl3) δ: 160.79, 159.69, 158.95, 156.61, 139.22, 130.10, 129.64, 129.13, 126.42, 126.08, 123.60, 115.33, 114.12, 110.86, 110.01, 107.17, 101.71, 55.44, 55.31, 50.43, 37.77. HPLC-MS: 5.21 min, 486.3 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C27H28O4N5: 486.2136. Found: 486.2123.
(5-(3-Methoxyphenoxy)-1H-1,2,3-triazol-4-yl)(4-methyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (25b)24b (70 mg, 144 µmol) was dissolved in trifluoroacetic acid (TFA) (5 mL), then heated to 65°C and stirred for 2 h. Upon completion of reaction, the reaction mixture was cooled to r.t. and concentrated. The residue was then re-dissolved in DCM and washed with water. Then the solvent was removed under reduced pressure, and the crude reaction mixture was purified by preparative TLC to yield the title compound 25b (35 mg, 66%) as a light yellow solid. mp 157°C. 1H-NMR (300 MHz, CDCl3) δ: 7.18 (1H, d, J=8.4 Hz), 7.02 (1H, d, J=7.8 Hz), 6.61 (3H, m), 6.36 (3H, m), 3.99 (2H, m), 3.73 (3H, s), 3.21 (2H, m), 2.87 (3H, s). HPLC-MS: 3.98 min, 366.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C19H20O3N5: 366.1561. Found: 366.1556.
(5-(2,5-Dichlorophenoxy)-1H-1,2,3-triazol-4-yl)(4-methyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (25a)The title compound 25a was prepared as a light yellow solid from 24a according to the similar procedure that preparation of the title compound 25b from 24b. mp 149°C. 1H-NMR (300 MHz, CDCl3) δ: 7.23 (1H, m), 6.97 (1H, m), 6.88 (1H, m), 6.55–6.39 (4H, m), 4.07 (2H, d, J=5.4 Hz), 3.48 (2H, d, J=5.4 Hz), 2.87 (3H, s). 13C-NMR (126 MHz, CDCl3) δ: 150.77, 139.67, 132.79, 130.65, 127.13, 124.97, 123.73, 123.02, 122.27, 118.83, 115.33, 111.24, 50.73, 40.13, 37.90. IR (KBr) cm−1: 1629.60, 1512.67, 1474.02, 1401.87, 1329.41, 1229.21, 733.17. HPLC-MS: 4.99 min, 404.1 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C18H16O2N5Cl2: 404.0676. Found: 404.0664.
Ethyl-4-hydroxy-2-methylthiazole-5-carboxylate (26)Compound 26 was synthesized with the method described in the patent.28) 1H-NMR (300 MHz, CDCl3) δ: 9.88 (1H, br), 4.34 (2H, q, J=7.2 Hz), 2.65 (1H, s), 1.35 (3H, t, J=7.2 Hz).
Ethyl-4-(5-chloro-2-nitrophenoxy)-2-methylthiazole-5-carboxylate (27a)To a solution of 26 in ethanol was added dropwise with fresh sodium ethoxide and then allowed to stir at 45°C for 0.5 h. The reaction mixture was cooled to r.t. and filtered to give sodium salt of 26 (2 g, 9.57 mmol). The residue was treated with 4-chloro-2-fluoro-1-nitrobenzene (1.7 g, 9.71 mmol) in NMP and heated by microwave at 125°C for 2 h. Then the reaction mixture was cooled to r.t., poured into water, extracted with EtOAc three times and combined the organic phases. The organic layer was washed with brine twice, dried over MgSO4, filtered and concentrated. The crude was purified by flash column chromatography to give compound 27a (2.21 g) in yield of 65%. 1H-NMR (300 MHz, CDCl3) δ: 8.02 (1H, d, J=8.7 Hz), 7.29 (1H, dd, J=2.1, 8.7 Hz), 7.27 (2H, d, J=2.1 Hz), 4.31 (2H, q, J=7.2 Hz), 2.57 (3H, s), 1.32 (3H, t, J=7.2 Hz).
Ethyl-4-(2-amino-5-chlorophenoxy)-2-methylthiazole-5-carboxylate (28a)To a solution of 27a (1.37 g, 4.0 mmol) in THF–H2O (2 : 1) was added iron powder (2.24 g, 40 mmol) and NH4Cl (2.14g, 40 mmol), and then stirred at 65°C overnight. After completion of the reaction as monitored by TLC, the mixture was cooled to r.t., filtered by siliceousearth and the filter cake was washed with DCM. The filtrate was extracted with DCM and the organic layer was combined. The residue was evaporated under reduced pressure to give crude product 28a and was used directly in the next step without further purification. HPLC-MS: 313.13 (M+H+).
Ethyl-4-(2,5-dichlorophenoxy)-2-methylthiazole-5-carboxylate (29a)To a solution of 28a (312 mg, 1.0 mmol) in ACN was added CuCl2 (202 mg, 1.5 mmol), and slowly added tBuONO (238 µL, 2.0 mmol). The reaction mixture was allowed to stir at r.t. for 24 h, then heated to 60°C and stirring was continued for 2 h. Upon completing, the reaction was quenched with 2 N aqueous HCl and diluted with water. The mixture was extracted with DCM (3×) and the organic phases were combined. The combined organic layer was washed twice with brine, dried over MgSO4 and concentrated. The residue was purified by flash column chromatography to give the product 29a (249 mg, 75%). 1H-NMR (300 MHz, CDCl3) δ: 7.36 (1H, d, J=6.9 Hz), 7.11 (1H, dd, J=1.8, 6.9 Hz), 7.10 (1H, d, J=1.8 Hz), 4.31 (2H, q, J=5.4 Hz), 2.61 (3H, s), 1.32 (3H, t, J=5.4 Hz).
Ethyl-4-(2-bromo-5-chlorophenoxy)-2-methylthiazole-5-carboxylate (29c)In addition to use CuBr2 instead of CuCl2, 29c was synthesized from 28a as following a similar procedure that preparation 29a from 28a. 1H-NMR (300 MHz, CDCl3) δ: 7.52 (1H, d, J=6.0 Hz), 7.04 (2H, m), 4.31 (2H, q, J=5.4 Hz), 2.62 (3H, s), 1.31 (3H, t, J=5.4 Hz).
Ethyl-4-(3-chlorophenoxy)-2-methylthiazole-5-carboxylate (29e)A solution of 28a (312 mg, 1.0 mmol) in ACN, was treated with tBuONO and held at reflux for 8 h. After the reaction was completed, the reaction mixture was cooled to r.t. and quenched with 2 N aqueous HCl, then diluted with water. The crude mixture was extracted with EtOAc, and the combined organic layer was washed twice with brine, dried over MgSO4, filtered and concentrated under reduced pressure. The residue was purified by flash column chromatography to yield 29e (194 mg) in 65%. 1H-NMR (300 MHz, CDCl3) δ: 7.27 (1H, t, J=6.0 Hz), 7.12 (1H, dd, J=1.5, 6.0 Hz), 7.09 (1H, d, J=1.5 Hz), 7.00 (1H, dd, J=1.5, 6.0 Hz), 4.29 (2H, q, J=5.4 Hz), 2.63 (3H, s), 1.30 (3H, t, J=5.1 Hz).
4-(2,5-Dichlorophenoxy)-2-methylthiazole-5-carboxylic AcidThe intermediate was prepared with the same method described that preparation of 17a from 16a. 1H-NMR (300 MHz, DMSO) δ: 13.21 (1H, br), 7.60 (1H, d, J=6.6 Hz), 7.38 (1H, d, J=1.5 Hz), 7.31 (1H, d, J=1.5, 6.6 Hz), 2.55 (3H, s).
4-(2-Bromo-5-chlorophenoxy)-2-methylthiazole-5-carboxylic AcidThe intermediate was prepared with the same method described that preparation of 17a from 16a. 1H-NMR (300 MHz, DMSO) δ: 13.19 (1H, br), 7.60 (1H, d, J=6.6 Hz), 7.38 (1H, d, J=1.5 Hz), 7.31 (1H, d, J=1.5, 6.6 Hz), 2.55 (3H, s).
4-(3-Chlorophenoxy)-2-methylthiazole-5-carboxylic AcidThe intermediate was prepared with the same method described that preparation of 17a from 16a. 1H-NMR (300 MHz, DMSO) δ: 13.18 (1H, br), 7.39 (1H, t, J=6.0 Hz), 7.22 (1H, dd, J=1.5, 6.0 Hz), 7.17 (1H, d, J=1.5 Hz), 7.03 (1H, dd, J=1.5, 6.0 Hz), 2.58 (3H, s).
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(4-(2,5-dichlorophenoxy)-2-methylthiazol-5-yl)methanone (30c)The title compound 30c was prepared as a light yellow solid according to the general procedure that preparation of 13c from 12c. mp 160°C. 1H-NMR (400 MHz, CDCl3) δ: 7.21 (1H, d, J=8.6 Hz), 6.95–6.89 (2H, m), 6.85 (1H, dd, J=8.3, 1.5 Hz), 6.79 (1H, d, J=7.9 Hz), 6.52 (1H, ddd, J=8.4, 7.2, 1.5 Hz), 6.13 (1H, s), 3.94 (2H, t, J=5.5 Hz), 3.49 (2H, t, J=5.5 Hz), 2.61 (3H, s), 2.30 (1H, tt, J=6.8, 3.7 Hz), 0.74–0.68 (2H, m), 0.45–0.39 (2H, m). 13C-NMR (101 MHz, CDCl3) δ: 166.75, 159.52, 154.26, 151.09, 139.83, 132.58, 130.40, 126.51, 125.52, 124.65, 122.95, 122.47, 119.93, 116.00, 113.22, 112.84, 48.98, 41.30, 31.22, 20.24, 8.04. IR (KBr) cm−1: 1646.23, 1536.75, 1506.15, 1472.16, 1405.36, 1331.26, 748.47. HPLC-MS: 6.49 min, 460.1 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C22H19Cl2N3O2S: 460.0648. Found: 460.0661.
(4-(2,5-Dichlorophenoxy)-2-methylthiazol-5-yl)(4-methyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (30d)In addition to replaced 1-cyclopropyl-1,2,3,4-tetrahydro-quinoxaline with 1-methyl-1,2,3,4-tetrahydro-quinoxaline, 30d was prepared as a light yellow solid according to the general procedure that preparation of 13c from 12c. mp 126°C. 1H-NMR (300 MHz, CDCl3) δ: 7.19 (1H, d, J=8.7 Hz), 6.90 (1H, dd, J=2.4, 8.4 Hz), 6.84 (1H, dt, J=1.2, 7.8 Hz), 6.74 (1H, d, J=7.2 Hz), 6.44 (1H, dt, J=1.2, 7.2, 7.8 Hz), 6.24 (1H, d, J=7.8 Hz), 6.17 (1H, s), 3.99 (2H, t, J=5.4 Hz), 3.49 (2H, t, J=5.4 Hz), 2.78 (3H, s), 2.63 (3H, s). 13C-NMR (126 MHz, CDCl3) δ: 166.64, 159.32, 153.62, 150.84, 139.48, 132.43, 130.15, 126.81, 124.77, 124.07, 122.92, 121.72, 119.05, 115.08, 113.50, 111.03, 50.78, 41.03, 37.73, 20.13. IR (KBr) cm−1: 1640.10, 1625.80, 1512.29, 1473.01, 1407.68, 1333.55, 742.15. HPLC-MS: 6.01 min, 434.1 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C20H18O2N3Cl2S: 434.0491. Found: 434.0487.
(4-(2-Chloro-5-methylphenoxy)-2-methylthiazol-5-yl)(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (30a)The title compound 30a was obtained as a light yellow solid according to the general procedure that preparation of 13c from 12c. mp 136°C. 1H-NMR (300 MHz, CDCl3) δ: 7.31 (1H, dd, J=1.2, 7.2 Hz), 7.03–6.97 (2H, m), 6.92–6.88 (3H, m), 6.51 (1H, m), 6.33 (1H, m), 3.96 (2H, t, J=5.4 Hz), 3.46 (2H, t, J=5.4 Hz), 2.59 (3H, s), 2.32 (1H, m), 0.71 (2H, m), 0.42 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.36, 159.89, 155.34, 150.44, 139.76, 137.49, 129.41, 125.87, 125.69, 125.46, 122.96, 120.98, 120.37, 115.79, 113.15, 111.52, 48.85, 41.70, 31.06, 20.86, 20.11, 7.87. IR (KBr) cm−1: 1628.74, 1506.62, 1415.80, 1332.32, 1067.33, 757.53. HPLC-MS: 6.27 min, 440.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C23H23O2N3ClS: 440.1194. Found: 440.1189.
(4-(2-Chloro-5-methylphenoxy)-2-methylthiazol-5-yl)(4-methyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (30b)In addition to replaced 1-cyclopropyl-1,2,3,4-tetrahydro-quinoxaline with 1-methyl-1,2,3,4-tetrahydro-quinoxaline, the title compound 30b was obtained as a light yellow solid according to the general procedure that preparation of 13c from 12c. mp 137°C. 1H-NMR (300 MHz, CDCl3) δ: 7.15 (1H, d, J=8.1 Hz), 6.87–6.82 (2H, m), 6.75 (1H, dd, J=1.2, 8.1 Hz), 6.45 (1H, m), 6.32 (1H, d, J=7.8 Hz), 6.10 (1H, s), 4.00 (2H, t, J=5.4 Hz), 3.46 (2H, t, J=5.4 Hz), 2.79 (3H, s), 2.60 (3H, s), 2.17 (3H, s). 13C-NMR (126 MHz, CDCl3) δ: 166.38, 159.83, 154.93, 150.41, 139.61, 137.46, 129.36, 126.30, 125.10, 123.09, 120.50, 119.75, 118.32, 115.11, 112.21, 111.30, 50.84, 41.58, 37.87, 20.93, 20.12. HPLC-MS: 5.76 min, 414.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C21H21O2N3ClS: 414.1038. Found: 414.1031.
(4-(2-Bromo-5-chlorophenoxy)-2-methylthiazol-5-yl)(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (30e)The title compound 30e was obtained as a light yellow solid according to the general procedure that preparation of 13c from 12c. mp 144°C. 1H-NMR (300 MHz, CDCl3) δ: 7.37 (1H, d, J=7.2 Hz), 6.94–6.76 (4H, m), 6.52 (1H, dt, J=1.5, 7.5 Hz), 6.04 (1H, s), 3.93 (2H, t, J=5.4 Hz), 3.51 (2H, t, J=5.4 Hz), 2.61 (3H, s), 2.31 (1H, m), 0.71 (2H, m), 0.42 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.64, 159.37, 154.06, 152.08, 139.66, 133.28, 133.19, 126.42, 125.32, 124.90, 122.83, 119.61, 115.78, 113.06, 112.90, 110.79, 48.89, 41.08, 31.14, 20.13, 7.94. IR (KBr) cm−1: 1645.76, 1534.69, 1505.39, 1466.46, 1403.98, 1331.27, 747.58. HPLC-MS: 6.55 min, 504.1 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C22H20O2N3BrClS: 504.0143. Found: 504.0130.
(4-(2-Bromo-5-chlorophenoxy)-2-methylthiazol-5-yl)(4-methyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (30f)In addition to replaced 1-cyclopropyl-1,2,3,4-tetrahydro-quinoxaline with 1-methyl-1,2,3,4-tetrahydro-quinoxaline, the title compound 30e was obtained as a light yellow solid according to the general procedure that preparation of 13c from 12c. mp 134°C. 1H-NMR (300 MHz, CDCl3) δ: 7.35 (1H, d, J=8.4 Hz), 6.86–6.82 (2H, m), 6.73 (1H, d, J=8.1 Hz), 6.44 (1H, d, J=7.8 Hz), 6.21 (1H, d, J=8.4 Hz), 6.10 (1H, s), 3.99 (2H, t, J=5.4 Hz), 3.51 (2H, t, J=5.4 Hz), 2.78 (3H, s), 2.63 (3H, s). 13C-NMR (126 MHz, CDCl3) δ: 166.66, 159.30, 153.57, 151.95, 146.03, 139.46, 133.25, 133.09, 126.86, 124.74, 124.46, 122.93, 118.91, 115.02, 113.71, 111.00, 50.80, 40.98, 37.79, 20.14. HPLC-MS: 6.09 min, 478.1 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C20H18O2N3BrClS: 477.9986. Found: 477.9980.
(4-(2-Bromo-5-methylphenoxy)-2-methylthiazol-5-yl)(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (30g)The title compound 30g was obtained as a light yellow solid according to the general procedure that preparation of 13c from 12c. mp 133°C. 1H-NMR (300 MHz, CDCl3) δ: 7.32 (1H, d, J=6.0 Hz), 6.88 (3H, m), 6.71 (1H, dd, J=0.9, 6.0 Hz), 6.53 (1H, m), 5.95 (1H, d, J=0.9 Hz), 3.95 (2H, t, J=4.2 Hz), 3.50 (2H, t, J=4.2 Hz), 2.59 (3H, s), 2.31 (1H, m), 2.15 (3H, s), 0.69 (2H, m), 0.41 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.37, 159.86, 155.25, 151.56, 139.74, 138.26, 132.38, 125.89, 125.66, 122.97, 120.24, 118.57, 115.68, 113.14, 111.76, 109.53, 48.89, 41.56, 31.08, 20.88, 20.13, 7.90. IR (KBr) cm−1: 1628.27, 1526.51, 1505.97, 1480.77, 1413.50, 1331.67, 1308.91, 756.78. HPLC-MS: 6.33 min, 484.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C23H23O2N3BrS: 484.0689. Found: 484.0683.
(4-(2-Bromo-5-methylphenoxy)-2-methylthiazol-5-yl)(4-methyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (30h)In addition to replaced 1-cyclopropyl-1,2,3,4-tetrahydro-quinoxaline with 1-methyl-1,2,3,4-tetrahydro-quinoxaline, the title compound 30h was obtained as a light yellow solid according to the general procedure that preparation of 13c from 12c. mp 129°C. 1H-NMR (300 MHz, CDCl3) δ: 7.32 (1H, d, J=6.0 Hz), 6.88 (3H, m), 6.71 (1H, dd, J=0.9, 6.0 Hz), 6.53 (1H, m), 6.10 (1H, s), 3.99 (2H, t, J=5.4 Hz), 3.49 (2H, t, J=5.4 Hz), 2.79 (3H, s), 2.60 (3H, s), 2.13 (3H, s). 13C-NMR (126 MHz, CDCl3) δ: 166.52, 159.81, 154.82, 151.51, 139.61, 138.21, 132.33, 128.75, 126.32, 125.53, 124.93, 122.99, 119.62, 117.76, 115.02, 111.28, 50.85, 41.16, 37.90, 20.96, 20.13. HPLC-MS: 6.16 min, 458.1 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C21H21O2N3BrS: 458.0532. Found: 458.0531.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(2-methyl-4-(m-tolyloxy)thiazol-5-yl)methanone (30i)The title compound 30i was obtained ass a light yellow solid according to the general procedure that preparation of 13c from 12c. mp 116°C. 1H-NMR (300 MHz, CDCl3) δ: 7.10 (1H, t, J=7.8 Hz), 7.01–6.91 (3H, m), 6.81 (1H, d, J=7.8 Hz), 6.55–6.50 (2H, m), 6.47 (1H, s), 3.92 (2H, t, J=5.4 Hz), 3.38 (2H, t, J=5.4 Hz), 2.60 (3H, s), 2.30 (1H, m), 0.74 (2H, m), 0.42 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.49, 159.61, 155.78, 154.88, 139.93, 134.37, 129.81, 126.23, 125.85, 123.81, 122.70, 118.58, 116.32, 116.21, 113.21, 112.50, 48.95, 41.51, 31.15, 20.09, 7.92. IR (KBr) cm−1: 1622.93, 1591.20, 1506.27, 1406.76, 1332.96, 1214.85, 1086.47, 746.28. HPLC-MS: 6.18 min, 426.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C22H21O2N3ClS: 426.1038. Found: 426.1033.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(2-methyl-4-(m-tolyloxy)thiazol-5-yl)methanone (30j)The title compound 30j was obtained as a light yellow solid according to the general procedure that preparation of 13c from 12c. mp 110°C. 1H-NMR (300 MHz, CDCl3) δ: 7.09–6.94 (3H, m), 6.88 (1H, d, J=7.8 Hz), 6.83 (1H, d, J=7.5 Hz), 6.57-6.51 (1H, m), 6.42 (1H, m), 6.34 (1H, s), 3.92 (2H, t, J=5.7 Hz), 3.36 (2H, t, J=5.7 Hz), 2.58 (3H, s), 2.30 (1H, m), 2.24 (3H, s), 0.71 (2H, m), 0.41 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.28, 160.07, 155.99, 155.41, 139.87, 139.19, 128.82, 125.98, 125.91, 124.49, 122.83, 118.68, 116.25, 115.13, 113.19, 111.65, 48.86, 41.83, 31.08, 21.28, 20.10, 7.89. IR (KBr) cm−1: 1623.00, 1506.34, 1405.22, 1334.80, 1306.90, 1248.01, 1146.39, 746.32. HPLC-MS: 5.98 min, 406.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C23H24O2N3S: 406.1584. Found: 406.1578.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(4-(2,4-dichlorophenoxy)-2-methylthiazol-5-yl)methanone (30k)The title compound 30k was synthesized as a light yellow solid in a manner similar to the title compound 30c. mp 87°C. 1H-NMR (400 MHz, CDCl3) δ: 7.31 (1H, d, J=2.5 Hz), 6.98 (1H, dd, J=8.8, 2.6 Hz), 6.95–6.88 (2H, m), 6.85 (1H, d, J=8.0 Hz), 6.50 (1H, ddd, J=8.2, 6.5, 2.2 Hz), 6.22 (1H, d, J=8.8 Hz), 3.95 (2H, t, J=5.5 Hz), 3.47 (2H, t, J=5.5 Hz), 2.57 (3H, s), 2.33 (1H, tt, J=6.8, 3.7 Hz), 0.77–0.70 (2H, m), 0.46–0.39 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.48, 159.60, 154.91, 149.57, 139.83, 129.58, 129.30, 127.36, 126.12, 125.72, 125.23, 122.77, 121.00, 115.99, 113.18, 111.48, 48.86, 41.56, 31.10, 20.05, 7.97. HPLC-MS: 6.62 min, 460.1 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C22H20O2N3Cl2S: 460.0648. Found: 460.0641.
(4-(4-Chlorophenoxy)-2-methylthiazol-5-yl)(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (30l)The title compound 30l was synthesized as a light yellow solid in a manner similar to the title compound 30i. mp 88°C. 1H-NMR (400 MHz, CDCl3) δ: 7.16–7.11 (2H, m), 7.03–6.94 (2H, m), 6.83 (1H, d, J=7.9 Hz), 6.52 (3H, ddd, J=12.6, 7.8, 1.8 Hz), 3.93 (2H, t, J=5.6 Hz), 3.38 (2H, t, J=5.6 Hz), 2.58 (3H, s), 2.31 (1H, tt, J=6.7, 3.7 Hz), 0.77–0.71 (2H, m), 0.44–0.38 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.43, 159.76, 155.50, 153.83, 140.00, 129.09, 128.85, 126.09, 122.71, 119.71, 116.35, 113.26, 111.62, 48.94, 41.62, 31.14, 20.07, 7.96. HPLC-MS: 6.18 min, 426.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C22H21O2N3ClS: 426.1038. Found: 426.1035.
(4-(2-Chlorophenoxy)-2-methylthiazol-5-yl)(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (30m)The title compound 30m was synthesized as a yellow solid in a manner similar to the title compound 30c. mp 93°C. 1H-NMR (300 MHz, CDCl3) δ: 7.31 (1H, dd, J=1.2, 7.2 Hz), 7.03–6.97 (2H, m), 6.92–6.88 (3H, m), 6.51 (1H, m), 6.33 (1H, m), 3.96 (2H, t, J=5.4 Hz), 3.46 (2H, t, J=5.4 Hz), 2.59 (3H, s), 2.32 (1H, m), 0.71 (2H, m), 0.42 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.37, 159.85, 155.32, 150.87, 139.77, 129.98, 127.28, 126.01, 125.68, 124.73, 124.34, 122.86, 120.07, 116.00, 113.15, 111.39, 48.83, 41.79, 31.08, 20.07, 7.90. HPLC-MS: 5.94 min, 426.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C22H21O2N3ClS: 426.1038. Found: 426.1029.
(4-(4-Bromo-2,5-dichlorophenoxy)-2-methylthiazol-5-yl)(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (30n)The title compound 30n was synthesized as a light yellow solid in a manner similar to the title compound 30e. mp 128°C. 1H-NMR (300 MHz, CDCl3) δ: 7.31 (1H, dd, J=1.2, 7.2 Hz), 7.03–6.97 (2H, m), 6.92–6.88 (3H, m), 6.51 (1H, m), 6.33 (1H, m), 3.96 (2H, t, J=5.4 Hz), 3.46 (2H, t, J=5.4 Hz), 2.59 (3H, s), 2.32 (1H, m), 0.71 (2H, m), 0.42 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.74, 159.21, 153.82, 150.10, 139.74, 133.34, 132.81, 126.43, 125.40, 123.27, 122.79, 120.98, 116.54, 115.86, 113.13, 112.73, 48.85, 41.15, 31.12, 20.10, 7.97. HPLC-MS: 7.14 min, 538.1 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C22H19O2N3BrCl2S: 537.9753. Found: 537.9753.
(4-(5-Chloro-2-methylphenoxy)-2-methylthiazol-5-yl)(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (31a)To a solution of 30e (102 mg, 0.2 mmol) in dry 1,4-dioxane, was added Zn(CH3)2 (333 µL, 0.4 mmol) and PdCl2(dppf)·CH2Cl2 (16.3 mg, 0.02 mmol). The reaction mixture was stirred at reflux under an atmosphere of nitrogen for 2 h. After completion, the reaction was quenched with CH3OH and stirring was continued for 10 min. The precipitate was removed by filtration and the filtrate was concentrated under reduced pressure. The residue was purified to give the title compound 31a (71 mg) as a light yellow solid in yield of 81%. mp 98°C. 1H-NMR (300 MHz, CDCl3) δ: 7.01–6.89 (4H, m), 6.83 (1H, d, J=5.7 Hz), 6.56 (1H, dt, J=1.2, 5.7 Hz), 6.05 (1H, s), 3.93 (2H, t, J=4.2 Hz), 3.42 (2H, t, J=4.2 Hz), 2.59 (3H, s), 2.27 (1H, m), 2.04 (3H, s), 0.70 (2H, m), 0.35 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.49, 159.81, 155.52, 153.78, 139.81, 131.31, 131.17, 126.43, 126.37, 125.83, 123.80, 122.81, 118.27, 116.26, 113.26, 111.54, 49.06, 41.37, 31.08, 20.11, 15.76, 7.83. HPLC-MS: 6.43 min, 440.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C23H23O2N3ClS: 440.1194. Found: 440.1190.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(4-(2,5-dimethylphenoxy)-2-methylthiazol-5-yl)methanone (31b)The title compound 31b was prepared as a light yellow solid from 30g according to the similar procedure that preparation of 31a from 30e. mp 90°C. 1H-NMR (300 MHz, CDCl3) δ: 6.98–6.96 (3H, m), 6.92 (1H, d, J=6.0 Hz), 6.75 (1H, d, J=6.0 Hz), 6.56 (1H, m), 5.99 (1H, s), 3.94 (2H, t, J=4.2 Hz), 3.39 (2H, t, J=4.2 Hz), 2.57 (3H, s), 2.29 (1H, m), 2.17 (3H, s), 2.01 (3H, s), 0.69 (2H, m), 0.37 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.28, 160.28, 156.60, 153.52, 139.80, 136.27, 130.36, 126.03, 125.87, 124.88, 124.65, 122.86, 118.70, 116.18, 113.27, 110.54, 48.94, 41.77, 31.08, 20.87, 20.13, 15.76, 7.83. HPLC-MS: 6.29 min, 420.3 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C24H26O2N3S: 420.1740. Found: 420.1732.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(4-(2,5-dichloro-4-methylphenoxy)-2-methylthiazol-5-yl)methanone (31c)The title compound 31c was prepared from 30n according to the similar procedure that preparation of 31a from 30e. mp 167°C. 1H-NMR (400 MHz, CDCl3) δ: 7.16 (1H, s), 6.97–6.87 (2H, m), 6.84 (1H, d, J=7.9 Hz), 6.58–6.49 (1H, m), 6.18 (1H, s), 3.95 (2H, t, J=5.5 Hz), 3.48 (2H, t, J=5.5 Hz), 2.58 (3H, s), 2.32 (1H, tt, J=7.1, 3.7 Hz), 2.26 (3H, s), 0.75–0.67 (2H, m), 0.45–0.37 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.52, 159.63, 154.87, 148.87, 139.77, 132.62, 132.29, 131.04, 126.27, 125.62, 122.84, 122.30, 120.58, 115.94, 113.13, 111.57, 48.86, 41.48, 31.07, 20.10, 19.28, 7.92. IR (KBr) cm−1: 1630.98, 1414.40, 1328.77, 1085.80, 741.82. HPLC-MS: 6.89 min, 474.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C23H22O2N3Cl2S: 474.0804. Found: 474.0800.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(4-(2,5-dichloro-4-hydroxyphenoxy)-2-methylthiazol-5-yl)methanone (31d)A flask was charged with 30n (85 mg, 0.16 mmol), PdCl2(dppf)·CH2Cl2 (26 mg, 0.032 mmol), bis(pinacolato)diboron (60 mg, 0.24 mmol) and KAc (31 mg, 0.31 mmol) in 1,4-dioxane under an atmosphere of nitrogen, and then heat at 130°C for 3 h. After completion, the resulting mixture was cooled to r.t. and filtered to remove the suspended solids. The filtrate was concentrated to dryness to give a crude intermediate and that was carried forward crude into the next step. The crude intermediate was redissolved in THF, then cooled to 0°C and H2O2 (357 µL, 3.15 mmol) was added dropwise. After the addition was completed, the reaction mixture was warmed to r.t. and stirring was continued for 3 h. Upon completion, the reaction was quenched with saturated aqueous Na2SO3 and extracted with EtOAc three times. The combined organic phases were washed with brine, dried over MgSO4, filtered and evaporated under reduced pressure. The residue was purified by flash column chromatography to obtain the title compound 31b (45 mg) as a light yellow solid in 60% yield. mp 187°C. 1H-NMR (400 MHz, CDCl3) δ: 7.00–6.89 (3H, m), 6.84 (1H, d, J=7.9 Hz), 6.54 (1H, d dd, J=8.4, 6.8, 1.9 Hz), 6.11 (1H, s), 5.93 (1H, s), 3.95 (2H, t, J=5.5 Hz), 3.48 (2H, t, J=5.5 Hz), 2.59 (3H, s), 2.32 (1H, tt, J=6.8, 3.7 Hz), 0.76–0.67 (2H, m), 0.44–0.37 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 167.01, 159.65, 155.37, 148.68, 143.87, 139.98, 126.35, 125.80, 123.94, 122.81, 120.94, 118.61, 117.07, 115.93, 113.25, 110.64, 48.89, 41.57, 31.01, 19.90, 7.95. HPLC-MS: 5.28 min, 476.1 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C22H20O3N3Cl2S: 476.0597. Found: 476.0601.
4-(5-Chloro-2-nitrophenoxy)-2-methylthiazole-5-carboxylic AcidThe carboxylic acid intermediate shown above was synthesized from 27a with the method described that preparation of 17a from 16a in yield of 93%. 1H-NMR (300 MHz, DMSO) δ: 10.28 (1H, br), 7.91 (1H, d, J=7.2 Hz), 7.15 (1H, d, J=1.2 Hz), 7.02 (1H, dd, J=1.2, 7.2 Hz), 2.54 (3H, s).
(4-(5-Chloro-2-nitrophenoxy)-2-methylthiazol-5-yl)(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (32a)The title compound 32a was obtained as a yellow solid from the intermediates shown above according to the general procedure that preparation of 13c from 12c. mp 159°C. 1H-NMR (300 MHz, CDCl3) δ: 7.83 (1H, d, J=8.7 Hz), 7.10 (1H, dd, J=2.1, 8.7 Hz), 6.93–6.81 (3H, m), 6.55–6.50 (1H, m), 6.29 (1H, s), 3.93 (2H, t, J=5.4 Hz), 3.48 (2H, t, J=5.4 Hz), 2.59 (3H, s), 2.28 (1H, m), 0.71 (2H, m), 0.38 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.92, 158.86, 153.11, 148.47, 139.76, 139.71, 138.51, 126.38, 126.06, 125.27, 124.06, 122.91, 121.36, 115.91, 113.47, 113.00, 48.81, 41.13, 31.16, 20.05, 7.95. IR (KBr) cm−1: 1629.14, 1600.68, 1528.36, 1503.64, 1410.83, 1331.04, 1229.17, 756.33. HPLC-MS: 6.02 min, 471.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C22H20O4N4ClS: 471.0888. Found: 471.0875.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(2-methyl-4-(5-methyl-2-nitrophenoxy)thiazol-5-yl)methanone (32b)The title compound 32b was obtained as a light yellow solid according to the procedure that preparation of the title compound 32a. mp 94°C. 1H-NMR (300 MHz, CDCl3) δ: 7.81 (1H, d, J=6.3 Hz), 6.97–6.88 (4H, m), 6.54 (1H, m), 6.16 (1H, s), 3.95 (2H, t, J=4.2 Hz), 3.47 (2H, t, J=4.2 Hz), 2.56 (3H, s), 2.28 (4H, m), 0.70 (2H, m), 0.38 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.63, 163.28, 159.39, 154.38, 147.97, 145.65, 139.78, 138.10, 127.08, 125.92, 125.21, 124.82, 123.10, 121.75, 115.86, 113.07, 48.84, 41.70, 31.13, 21.45, 20.03, 7.91. HPLC-MS: 5.72 min, 451.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C23H23O4N4S: 451.1435. Found: 451.1430.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(4-(2,5-dichlorophenoxy)-2-ethylthiazol-5-yl)methanone (37a)The title compound 37a was obtained as a light yellow solid according to the procedure that preparation of the title compound 30c. mp 102°C. 1H-NMR (400 MHz, CDCl3) δ: 7.20 (1H, d, J=8.5 Hz), 6.95–6.87 (2H, m), 6.87–6.82 (1H, m), 6.79 (1H, d, J=7.8 Hz), 6.52 (1H, td, J=8.1, 7.6, 1.5 Hz), 6.14 (1H, s), 3.94 (2H, t, J=5.5 Hz), 3.49 (2H, t, J=5.4 Hz), 2.91 (2H, q, J=7.5 Hz), 2.34–2.27 (1H, m), 1.33 (3H, t, J=7.6 Hz), 0.75–0.69 (2H, m), 0.46–0.40 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 173.34, 159.55, 153.98, 150.97, 139.63, 132.40, 130.24, 126.32, 125.33, 124.37, 122.78, 122.23, 119.64, 115.85, 113.06, 112.40, 48.82, 41.26, 31.12, 27.58, 13.45, 7.91. IR (KBr) cm−1: 1632.63, 1475.48, 1414.25, 1329.43, 734.26. HPLC-MS: 7.33 min, 474.1 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C23H22O2N3Cl2S: 474.0804. Found: 474.0797.
(4-(3-Chlorophenoxy)-2-ethylthiazol-5-yl)(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (37b)The title compound 37b was obtained as a light yellow solid according to the procedure that preparation of the title compound 30i. mp 84°C. 1H-NMR (400 MHz, CDCl3) δ: 7.10 (1H, t, J=8.1 Hz), 7.02–6.93 (3H, m), 6.81 (1H, d, J=8.0 Hz), 6.55–6.47 (3H, m), 3.92 (2H, t, J=5.6 Hz), 3.37 (2H, t, J=5.6 Hz), 2.91 (2H, q, J=7.5 Hz), 2.35–2.28 (1H, m), 1.33 (3H, t, J=7.6 Hz), 0.78–0.72 (2H, m), 0.47–0.41 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 173.23, 159.77, 155.85, 154.73, 139.87, 134.35, 129.78, 126.18, 125.81, 123.69, 122.67, 118.47, 116.33, 116.06, 113.20, 112.24, 48.94, 41.55, 31.19, 29.70, 27.58, 13.47, 7.94. HPLC-MS: 7.02 min, 440.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C23H23O2N3ClS: 440.1194. Found: 440.1188.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(4-(2,5-dichlorophenoxy)-2-isopropylthiazol-5-yl)methanone (37c)The title compound 37c was obtained as a light yellow solid according to the procedure that preparation of the title compound 30c. mp 89°C. 1H-NMR (400 MHz, CDCl3) δ: 7.20 (1H, d, J=8.5 Hz), 6.92 (1H, dd, J=8.5, 2.3 Hz), 6.90–6.82 (2H, m), 6.79 (1H, d, J=7.8 Hz), 6.51 (1H, ddd, J=8.1, 7.0, 1.8 Hz), 6.17 (1H, s), 3.95 (2H, t, J=5.4 Hz), 3.49 (2H, t, J=5.5 Hz), 3.17 (1H, p, J=6.9 Hz), 2.34–2.27 (1H, m), 1.33 (6H, d, J=6.9 Hz), 0.76–0.68 (2H, m), 0.48–0.43 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 178.20, 159.69, 153.79, 151.00, 139.58, 132.34, 130.22, 126.26, 125.26, 124.21, 122.76, 122.14, 119.51, 115.84, 113.05, 112.15, 48.79, 41.32, 33.82, 31.16, 29.70, 22.49, 14.13, 7.93. HPLC-MS: 7.74 min, 488.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C24H24O2N3Cl2S: 488.0961. Found: 488.0951.
(4-(3-Chlorophenoxy)-2-isopropylthiazol-5-yl)(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (37d)The title compound 37d was obtained as a light yellow solid according to the procedure that preparation of the title compound 30i. mp 81°C. 1H-NMR (400 MHz, CDCl3) δ: 7.12–7.06 (1H, m), 7.02–6.92 (3H, m), 6.81 (1H, d, J=7.8 Hz), 6.55–6.49 (3H, m), 3.92 (2H, t, J=5.6 Hz), 3.36 (2H, t, J=5.5 Hz), 3.17 (1H, p, J=6.9 Hz), 2.35–2.28 (1H, m), 1.34 (6H, d, J=6.9 Hz), 0.79–0.72 (2H, m), 0.49–0.44 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 178.12, 159.89, 155.93, 154.48, 139.81, 134.30, 129.73, 126.14, 125.73, 123.52, 122.63, 118.33, 116.34, 115.86, 113.19, 112.14, 48.90, 41.64, 33.82, 31.24, 29.70, 22.50, 7.95. HPLC-MS: 7.42 min, 454.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C24H25O2N3ClS: 454.1351. Found: 454.1349.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(4-(2,5-dichlorophenoxy)-2-phenylthiazol-5-yl)methanone (37e)The title compound 37e was obtained as a yellow solid according to the procedure that preparation of the title compound 30c. mp 136°C. 1H-NMR (400 MHz, CDCl3) δ: 7.84–7.78 (2H, m), 7.47–7.37 (3H, m), 7.24 (1H, d, J=8.6 Hz), 6.99–6.81 (4H, m), 6.55–6.47 (1H, m), 6.27 (1H, s), 3.98 (2H, t, J=5.5 Hz), 3.52 (2H, t, J=5.5 Hz), 2.36–2.29 (1H, m), 0.77–0.69 (2H, m), 0.49–0.42 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.87, 159.42, 155.20, 150.94, 139.69, 132.54, 132.37, 131.11, 130.22, 129.01, 126.41, 126.17, 125.38, 124.55, 122.73, 122.53, 120.07, 115.97, 113.10, 112.96, 48.82, 41.25, 31.14, 7.95. IR (KBr) cm−1: 1639.69, 1472.36, 1407.45, 1336.07, 731.61. HPLC-MS: 8.15 min, 522.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C27H22O2N3Cl2S: 522.0804. Found: 522.0800.
(4-(3-Chlorophenoxy)-2-phenylthiazol-5-yl)(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (37f)The title compound 37f was obtained as a yellow solid according to the procedure that preparation of the title compound 30i. mp 123°C. 1H-NMR (400 MHz, CDCl3) δ: 7.87–7.80 (2H, m), 7.47–7.36 (3H, m), 7.12 (1H, t, J=7.9 Hz), 7.06–6.93 (3H, m), 6.86 (1H, d, J=7.9 Hz), 6.59 (2H, d, J=8.4 Hz), 6.56–6.48 (1H, m), 3.97–3.94 (2H, m), 3.40 (2H, t, J=5.6 Hz), 2.38–2.30 (1H, m), 0.81–0.71 (2H, m), 0.51–0.43 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 166.81, 159.60, 155.85, 155.77, 139.92, 134.29, 132.61, 131.04, 129.72, 128.98, 126.28, 126.13, 125.85, 123.80, 122.58, 118.75, 116.44, 116.33, 113.24, 112.95, 48.94, 41.57, 31.22, 7.97. HPLC-MS: 7.05 min, 488.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C27H23O2N3ClS: 488.1194. Found: 488.1186.
(4-Cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)(4-(2,5-dichlorophenoxy)-2-(pyridin-2-yl)thiazol-5-yl)methanone (37g)The title compound 37g was obtained as a yellow solid according to the procedure that preparation of the title compound 30c. mp 169°C. 1H-NMR (400 MHz, CDCl3) δ: 8.60 (1H, d, J=5.0 Hz), 7.90 (1H, d, J=7.9 Hz), 7.72 (1H, t, J=7.9 Hz), 7.40–7.18 (2H, m), 7.03–6.77 (4H, m), 6.49 (1H, t, J=7.5 Hz), 6.21 (1H, s), 3.98 (2H, d, J=5.7 Hz), 3.52 (2H, t, J=5.4 Hz), 2.32 (1H, s), 0.73 (2H, d, J=6.5 Hz), 0.44 (2H, s). 13C-NMR (126 MHz, CDCl3) δ: 167.27, 159.52, 155.16, 150.98, 150.06, 149.55, 139.70, 137.05, 132.38, 130.24, 126.46, 125.31, 125.24, 124.48, 122.88, 122.46, 119.88, 119.79, 115.91, 115.72, 113.09, 48.81, 41.13, 31.12, 7.93. IR (KBr) cm−1: 1627.25, 1471.82, 1398.62, 1230.21, 1075.35, 738.43. HPLC-MS: 7.44 min, 523.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C26H21O2N4Cl2S: 523.0757. Found: 523.0747.
(4-(3-Chlorophenoxy)-2-(pyridin-2-yl)thiazol-5-yl)(4-cyclopropyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone (37h)The title compound 37h was obtained as a yellow solid according to the procedure that preparation of the title compound 30i. mp 142°C. 1H-NMR (400 MHz, CDCl3) δ: 8.60 (1H, dt, J=4.8, 1.3 Hz), 7.95–7.91 (1H, m), 7.72 (1H, td, J=7.8, 1.8 Hz), 7.36–7.30 (1H, m), 7.13 (1H, t, J=8.0 Hz), 7.05–6.93 (3H, m), 6.83 (1H, d, J=7.9 Hz), 6.58 (2H, d, J=10.2 Hz), 6.50 (1H, td, J=8.0, 7.3, 1.9 Hz), 3.96 (2H, t, J=5.6 Hz), 3.40 (2H, t, J=5.6 Hz), 2.38–2.30 (1H, m), 0.79–0.72 (2H, m), 0.49–0.42 (2H, m). 13C-NMR (126 MHz, CDCl3) δ: 167.18, 159.72, 155.86, 150.16, 149.55, 139.93, 137.04, 134.34, 130.01, 129.77, 126.33, 125.70, 125.25, 123.78, 122.75, 119.75, 118.67, 116.37, 116.23, 115.68, 113.23, 48.92, 41.45, 31.21, 7.95. HPLC-MS: 7.26 min, 489.2 (M+H+). HR-MS (ESI): m/z (M+H+) Calcd for C26H22O2N4ClS: 489.1147. Found: 489.1137.
This work was financially supported by Grant from “National Science and Technology Major Project-Key New Drug Creation and Manufacturing program, China” (2012ZX09103101-049) and National Nature Science Foundation of China (Grant 81202571).
The authors declare no conflict of interest.