2016 Volume 64 Issue 5 Pages 512-516
2016 Volume 64 Issue 5 Pages 512-516
Sticking is a failure of pharmaceutical production that occurs when a powder containing a large amount of adhesive is being tableted. This is most frequently observed when long-term tableting is carried out, making it extremely difficult to predict its occurrence during the tablet formula design stage. The efficiency of the pharmaceutical production process could be improved if it were possible to predict whether a particular formulation was likely to stick during tableting. To address this issue, in the present study we prepared tablets composed of blended ibuprofen (Ibu), a highly adhesive drug, and measured the degree of adherence of powder particles to the surface of the tablet punch. We also measured the shear stress of the powder to determine the practical angle of internal friction (Φp) of the powder bed as well as the angle of wall friction (Φw) relative to the punch surface. These values were used to define a sticking index (SI), which showed a high correlation with the amount of Ibu that adhered to the punch during tableting; sticking occurred at SI >0.3. When the amount of lubricant added to the formulation was changed to yield tablets exhibiting different SI values without changing the compounding ratio, sticking did not occur at SI ≤0.3. These results suggest that determining the SI of a pharmaceutical powder before tableting allows prediction of the likelihood of sticking during tableting.
Many forms of dosage are available for pharmaceutical formulations; among the most frequently used are tablets made by compacting powdered or granular ingredients into a shape of a certain size. Tablets are convenient to package, transport, and store, and allows the patient to count the number of unit doses that they take; they are also easy to coat or otherwise treat in order to add functionalities such as bitter taste and odor masking, enteric coating, or sustained release. Tablets are manufactured by automated high-speed tablet presses in order to meet the high demand. This process requires quality assurance to avoid failures such as capping, lamination, or sticking.
Sticking refers to the phenomenon where part of a tablet sticks to the punch surface or detaches, resulting in clouding or an indentation of the surface; it has a major impact on the tablet’s outer appearance, to the extent that it may no longer be possible to read tablet markings such as a logo or product code. Mild sticking is difficult to detect by visual inspection, and with the current automation of tablet presses, tablet production is greatly affected by sticking. Causes of sticking include an insufficient amount of lubricant, an excessive amount of binder, excessive moisture in the powder, or melting due to frictional heat when a low-melting-point drug is being tableted.1–4) Physicochemical factors such as particle size or the crystallinity of the material constituting the tablet formulation5,6); mechanical factors such as the condition of the punch surface or compaction pressure7,8); and environmental factors such as temperature or relative humidity around the machinery can also play a role.9)
Given its potential impact on pharmaceutical production, sticking has been the subject of various studies. For example, the extent of sticking has been examined by evaluating tablet surface roughness,10) and whether or not sticking occurs has been investigated by measuring scraper pressure.11,12) The effectiveness of adding various types of lubricant or fluidizer has also been studied3); it has been shown that using an external lubricant reduces the occurrence of sticking.13) Punch surface treatments have also been considered, and studies have been carried out using punches with hard chrome plating, chromium nitride coating, or that have otherwise been treated.7,14) These studies have provided a basis for understanding why sticking occurs and how it can be counteracted.15,16) However, sticking often occurs when long-term tableting is practiced, and a means of predicting the occurrence of sticking is needed so that it can be avoided.
Sticking can occur when powder particles that form the tablet adhere to the punch surface; as such, evaluating the degree of adherence can provide insight into the underlying mechanism. This can be done by measuring shear stress. The Japanese Pharmacopoeia (16th edition) describes a shear cell method for assessing the fluidity of a powder,17) and Japanese Industrial Standards is currently developing four different shear tests based on different measurement principles.18)
Avoiding sticking by anticipating its occurrence based on powder properties ascertained through small-scale testing prior to tableting can potentially reduce time and minimize production losses. In the present study, ibuprofen (Ibu)—a drug that has a low melting point and is prone to sticking—was selected as a model drug and tableted by direct compression using a rotary tablet press, representing conditions similar to those of the actual manufacturing process. A sticking index (SI) was defined from the measured value of shear stress for the pharmaceutical powder, and the relationship between SI and the amount of Ibu that adhered to the punch surface after tableting was analyzed in order to determine whether the occurrence of sticking can be predicted using the SI.
We used Ibu (Ibuprofen 25; BASF, Ludwigshafen, Germany) as a model drug, lactose monohydrate (Lac) (FlowLac 100; Meggle, Wasserburg, Germany) as an excipient, crystalline cellulose (CC) (Ceolus UF-702; Asahi Kasei Chemicals, Tokyo, Japan) as a binding agent, cornstarch (CS) (Nihon Shokuhin Kako, Tokyo, Japan) as a disintegrant, light anhydrous silicic acid (LS) (Aerosil 200; Nippon Aerosil, Tokyo, Japan) as a fluidizing agent, and magnesium stearate (Mg-St) (Mallinckrodt, Dublin, Ireland) as a lubricant.
Particle Size Distribution and Average Particle Diameter MeasurementsThe particle size distribution for each powder sample was measured using a laser diffraction particle size analyzer (SALD-2200; Shimadzu, Kyoto, Japan) and a cyclone injection-type dry measurement unit (SALD-DS5; Shimadzu) with a stage elevation rate of 10 mm/s, a compressed air pressure of 0.5 MPa, and a refractive index of 1.60–0.10i. Median diameter was determined based on particle size distribution; average particle diameter and geometric standard deviation are listed in Table 1.
| Average particle diameter (µm) | Geometric standard deviation (−) | |
|---|---|---|
| Ibu | 25.51±0.60 | 0.37 |
| Lac | 144.68±2.71 | 0.19 |
| CC | 173.84±2.98 | 0.20 |
| CS | 15.14±0.09 | 0.15 |
| LS | ND* | ND* |
| Mg-St | 3.21±0.25 | 0.30 |
Data represent the mean±S.D. (n=3). CC, crystalline cellulose; CS, cornstarch; Ibu, ibuprofen; Lac, lactose monohydrate; LS, light anhydrous silicic acid; Mg-St, magnesium stearate. *ND, not determined. LS particle size could not be measured due to the small size of the particles.
Table 2 shows the compositions of each of the pharmaceutical powders used in the experiment. In all formulations, Ibu, LS, and Mg-St were first measured; 30% of the remaining material was CC and the rest was Lac : CS=7 : 3. Ibu, Lac, CC, CS, and LS were weighed for a total amount of 300 g, and then gently mixed in a metal bowl while breaking up any agglomerates of Ibu or LS. The powder was fed into a V-type mixer (DV-5; Dalton, Tokyo, Japan) and pre-mixed for 5 min. Mg-St was then added, followed by additional mixing for 5 min.
| (a) Formulations in which Ibu content was altered | |||||
|---|---|---|---|---|---|
| Ibu | 0.0 | 10.0 | 30.0 | 50.0 | 70.0 |
| Lac | 48.5 | 43.6 | 33.8 | 24.0 | 14.2 |
| CC | 29.7 | 26.7 | 20.7 | 14.7 | 8.7 |
| CS | 20.8 | 18.7 | 14.5 | 10.3 | 6.1 |
| LS | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Mg-St | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| (b) Formulations in which Ibu content was kept constant and Mg-St content was altered | |||||
| Ibu | 30.0 | 30.0 | 30.0 | 30.0 | |
| Lac | 33.8 | 33.6 | 33.1 | 32.6 | |
| CC | 20.7 | 20.5 | 20.2 | 19.9 | |
| CS | 14.5 | 14.4 | 14.2 | 14.0 | |
| LS | 0.5 | 0.5 | 0.5 | 0.5 | |
| Mg-St | 0.5 | 1.0 | 2.0 | 3.0 | |
CC, crystalline cellulose; CS, cornstarch; Ibu, ibuprofen; Lac, lactose monohydrate; LS, light anhydrous silicic acid; Mg-St, magnesium stearate.
Tablets were produced using a rotary tablet press (VELA 5; Kikusui Seisakusho, Kyoto, Japan). We used four hard, chrome-plated and specially polished flat punches (PharmaCote HC+; I Holland, Nottingham, U.K.) with a diameter of 8 mm. The settings for the tablet press were as follows: no preload; compaction pressure=10 kN; turntable speed=10 rpm; tablet weight=250 mg/tablet; and tableting time=10 min. The tableting environment had a temperature of 25±2°C and a relative humidity of 35±10%.
Examination of Punch Surfaces after TabletingAfter tableting, the upper punches were removed from the tablet press and their surfaces were photographed with a digital camera.
Quantifying the Ibu Adhering to the Punch SurfaceMethanol-soaked gauze was used to wipe the upper punch surface after tableting and allowed to dry overnight under vacuum. The gauze was immersed in 1 mL methanol and ultrasonically vibrated for 10 min. The resultant solution was centrifuged for 15 min, and the absorbance of the supernatant at 263.4 nm was measured using a UV-visible spectrophotometer (U-1800; Hitachi High-Technologies, Tokyo, Japan). Any deviation from the baseline caused by the gauze used for wiping was corrected for by subtracting from this value the absorbance of unused gauze that was similarly handled. The amount of Ibu adhered to the upper punch surface was determined from a calibration curve.
Measuring the Practical Angle of Internal Friction (Φp) and Angle of Wall Friction (Φw)Various methods of measuring Φp that are much simpler than Jenike’s method19,20) have been proposed, and devices that can complete measurements in a short period of time have been developed.21) Such devices measure shear stress with a constant volume direct shear test, and offer the advantage that after a constant vertical load (σ) has been applied, shear stress (τ) can be directly plotted onto the σ–τ coordinate plane. The regression line obtained from these plots corresponds to the critical state line in Roscoe’s diagram22,23) and can be handled in a manner similar to the effective yield locus obtained by Jenike’s method.21) A shear stress measurement device (NS-V100; Nanoseeds, Aichi, Japan) was used to carry out a constant volume direct shear test. Using a cylindrical shear cell with a diameter of 7 mm, the device conditions were set to a 38.5-mm2 shear area and a 0.2-mm interval between cells of the shearing plane. For measurement of the shear stress of a powder bed, we used a lower operational stage with receiving holes (diameter=7 mm) directly below the shear cells. For measurement of the shear stress of a powder relative to the punch surface, we used a lower operational stage with test pieces (diameter=8 mm)—obtained by cutting the tips of the punches—that were fixed directly below the shear cells and were filled with 250 mg of powder. The measurement environment had a temperature of 25±2°C and a relative humidity of 35±10%. A vertical load of 60 N (1.56 N/mm2) was applied to the powder packed into the shear cells, and after allowing it to relax for 90 s the lower operational stage was moved at a speed of 0.1 mm/s and the shear stress was measured. The vertical load was then changed to 120 N (3.12 N/mm2) and then to 180 N (4.68 N/mm2), and the shear stress was measured in the same manner. The relationship between vertical load (σ) after stress relaxation and shear stress (τ) was plotted onto σ–τ coordinates, and a regression line was drawn by the method of least squares. The angle formed by this regression line and the σ-axis was taken as Φp for the measurement of shearing of the powder bed and as Φw for the measurement of shearing relative to the punch surface.
Defining SI for Assessing Adhesion of Powder to the PunchOne cause of sticking is that when a powder is being compacted, the adhesive force between powder particles of the tablet and the punch surface exceeds the adhesive forces of powder particles within the tablet. Φp represents the frictional force acting between particles in the powder bed, and Φw represents the frictional force between the powder and the punch surface. The larger these values, the higher the friction and adhesion of the powder. We defined SI, which represents the degree of adhesion of a powder to the punch surface, as the value obtained by dividing Φw by Φp according to the following formula.
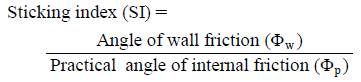 |
Pharmaceutical powders (Table 2a) were compacted by direct compression and the effect of Ibu content on the adhesion of the powder to the punch was assessed. The upper punch surface after 10 min of tableting with a compaction pressure of 10 kN is shown in Fig. 1. For the formulation containing no Ibu (Ibu 0%), no powder adhered to the punch surface after tableting. When the Ibu content was 10%, the punch surface was slightly clouded, and when Ibu content was 30%, powder was detected near the center of the punch. Further increases in Ibu content resulted in progressively thicker layers of powder at the center of the punch. We conjectured that this was due to an unevenness in the distribution of internal pressure inside the tablet during tableting at higher Ibu contents, which caused the middle of the tablet to have a lower compaction pressure than the periphery so that the powder at the center of the tableting surface became laminated and adhered to the punch.

The mean amount of Ibu per punch that adhered to the upper punch surface during tableting was positively correlated with Ibu content of the formulation (Fig. 2). This indicated that the amount of powder adhering to the punch is dependent on the Ibu content of the tablet surface; sticking is likely to have occurred as a result of melting or plastic deformation of the Ibu contacting the punch during tableting. In general, tablets containing a large amount of drug with a low melting point readily undergo plastic deformation and are highly adhesive, and are therefore difficult to compact by direct compression. We found here that sticking mainly occurred when the Ibu content of tablets was ≥30%.

Ibu adhered to the upper punch surface after 10 min of tableting pharmaceutical powders with different Ibu contents and 0.5% Mg-St at a compaction pressure of 10 kN. Data represent the mean±S.D. (n=4).
We obtained values for Φp and Φw from shear tests of the powder bed and of powder to the punch surface, respectively. Figure 3 shows the Φp and Φw values of pharmaceutical powders with different Ibu contents. As Ibu content increased, Φp decreased and approached the Φp value of raw Ibu powder (10.6±1.1°). The other additives in the formulation improved the fluidity of the powder and the rate of filling of shear cells with the powder, resulting in increased friction and adhesion along the shear plane.

Data represent the mean±S.D. (n=3).
Unlike Φp, the Φw value increased with Ibu content and was 7.2±0.2° for the powder consisting of Ibu only. The fact that Φw had a greater value when there was more Ibu in the tablet was presumed to be because applying a vertical load caused the Ibu powder—which readily undergoes plastic deformation—to follow the convexities and concavities of the punch surface, increasing the surface area of contact between the two materials. Since the changes in Φp and Φw showed opposite trends with increases in Ibu content, a direct comparison of their magnitudes does not provide adequate information for describing the adhesion between powder particles and the punch surface.
SI as a Measure of Adhesion of Powder to the Punch SurfaceWe assessed the relationship between SI obtained from shear tests of pharmaceutical powders and the mean amount of Ibu adhering to the punch surface during tableting (Fig. 4). Linear regression by the method of least squares revealed a strong correlation between SI and the mean amount of adhered Ibu. When the regression line was extrapolated to the longitudinal vertical axis, the intercept corresponding to the point at which the mean amount of adhered Ibu was zero had a value of 0.3, implying that for a powder with an SI of ≤0.3, the Ibu does not adhere to the punch during tableting, i.e., sticking will not occur. Formulations for which adhesion of the powder to the punch was observed during tableting had an SI >0.3; however, when Ibu content was 0%, no adhesion was observed and the SI was 0.29. These findings suggest that the SI value obtained from shear testing can be used to predict the occurrence of sticking during tableting.

Data represent the mean±S.D. (n=4 for amount of adhered Ibu; n=9 for SI).
To test the predictive value of SI obtained by measuring the shear stress of pharmaceutical powders, we prepared formulations with increasing amounts of Mg-St (Table 2b) to reduce adhesion to the punch and measured shear stress to obtain SI values (Fig. 5). When Mg-St content was ≥2%, SI was ≤0.3. Pharmaceutical powders with an SI ≤0.3 (dotted line in Fig. 5) were predicted not to adhere to the punch; thus, an Mg-St content of about 1.5% Mg-St would make it possible to avoid sticking.

Data represent the mean±S.D. (n=9).
The state of the punch surface after tableting pharmaceutical powders containing various amounts of Mg-St is shown in Fig. 6. When the Mg-St content was ≤1%, a layer of powder was observed near the center of the punch, and the SI was >0.3. When Mg-St content was ≥2%, the enhanced fluidity of the powder caused it to soar with the rotation of the tablet press, resulting in the electrostatic adherence of powder to the punch surface without firm sticking. Figure 7 shows the mean amount of Ibu that adhered to the upper punch surface during tableting. This amount decreased with increasing Mg-St content of the formulation; the differences were statistically significant when Mg-St content was increased from 0.5% to 2% (p<0.05) and 3% (p<0.01) (t-test). Thus, a small amount of Ibu adhered to the upper punch surface owing to static electricity, when Mg-St content was ≥2%. Thus, pharmaceutical powders with an SI ≤0.3 did not stick during tableting.


Ibu adhered to the upper punch surface after 10 min of tableting pharmaceutical powders with 30% Ibu and different Mg-St amounts at a compaction pressure of 10 kN. Data represent the mean±S.D. (n=4). Differences between groups after adding 0.5% Mg-St and ≥1% Mg-St were assessed by the t-test.
These results demonstrate that shear stress of pharmaceutical powders can be measured and used to calculate SI, which makes it possible to predict the occurrence of sticking during tableting without the need to actually perform tableting. This makes the SI a useful tool for predicting and avoiding the occurrence of sticking.
Shear stress of pharmaceutical powders was measured in order to determine Φp between powder particles and Φw between powder particles and the punch surface. The two values were used to define SI as a measure of the adhesion of powder to the punch surface. SI was strongly correlated with the mean amount of Ibu adhering to the punch surface after tableting, and extrapolation of the regression line showed zero Ibu adherence at an SI of 0.3, suggesting that the occurrence of sticking during tableting can be predicted in advance by determining the SI. Moreover, SI can be used as a basis for avoiding sticking, such as by switching the formulation of the pharmaceutical powder. Therefore, measuring shear stress is a useful means of optimizing formulations and manufacturing conditions of tablets containing drugs that are susceptible to sticking. Future studies should clarify the effects of differences in the conditions of pharmaceutical powders—such as a drug type and mixing time—on SI.
The authors declare no conflict of interest.