Experimental
General Chemical ProcedureMelting points (mp) of the compounds were obtained using Mettler-toledo Excellence MP70 melting-point apparatus. 1H- and 13C-NMR spectra were recorded on a JEOL AL-400 (at 400 and 100 MHz, respectively) by using dimethylsulfoxide (DMSO)-d6 with tetramethylsilane (TMS) as the internal standard. The spin multiplicities are indicated by the following symbols s (singlet), d (doublet), dd (doublet of doublets), t (triplet), dt (doublet of triplets), ddd (doublet of doublet of doublets), ddt (doublet of doublet of triplets), q (quartet), m (multiplet). Chemical shift (δ) is expressed in ppm. LC/MS analyses were performed with Waters 2767 sample manager, 2996 photodiode array detector and 600 controller ZQ. Electrospray ionization (ESI) MS spectra were recorded at 60 eV on a Waters ZQ2000. A Cosmosil 5C18-ARII column (2.0×50 mm, Nacalai Tesque) was employed with a linear gradient of CH3CN containing 0.05% (v/v) trifluoroacetic acid (TFA) at a flow rate of 1 mL/min, and eluting products were detected by UV at 254 nm. The purity of the compounds was determined by HPLC analysis. Reactions were monitored by TLC using E. MERCK silica gel 60 F254 glass plate. Preparative layer chromatography was performed using E. MERCK PLC silica gel 60 F254, 2 mm glass plate. Column chromatography was carried out on FUJI SILYSIA CHEMICAL CHROMATOREX NH DM1020 and/or silica gel. 2′-CMC was purchased from Sigma-Aldrich. The commercial bleach (Oyalox), which was contained 6% sodium hypochlorite, was purchased locally. GC376 was synthesized by a reported procedure.15)
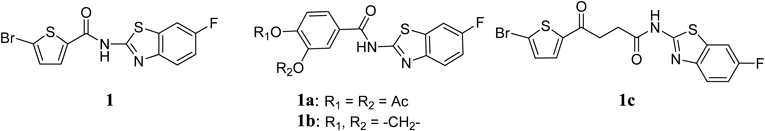
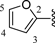
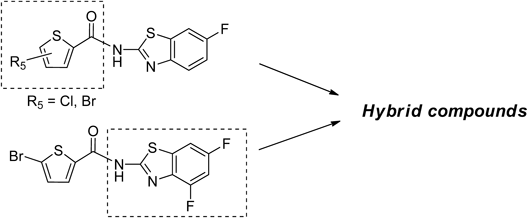
5-Bromo-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (1)A mixture of 5-bromothiophene-2-carboxylic acid (203 mg, 0.98 mmol), 6-fluorobenzo[d]thiazol-2-amine (188 mg, 1.12 mmol), DMAP (133 mg, 1.09 mmol) and EDC·HCl (208 mg, 1.08 mmol) in CH2Cl2 (2 mL) was stirred for 4 h at room temperature (rt). The reaction mixture was concentrated and the residue was washed several times with cold MeOH. The solid was crystallized from MeOH to give 1 (67 mg, 19%) as light yellow powder. mp: 257–258°C. 1H-NMR (DMSO-d6) δ: 7.32 (1H, dt, J=8.8, 2.4 Hz), 7.43 (1H, d, J=4.8 Hz), 7.77 (1H, dd, J=8.8, 4.8 Hz), 7.93 (1H, dd, J=8.8, 2.4 Hz), 8.09 (1H, d, J=3.6 Hz). 13C-NMR (DMSO-d6) δ: 108.3 (d, J=27.3 Hz), 114.4 (d, J=24.8 Hz), 120.1, 121.1, 132.2, 132.3, 132.5 (d, J=9.9 Hz), 138.9, 144.5, 158.7 (d, J=240.6 Hz), 158.8, 159.8. HPLC purity: >99%, ESI-MS m/z: 357 [M+H]+.
N-(6-Fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2a)A mixture of thiophene-2-carboxylic acid (143 mg, 1.12 mmol), 6-fluorobenzo[d]thiazol-2-amine (207 mg, 1.23 mmol), DMAP (155 mg, 1.27 mmol) and EDC·HCl (230 mg, 1.20 mmol) in CH2Cl2 (2 mL) was stirred overnight at rt. The reaction mixture was diluted with CH2Cl2 and washed with 2 N HCl, H2O, and brine. The organic layer was dried over MgSO4 and concentrated. The residue was washed several times with CHCl3 and MeOH to give 2a (126 mg, 40%) as white powder. 1H-NMR (DMSO-d6) δ: 7.28–7.35 (2H, m), 7.79 (1H, dd, J=8.8, 4.8 Hz), 7.94 (1H, dd, J=8.8, 2.4 Hz), 8.03 (1H, d, J=4.8 Hz), 8.32 (1H, d, J=3.2 Hz). 13C-NMR (DMSO-d6) δ: 108.2 (d, J=28.1 Hz), 114.3 (d, J=24.0 Hz), 121.4, 128.7, 131.5, 132.7 (d, J=8.2 Hz), 134.2, 136.9, 145.1, 158.5, 158.7 (d, J=240.6 Hz), 160.6. HPLC purity: >99%, ESI-MS m/z 279 [M+H]+.
5-Chloro-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2b)A mixture of 5-chlorothiophene-2-carboxylic acid (163 mg, 1.00 mmol), 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), DMAP (183 mg, 1.50 mmol) and EDC·HCl (287 mg, 1.50 mmol) in CH2Cl2 (2 mL) was stirred overnight at rt. The reaction mixture was diluted with CH2Cl2 and washed with 2 N HCl, H2O, and brine. The organic layer was dried over MgSO4 and concentrated. The residue was purified by NH-silica-gel column chromatography (eluent: CH2Cl2/MeOH=10 : 1) and the solid was washed several times with CHCl3 and MeOH to give 2b (136 mg, 43%) as white powder. mp: 244–246°C. 1H-NMR (DMSO-d6) δ: 7.30–7.35 (2H, m), 7.77 (1H, dd, J=8.8, 4.8 Hz), 7.93 (1H, dd, J=8.8, 2.4 Hz), 8.15 (1H, s). 13C-NMR (DMSO-d6) δ: 108.3 (d, J=27.3 Hz), 114.4 (d, J=24.8 Hz), 121.3 (d, J=24.8 Hz), 128.8, 131.5, 131.8, 132.6, 136.0, 145.1, 158.7 (d, J=240.5 Hz), 159.0, 159.6. HPLC purity: 98%, ESI-MS m/z 313 [M+H]+.
5-Fluoro-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2c)According to the same procedure used for 2a, starting from 5-fluorothiophene-2-carboxylic acid (146 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2c (76 mg, 26%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 6.97 (1H, dd, J=4.8, 1.6 Hz), 7.32 (1H, dt, J=8.8, 2.4 Hz), 7.77 (1H, dd, J=8.8, 4.8 Hz), 7.92 (1H, dd, J=8.8, 2.4 Hz), 8.07 (1H, s). 13C-NMR (DMSO-d6) δ: 108.3 (d, J=27.3 Hz), 110.9 (d, J=11.5 Hz), 114.3 (d, J=24.0 Hz), 121.1 (d, J=9.1 Hz), 126.5, 129.8 (d, J=4.1 Hz), 132.6 (d, J=8.3 Hz), 144.9, 158.6, 158.7 (d, J=240.6 Hz), 160.3, 169.1 (d, J=293.5 Hz). HPLC purity: >99%, ESI-MS m/z 297 [M+H]+.
5-(tert-Butyl)-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2d)A mixture of 5-(tert-butyl)thiophene-2-carboxylic acid (184 mg, 1.00 mmol), 6-fluorobenzo[d]thiazol-2-amine (186 mg, 1.10 mmol), DMAP (183 mg, 1.50 mmol) and EDC·HCl (287 mg, 1.50 mmol) in CH2Cl2 (2 mL) was stirred overnight at rt. CH2Cl2 and 2 N HCl were added, the precipitate was filtered and washed several times with CH2Cl2 and MeOH to give 2d (195 mg, 58%) as white powder. 1H-NMR (DMSO-d6) δ: 1.39 (9H, s), 7.09 (1H, dd, J=3.6, 0.8 Hz), 7.31 (1H, dt, J=8.8, 2.4 Hz), 7.77 (1H, dd, J=8.8, 4.8 Hz), 7.92 (1H, dd, J=8.8, 2.4 Hz), 8.14 (1H, d, J=3.6 Hz). 13C-NMR (DMSO-d6) δ: 31.8, 34.8, 108.2 (d, J=27.2 Hz), 114.2 (d, J=24.0 Hz), 121.3 (d, J=9.1 Hz), 123.8, 131.6, 132.8 (d, J=9.1 Hz), 133.3, 145.3, 158.7, 158.7 (d, J=239.7 Hz), 160.5, 165.4. HPLC purity: >99%, ESI-MS m/z 335 [M+H]+.
4-Chloro-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2e)According to the same procedure used for 2d, starting from 4-chlorothiophene-2-carboxylic acid (163 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2e (182 mg, 58%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.33 (1H, dt, J=8.8, 2.4 Hz), 7.79 (1H, dd, J=8.8, 4.8 Hz), 7.94 (1H, dd, J=8.8, 2.4 Hz), 8.06 (1H, s), 8.26 (1H, s). 13C-NMR (DMSO-d6) δ: 108.3 (d, J=26.4 Hz), 114.5 (d, J=24.8 Hz), 121.4 (d, J=10.7 Hz), 124.4, 129.1, 130.7, 132.7, 137.5 (d, J=9.1 Hz), 144.9, 158.5, 158.8 (d, J=240.6 Hz), 160.6. HPLC purity: >99%, ESI-MS m/z 313 [M+H]+.
4-Bromo-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2f)A mixture of 4-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol), 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), DMAP (184 mg, 1.51 mmol) and EDC·HCl (290 mg, 1.51 mmol) in CH2Cl2 (2 mL) was stirred overnight at rt. CH2Cl2 and 2 N HCl were added, the precipitate was filtered and washed several times with CHCl3 and MeOH to give 2f (150 mg, 42%) as white powder. 1H-NMR (DMSO-d6) δ: 7.33 (1H, dt, J=8.8, 2.4 Hz), 7.79 (1H, dd, J=8.8, 4.8 Hz), 7.94 (1H, dd, J=8.8, 2.4 Hz), 8.15 (1H, s), 8.30 (1H, s). 13C-NMR (DMSO-d6) δ: 108.3 (d, J=27.2 Hz), 109.6, 114.4 (d, J=24.0 Hz), 121.4 (d, J=11.5 Hz), 131.6, 132.6 (d, J=9.0 Hz), 133.1, 138.2 (d, J=5.8 Hz), 145.0, 158.5, 158.8 (d, J=239.8 Hz), 159.4. HPLC purity: >99%, ESI-MS m/z 357 [M+H]+.
3-Fluoro-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2g)According to the same procedure used for 2b, starting from 3-fluorothiophene-2-carboxylic acid (147 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2g (120 mg, 40%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.19 (1H, d, J=4.8 Hz), 7.33 (1H, dt, J=8.8, 2.4 Hz), 7.70 (1H, s), 7.91 (1H, dd, J=8.8, 2.4 Hz), 7.96 (1H, s). 13C-NMR (DMSO-d6) δ: 108.6 (d, J=26.5 Hz), 114.5 (d, J=24.8 Hz), 116.1, 118.7 (d, J=25.6 Hz), 119.9, 131.7, 131.8, 141.7, 156.6, 157.9 (d, J=271.1 Hz), 158.8 (d, J=241.4 Hz), 160.8. HPLC purity: >99%, ESI-MS m/z 297 [M+H]+.
3-Chloro-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2h)According to the same procedure used for 2a, starting from 3-chlorothiophene-2-carboxylic acid (163 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2h (131 mg, 42%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.25 (1H, d, J=4.8 Hz), 7.33 (1H, dt, J=8.8, 2.4 Hz), 7.66 (1H, s), 7.91 (1H, dd, J=8.8, 2.4 Hz), 7.97 (1H, d, J=4.8 Hz). 13C-NMR (DMSO-d6) δ: 108.8 (d, J=27.3 Hz), 114.6 (d, J=24.8 Hz), 118.9 (d, J=9.0 Hz), 127.7, 128.0, 129.8, 130.0, 131.0, 131.3, 140.4, 158.7 (d, J=239.7 Hz), 161.4. HPLC purity: >99%, ESI-MS m/z 313 [M+H]+.
3-Bromo-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2i)According to the same procedure used for 2a, starting from 3-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2i (41 mg, 11%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.29 (1H, d, J=5.2 Hz), 7.35 (1H, dt, J=8.8, 2.4 Hz), 7.66 (1H, s), 7.91 (1H, dd, J=8.8, 2.4 Hz), 7.95 (1H, d, J=5.2 Hz). 13C-NMR (DMSO-d6) δ: 108.9 (d, J=26.4 Hz), 113.9, 114.6 (d, J=24.8 Hz), 119.2, 130.9, 131.5, 132.0, 132.5, 132.8, 141.7, 158.7 (d, J=239.7 Hz), 163.0. HPLC purity: 99%, ESI-MS m/z 357 [M+H]+.
3,5-Dibromo-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2j)According to the same procedure used for 2a, starting from 3,5-dibromothiophene-2-carboxylic acid (286 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2j (266 mg, 61%) was obtained as light yellow powder. mp: 212–213°C. 1H-NMR (DMSO-d6) δ: 7.34 (1H, dt, J=8.8, 2.4 Hz), 7.49 (1H, s), 7.56 (1H, s), 7.88 (1H, dd, J=8.8, 2.4 Hz). 13C-NMR (DMSO-d6) δ: 109.2 (d, J=28.9 Hz), 113.6, 114.8 (d, J=29.4 Hz), 116.6 (d, J=11.5 Hz), 118.2, 129.6 (d, J=4.1 Hz), 134.6 (d, J=11.6 Hz), 135.4, 135.7, 158.7 (d, J=248.0 Hz), 164.0, 164.6. HPLC purity: >99%, ESI-MS m/z 435 [M+H]+.
3,5-Dichloro-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2k)According to the same procedure used for 2d, starting from 3,5-dichlorothiophene-2-carboxylic acid (197 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2k (192 mg, 55%) was obtained as beige powder. mp: 259–261°C. 1H-NMR (DMSO-d6) δ: 7.29 (1H, dt, J=8.8, 2.4 Hz), 7.73 (1H, dd, J=8.8, 4.8 Hz), 7.85 (1H, dd, J=8.8, 2.4 Hz), 8.17 (1H, s). 13C-NMR (DMSO-d6) δ: 108.4 (dd, J=27.2, 8.0 Hz), 114.5 (dd, J=24.8, 8.0 Hz), 120.7 (dd, J=15.6, 4.1 Hz), 124.1, 130.3, 130.7, 132.3 (d, J=6.6 Hz), 134.6, 135.1 (d, J=4.9 Hz), 142.3, 158.8 (d, J=240.6 Hz), 159.9. HPLC purity: 99%, ESI-MS m/z 347 [M+H]+.
4,5-Dibromo-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2l)According to the same procedure used for 2d, starting from 4,5-dibromothiophene-2-carboxylic acid (287 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2l (321 mg, 74%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.29 (1H, dt, J=8.8, 2.4 Hz), 7.74 (1H, dd, J=8.8, 4.8 Hz), 7.86 (1H, dd, J=8.8, 2.4 Hz), 8.16 (1H, s). 13C-NMR (DMSO-d6) δ: 108.4 (d, J=27.3 Hz), 114.5 (d, J=24.8 Hz), 114.7, 119.3, 121.4, 129.8, 132.3, 133.2, 138.5, 145.7, 158.8 (d, J=236.4 Hz), 159.1. HPLC purity: 96%, ESI-MS m/z 435 [M+H]+.
4,5-Dichloro-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2m)According to the same procedure used for 2d, starting from 4,5-dichlorothiophene-2-carboxylic acid (197 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2m (207 mg, 60%) was obtained as white powder. mp: 260–261°C. 1H-NMR (DMSO-d6) δ: 7.30 (1H, dt, J=8.8, 2.4 Hz), 7.73 (1H, dd, J=8.8, 4.8 Hz), 7.86 (1H, dd, J=8.8, 2.4 Hz), 8.18 (1H, s). 13C-NMR (DMSO-d6) δ: 108.0 (d, J=27.2 Hz), 114.1 (d, J=24.8 Hz), 120.2 (d, J=7.4 Hz), 123.8, 130.1, 130.3, 131.9 (d, J=8.3 Hz), 134.7 (d, J=3.3 Hz), 142.8, 158.6 (d, J=240.6 Hz), 159.5, 159.7. HPLC purity: >99%, ESI-MS m/z 347 [M+H]+.
3,4,5-Trichloro-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (2n)According to the same procedure used for 2d, starting from 3,4,5-trichlorothiophene-2-carboxylic acid (232 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2n (305 mg, 80%) was obtained as white powder. mp: 299–301°C. 1H-NMR (DMSO-d6) δ: 7.31 (1H, dt, J=8.8, 2.4 Hz), 7.55 (1H, dd, J=8.8, 4.8 Hz), 7.82 (1H, dd, J=8.8, 2.4 Hz). 13C-NMR (DMSO-d6) δ: 109.0 (d, J=27.2 Hz), 114.6 (d, J=24.7 Hz), 115.9 (d, J=9.1 Hz), 116.0, 124.1, 125.0, 128.1, 129.1 (d, J=9.0 Hz), 130.7, 143.9, 156.4, 158.6 (d, J=230.6 Hz). HPLC purity: >99%, ESI-MS m/z 381 [M+H]+.
N-(6-Fluorobenzo[d]thiazol-2-yl)furan-2-carboxamide (2o)According to the same procedure used for 2a, starting from furan-2-carboxylic acid (112 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (186 mg, 1.11 mmol), 2o (167 mg, 64%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 6.78 (1H, dd, J=3.6, 1.6 Hz), 7.32 (1H, dt, J=8.8, 2.4 Hz), 7.75 (1H, d, J=3.6 Hz), 7.78 (1H, dd, J=8.8, 4.8 Hz), 7.93 (1H, dd, J=8.8, 2.4 Hz), 8.07 (1H, d, J=1.6 Hz). 13C-NMR (DMSO-d6) δ: 108.2 (d, J=27.2 Hz), 112.4, 114.3 (d, J=24.0 Hz), 117.2, 121.4 (d, J=8.2 Hz), 132.7 (d, J=13.3 Hz), 145.1 (d, J=5.0 Hz), 145.4, 147.7, 156.4, 158.2, 158.8 (d, J=249.7 Hz). HPLC purity: 98%, ESI-MS m/z 263 [M+H]+.
5-Chloro-N-(6-fluorobenzo[d]thiazol-2-yl)furan-2-carboxamide (2p)According to the same procedure used for 2a, starting from 5-chlorofuran-2-carboxylic acid (147 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2p (97 mg, 33%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 6.82–6.84 (1H, m), 7.32 (1H, ddt, J=8.8, 1.6 Hz), 7.76–7.79 (2H, m), 7.93 (1H, dt, J=8.8, 2.4 Hz). 13C-NMR (DMSO-d6) δ: 108.2 (d, J=27.3 Hz), 109.9, 114.3 (d, J=24.8 Hz), 119.3, 121.3 (d, J=9.5 Hz), 132.6, 132.7, 140.0, 145.1, 155.6, 158.3, 158.7 (d, J=239.8 Hz). HPLC purity: >99%, ESI-MS m/z 297 [M+H]+.
5-Bromo-N-(6-fluorobenzo[d]thiazol-2-yl)furan-2-carboxamide (2q)According to the same procedure used for 2a, starting from 5-bromofuran-2-carboxylic acid (191 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2q (78 mg, 23%) was obtained as beige powder. 1H-NMR (DMSO-d6) δ: 6.92 (1H, d, J=4.0 Hz), 7.32 (1H, dt, J=8.8, 2.4 Hz), 7.79–7.75 (2H, m), 7.93 (1H, dd, J=8.8, 2.4 Hz). 13C-NMR (DMSO-d6) δ: 108.2 (d, J=27.3 Hz), 114.4 (d, J=24.8 Hz), 114.6, 119.4, 121.4 (d, J=8.3 Hz), 127.8, 132.7 (d, J=8.3 Hz), 145.4, 147.3, 155.5, 158.2, 158.7 (d, J=239.8 Hz). HPLC purity: 97%, ESI-MS m/z 341 [M+H]+.
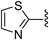
N-(6-Fluorobenzo[d]thiazol-2-yl)thiazole-2-carboxamide (2r)According to the same procedure used for 2a, starting from thiazole-2-carboxylic acid (129 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (186 mg, 1.11 mmol), 2r (46 mg, 17%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.34 (1H, dt, J=8.8, 2.4 Hz), 7.82 (1H, dd, J=8.8, 4.8 Hz), 7.96 (1H, dd, J=8.8, 2.4 Hz), 8.19 (1H, d, J=2.8 Hz), 8.25 (1H, d, J=2.8 Hz). 13C-NMR (DMSO-d6) δ: 108.3 (d, J=27.3 Hz), 114.5 (d, J=24.8 Hz), 121.6, 127.8, 132.7 (d, J=8.3 Hz), 132.9, 144.7, 157.9, 158.9 (d, J=240.5 Hz), 159.3, 160.9. HPLC purity: >99%, ESI-MS m/z 280 [M+H]+.
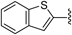
N-(6-Fluorobenzo[d]thiazol-2-yl)benzo[b]thiophene-2-carboxamide (2s)According to the same procedure used for 2a, starting from benzo[b]thiophene-2-carboxylic acid (179 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (186 mg, 1.11 mmol), 2s (139 mg, 42%) was obtained as white powder. mp: 235–236°C. 1H-NMR (DMSO-d6) δ: 7.34 (1H, dt, J=8.8, 2.4 Hz), 7.48–7.57 (2H, m), 7.81 (1H, dd, J=8.8, 4.8 Hz), 7.95 (1H, dd, J=8.8, 2.4 Hz), 8.03 (1H, d, J=7.2 Hz), 8.10 (1H, d, J=8.8 Hz), 8.64 (1H, s). 13C-NMR (DMSO-d6) δ: 108.3 (d, J=26.4 Hz), 114.4 (d, J=24.8 Hz), 121.3 (d, J=10.7 Hz), 122.9, 125.3, 126.0, 127.2, 128.5, 132.6 (d, J=9.1 Hz), 137.1 (d, J=4.9 Hz), 139.0, 141.1, 144.9, 158.7 (d, J=239.8 Hz), 158.8, 161.6. HPLC purity: >99%, ESI-MS m/z 329 [M+H]+.
2-Bromo-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-3-carboxamide (2t)A mixture of 2-bromothiophene-3-carboxylic acid (207 mg, 1.10 mmol), 6-fluorobenzo[d]thiazol-2-amine (186 mg, 1.11 mmol), DMAP (183 mg, 1.50 mmol) and EDC·HCl (288 mg, 1.50 mmol) in CH2Cl2 (2 mL) was stirred overnight at rt. The reaction mixture was diluted with CH2Cl2 and washed with 2 N HCl, H2O, and brine. The organic layer was dried over MgSO4 and concentrated. The residue was washed several times with CH2Cl2 and MeOH to give 2t (72 mg, 20%) as white powder. 1H-NMR (DMSO-d6) δ: 7.33 (1H, ddt, J=8.8, 2.4, 0.8 Hz), 7.61 (1H, dd, J=6.0, 1.2 Hz), 7.75 (1H, dd, J=6.0, 1.2 Hz), 7.80 (1H, dd, J=8.8, 4.8 Hz), 7.94 (1H, dd, J=8.8, 2.4 Hz). 13C-NMR (DMSO-d6) δ: 108.2 (d, J=27.2 Hz), 114.4 (d, J=24.8 Hz), 117.5, 121.6, 128.0, 128.1, 132.7 (d, J=10.7 Hz), 133.0, 145.1 (d, J=10.7 Hz), 158.2, 158.8 (d, J=239.8 Hz), 161.3. HPLC purity: >99%, ESI-MS m/z 357 [M+H]+.
4-Bromo-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-3-carboxamide (2u)According to the same procedure used for 2t, starting from 4-bromothiophene-3-carboxylic acid (207 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2u (134 mg, 37%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.33 (1H, dt, J=8.8, 2.4 Hz), 7.80 (1H, dd, J=8.8, 4.8 Hz), 7.86 (1H, dd, J=3.6, 1.2 Hz), 7.94 (1H, dd, J=8.8, 2.4 Hz), 8.48 (1H, dd, J=3.6, 1.2 Hz). 13C-NMR (DMSO-d6) δ: 108.2 (d, J=26.4 Hz), 109.0, 114.3 (d, J=24.8 Hz), 121.6 (d, J=9.1 Hz), 126.5, 132.7 (d, J=10.7 Hz), 132.8, 132.9, 145.1, 158.2, 158.7 (d, J=239.7 Hz), 161.2. HPLC purity: >99%, ESI-MS m/z 357 [M+H]+.
5-Bromo-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-3-carboxamide (2v)According to the same procedure used for 2d, starting from 5-bromothiophene-3-carboxylic acid (207 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (186 mg, 1.11 mmol), 2v (278 mg, 78%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.32 (1H, dt, J=8.8, 2.4 Hz), 7.79 (1H, dd, J=8.8, 4.8 Hz), 7.87 (1h, d, J=1.6 Hz), 7.93 (1H, dd, J=8.8, 2.4 Hz), 8.64 (1H, d, J=1.6 Hz). 13C-NMR (DMSO-d6) δ: 108.2 (d, J=27.2 Hz), 112.8, 114.3 (d, J=24.8 Hz), 121.5 (d, J=9.1 Hz), 129.8, 132.8 (d, J=11.5 Hz), 134.6, 135.1, 145.2, 158.4, 158.7 (d, J=239.8 Hz), 159.9. HPLC purity: >99%, ESI-MS m/z 357 [M+H]+.
2,5-Dibromo-N-(6-fluorobenzo[d]thiazol-2-yl)thiophene-3-carboxamide (2w)According to the same procedure used for 2d, starting from 2,5-dibromothiophene-3-carboxylic acid (286 mg, 1.00 mmol) and 6-fluorobenzo[d]thiazol-2-amine (185 mg, 1.10 mmol), 2w (313 mg, 72%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.33 (1H, dt, J=8.8, 2.4 Hz), 7.78–7.82 (2H, m), 7.94 (1H, dd, J=8.8, 2.4 Hz). 13C-NMR (DMSO-d6) δ: 108.2 (d, J=27.2 Hz), 111.3, 114.4 (d, J=24.8 Hz), 117.0, 121.6 (d, J=9.8 Hz), 131.1, 132.7 (d, J=11.5 Hz), 134.2, 144.7, 158.1, 158.8 (d, J=239.7 Hz), 160.2. HPLC purity: >99%, ESI-MS m/z 435 [M+H]+.
N-(Benzo[d]thiazol-2-yl)-5-bromothiophene-2-carboxamide (3a)A mixture of 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol), benzo[d]thiazol-2-amine (166 mg, 1.10 mmol), DMAP (183 mg, 1.50 mmol) and EDC·HCl (288 mg, 1.50 mmol) in CH2Cl2 (2 mL) was stirred overnight at rt. CH2Cl2 and 2 N HCl were added, the precipitate was filtered and washed several times with CH2Cl2 and MeOH. The residue was purified by NH-silica-gel column chromatography (eluent: CH2Cl2/MeOH=4 : 1 to 10 : 3) to give 3a (106 mg, 31%) as white powder. 1H-NMR (DMSO-d6) δ: 7.34 (1H, dt, J=8.4, 1.6 Hz), 7.43 (1H, d, J=3.6 Hz), 7.47 (1H, dt, J=8.4, 1.6 Hz), 7.74 (1H, d, J=8.0 Hz), 8.00 (1H, d, J=8.0 Hz), 8.06 (1H, s). 13C-NMR (DMSO-d6) δ: 119.9, 121.9, 123.7, 126.4, 130.8, 131.2, 132.1, 132.2, 139.5, 152.3, 160.3, 160.7. HPLC purity: >99%, ESI-MS m/z 339 [M+H]+.
5-Bromo-N-(6-chlorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3b)According to the same procedure used for 1, starting from 5-bromothiophene-2-carboxylic acid (205 mg, 0.99 mmol) and 6-chlorobenzo[d]thiazol-2-amine (202 mg, 1.10 mmol), 3b (143 mg, 38%) was obtained as light yellow powder. mp: 254–256°C. 1H-NMR (DMSO-d6) δ: 7.43 (1H, d, J=3.6 Hz), 7.48 (1H, dd, J=8.4, 1.6 Hz), 7.74 (1H, d, J=8.4 Hz), 8.09 (1H, d, J=3.6 Hz), 8.15 (1H, d, J=1.6 Hz). 13C-NMR (DMSO-d6) δ: 120.3, 121.1, 121.5, 126.6, 127.8, 132.3, 132.4, 132.9, 138.9, 150.4, 158.4, 159.9. HPLC purity: >99%, ESI-MS m/z 373 [M+H]+.
5-Bromo-N-(6-bromobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3c)According to the same procedure used for 2a, starting from 5-bromothiophene-2-carboxylic acid (209 mg, 1.01 mmol) and 6-bromobenzo[d]thiazol-2-amine (256 mg, 1.10 mmol), 3c (40 mg, 10%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.43 (1H, d, J=3.6 Hz), 7.60 (1H, dd, J=8.4, 1.6 Hz), 7.69 (1H, d, J=8.0 Hz), 8.09 (1H, s), 8.28 (1H, d, J=1.6 Hz). 13C-NMR (DMSO-d6) δ: 115.7, 120.3, 121.6, 121.8, 124.3, 129.3, 129.6, 132.3, 133.4, 138.9, 147.8, 159.8. HPLC purity: 97%, ESI-MS m/z 417 [M+H]+.
5-Bromo-N-(5-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3d)According to the same procedure used for 2a, starting from 5-bromothiophene-2-carboxylic acid (206 mg, 1.00 mmol) and 5-fluorobenzo[d]thiazol-2-amine (191 mg, 1.10 mmol), 3d (30 mg, 8%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.23 (1H, dt, J=8.8, 2.4 Hz), 7.44 (1H, d, J=3.6 Hz), 7.59 (1H, d, J=9.6 Hz), 8.04 (1H, dd, J=8.8, 5.6 Hz), 8.11 (1H, s). 13C-NMR (DMSO-d6) δ: 106.3, 111.8 (d, J=24.0 Hz), 120.3, 123.2 (d, J=9.9 Hz), 127.1 (d, J=4.1 Hz), 132.3, 132.6, 138.9 (d, J=6.6 Hz), 148.7, 159.4, 161.0, 161.4 (d, J=239.7 Hz). HPLC purity: >99%, ESI-MS m/z 357 [M+H]+.
5-Bromo-N-(5-chlorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3e)According to the same procedure used for 2d, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 5-chlorobenzo[d]thiazol-2-amine (203 mg, 1.10 mmol), 3e (214 mg, 57%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.37 (1H, dd, J=8.8, 1.6 Hz), 7.43 (1H, d, J=3.6 Hz), 7.81 (1H, s), 8.04 (1H, d, J=8.8 Hz), 8.10 (1H, s). 13C-NMR (DMSO-d6) δ: 119.5, 120.3, 123.4, 123.7, 130.1, 130.9, 132.2, 132.3, 138.8, 149.0, 159.8, 160.8. HPLC purity: >99%, ESI-MS m/z 373 [M+H]+.
5-Bromo-N-(5-bromobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3f)According to the same procedure used for 2f, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 5-bromobenzo[d]thiazol-2-amine (252 mg, 1.10 mmol), 3f (220 mg, 53%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.43 (1H, d, J=3.6 Hz), 7.49 (1H, ddd, J=8.4, 1.6, 0.8 Hz), 7.95 (1H, s), 7.98 (1H, d, J=8.4 Hz), 8.10 (1H, d, J=3.6 Hz). 13C-NMR (DMSO-d6) δ: 119.0, 120.4, 122.4, 123.7, 126.4, 130.5, 132.3, 132.4, 138.8, 149.4, 159.9, 160.3. HPLC purity: >99%, ESI-MS m/z 417 [M+H]+.
5-Bromo-N-(4-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3g)According to the same procedure used for 2a, starting from 5-bromothiophene-2-carboxylic acid (212 mg, 1.02 mmol) and 4-fluorobenzo[d]thiazol-2-amine (186 mg, 1.11 mmol), 3g (52.4 mg, 15%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.39 (1H, d, J=3.6 Hz), 7.53 (1H, m), 7.73 (1H, dd, J=8.4, 1.6 Hz), 7.78 (1H, t, J=8.4 Hz), 7.88 (1H, d, J=3.6 Hz), 10.42 (1H, s). 13C-NMR (DMSO-d6) δ: 111.3, 118.3, 118.5 (d, J=2.5 Hz), 122.2 (d, J=8.3 Hz), 126.6 (d, J=12.4 Hz), 126.9 (d, J=4.2 Hz), 128.0, 130.8, 131.9, 140.3, 155.1 (d, J=252.1 Hz), 158.9. HPLC purity: >99%, ESI-MS m/z 357 [M+H]+.
5-Bromo-N-(4-chlorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3h)According to the same procedure used for 2d, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 4-chlorobenzo[d]thiazol-2-amine (204 mg, 1.10 mmol), 3h (132 mg, 35%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.33 (1H, dt, J=8.4, 0.4 Hz), 7.44 (1H, dd, J=4.0, 0.4 Hz), 7.55 (1H, d, J=8.0 Hz), 8.00 (1H, d, J=8.0 Hz), 8.21 (1H, d, J=4.0 Hz). 13C-NMR (DMSO-d6) δ: 120.6, 120.8, 124.4, 124.6, 129.3, 132.4, 132.6, 133.3, 138.4, 145.4, 159.3, 159.4. HPLC purity: >99%, ESI-MS m/z 373 [M+H]+.
5-Bromo-N-(4-bromobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3i)According to the same procedure used for 2t, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 4-bromobenzo[d]thiazol-2-amine (253 mg, 1.10 mmol), 3i (257 mg, 61%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.26 (1H, t, J=8.4 Hz), 7.44 (1H, d, J=4.0 Hz), 7.70 (1H, d, J=8.0 Hz), 8.03 (1H, d, J=8.0 Hz), 8.22 (1H, d, J=4.0 Hz). 13C-NMR (DMSO-d6) δ: 113.6, 120.6, 121.3, 125.0, 129.4, 132.4, 132.6, 132.7, 138.4, 146.7, 159.1, 159.5. HPLC purity: >99%, ESI-MS m/z 417 [M+H]+.
5-Bromo-N-(4,6-difluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3j)According to the same procedure used for 2a, starting from 5-bromothiophene-2-carboxylic acid (212 mg, 1.02 mmol) and 4,6-difluorobenzo[d]thiazol-2-amine (205 mg, 1.11 mmol), 3j (95 mg, 25%) was obtained as white powder. mp: 252–254°C. 1H-NMR (DMSO-d6) δ: 7.31 (1H, dt, J=10.4, 2.4 Hz), 7.40 (1H, d, J=4.0 Hz), 7.76 (1H, dd, J=8.4, 1.6 Hz), 8.11 (1H, d, J=4.0 Hz). 13C-NMR (DMSO-d6) δ: 101.6 (dd, J=28.9, 22.4 Hz), 104.0 (dd, J=26.5, 4.2 Hz), 120.0 (d, J=5.8 Hz), 131.9, 132.2, 133.6 (d, J=8.2 Hz), 134.7 (dd, J=11.9, 6.6 Hz), 138.1 (d, J=9.9 Hz), 153.1 (dd, J=254.2, 12.8 Hz), 157.9 (dd, J=243.2, 10.7 Hz), 158.4, 158.5. HPLC purity: >99%, ESI-MS m/z 375 [M+H]+.
5-Bromo-N-(4,6-dichlorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3k)According to the same procedure used for 2d, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 4,6-dichlorobenzo[d]thiazol-2-amine (241 mg, 1.10 mmol), 3k (276 mg, 68%) was obtained as light yellow powder. 1H-NMR (DMSO-d6) δ: 7.44 (1H, d, J=4.0 Hz), 7.67 (1H, d, J=1.6 Hz), 8.16 (1H, d, J=1.6 Hz), 8.19 (1H, d, J=4.0 Hz). 13C-NMR (DMSO-d6) δ: 120.6, 120.8, 125.0, 126.1, 127.7, 132.4, 132.7, 134.3, 138.3, 144.6, 159.6, 160.2. HPLC purity: >99%, ESI-MS m/z 407 [M+H]+.
5-Bromo-N-(4-bromo-6-fluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3l)According to the same procedure used for 3a, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 4-bromo-6-fluoro benzo[d]thiazol-2-amine (272 mg, 1.10 mmol), 3l (303 mg, 69%) was obtained as light yellow powder. mp: 231°C. 1H-NMR (DMSO-d6) δ: 7.44 (1H, d, J=4.0 Hz), 7.69 (1H, dd, J=8.8, 2.4 Hz), 8.00 (1H, dd, J=8.8, 2.4 Hz), 8.20 (1H, d, J=4.0 Hz), 8.32 (1H, s). 13C-NMR (DMSO-d6) δ: 107.9 (d, J=26.4 Hz), 113.5 (d, J=10.7 Hz), 117.7 (d, J=28.1 Hz), 120.6, 132.3, 132.6, 133.2 (d, J=11.5 Hz), 138.3, 143.8, 158.0 (d, J=243.9 Hz), 159.0, 159.5. HPLC purity: >99%, ESI-MS m/z 435 [M+H]+.
5-Bromo-N-(5,6-difluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3m)According to the same procedure used for 2d, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.02 mmol) and 5,6-difluorobenzo[d]thiazol-2-amine (205 mg, 1.11 mmol), 3m (210 mg, 56%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.43 (1H, dd, J=4.0, 1.6 Hz), 7.84 (1H, dd, J=11.2, 7.2 Hz), 8.11 (1H, d, J=4.0 Hz), 8.16 (1H, t, J=8.0 Hz). 13C-NMR (DMSO-d6) δ: 108.3 (d, J=13.3 Hz), 110.0 (dd, J=23.0, 5.8 Hz), 120.5, 127.2 (d, J=6.6 Hz), 132.2, 132.3, 132.4, 138.5, 147.0 (dd, J=242.7, 14.5 Hz), 149.2 (dd, J=242.7, 14.5 Hz), 159.4, 160.3. HPLC purity: >99%, ESI-MS m/z 375 [M+H]+.
5-Bromo-N-(6-methylbenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3n)According to the same procedure used for 3a, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 6-methylbenzo[d]thiazol-2-amine (180 mg, 1.10 mmol), 3n (92 mg, 26%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 2.42 (3H, s), 7.28 (1H, d, J=8.0 Hz), 7.41 (1H, d, J=4.0 Hz), 7.62 (1H, d, J=8.0 Hz), 7.78 (1H, s), 8.04 (1H, s). 13C-NMR (DMSO-d6) δ: 21.0, 119.3, 119.7, 121.5, 127.6, 131.1, 131.9, 132.2, 133.2, 139.6, 139.7, 159.4, 162.6. HPLC purity: >99%, ESI-MS m/z 353 [M+H]+.
5-Bromo-N-(4-methylbenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3o)According to the same procedure used for 2a, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 4-methylbenzo[d]thiazol-2-amine (181 mg, 1.10 mmol), 3o (159 mg, 45%) was obtained as light yellow powder. 1H-NMR (DMSO-d6) δ: 2.62 (3H, s), 7.21–7.29 (2H, m), 7.43 (1H, d, J=4.0 Hz), 7.81 (1H, d, J=8.0 Hz), 8.17 (1H, s). 13C-NMR (DMSO-d6) δ: 18.1, 119.1, 120.2, 123.7, 126.8, 129.9, 131.3, 132.1, 132.3, 138.8, 147.7, 157.3, 159.2. HPLC purity: >99%, ESI-MS m/z 353 [M+H]+.
5-Bromo-N-(5,6-dimethylbenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3p)According to the same procedure used for 3a, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 5,6-dimethylbenzo[d]thiazol-2-amine (196 mg, 1.10 mmol), 3p (121 mg, 33%) was obtained as beige powder. 1H-NMR (DMSO-d6) δ: 2.32 (3H, s), 2.33 (3H, s), 7.41 (1H, d, J=4.0 Hz), 7.52 (1H, s), 7.72 (1H, s), 8.02 (1H, s). 13C-NMR (DMSO-d6) δ: 19.5, 19.7, 119.6, 121.7, 128.0, 128.2, 131.8, 132.2, 132.6, 135.1, 139.2, 139.7, 159.5, 160.2. HPLC purity: >99%, ESI-MS m/z 367 [M+H]+.
5-Bromo-N-(6-methoxybenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3q)According to the same procedure used for 1, starting from 5-bromothiophene-2-carboxylic acid (208 mg, 1.01 mmol) and 6-methoxylbenzo[d]thiazol-2-amine (205 mg, 1.14 mmol), 3q (143 mg, 39%) was obtained as light yellow powder. 1H-NMR (DMSO-d6) δ: 3.82 (3H, s), 7.06 (1H, dd, J=8.8, 2.4 Hz), 7.42 (1H, d, J=4.0 Hz), 7.60 (1H, d, J=2.4 Hz), 7.64 (1H, d, J=8.8 Hz), 8.06 (1H, s). 13C-NMR (DMSO-d6) δ: 55.6, 104.9, 115.1, 119.8, 120.4, 120.7, 131.9, 132.2, 132.4, 139.4, 156.3, 156.7, 160.9. HPLC purity: >99%, ESI-MS m/z 369 [M+H]+.
5-Bromo-N-(4-methoxybenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3r)According to the same procedure used for 3a, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 4-methoxylbenzo[d]thiazol-2-amine (198 mg, 1.10 mmol), 3r (170 mg, 46%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 3.93 (3H, s), 7.02 (1H, d, J=8.0 Hz), 7.29 (1H, t, J=8.0 Hz), 7.43 (1H, d, J=4.0 Hz), 7.55 (1H, d, J=8.0 Hz), 8.12 (1H, s). 13C-NMR (DMSO-d6) δ: 55.7, 107.6, 113.4, 120.1, 124.8, 132.0, 132.3, 132.9, 138.4, 138.8, 151.9, 156.6, 159.1. HPLC purity: >99%, ESI-MS m/z 369 [M+H]+.
5-Bromo-N-(6-ethoxybenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3s)According to the same procedure used for 2b, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 6-ethoxylbenzo[d]thiazol-2-amine (214 mg, 1.10 mmol), 3s (163 mg, 43%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 1.36 (3H, t, J=2.8 Hz), 4.08 (2H, q, J=2.8 Hz), 7.05 (1H, ddd, J=8.4, 2.4, 0.8 Hz), 7.42 (1H, d, J=4.0 Hz), 7.58 (1H, d, J=2.4 Hz), 7.63 (1H, d, J=8.4 Hz), 8.06 (1H, s). 13C-NMR (DMSO-d6) δ: 14.7, 63.6, 105.5, 115.5, 119.8, 120.6, 131.9, 132.2, 132.5, 139.4, 142.4, 155.5, 156.7, 159.5. HPLC purity: >99%, ESI-MS m/z 383 [M+H]+.
5-Bromo-N-(6-nitrobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (3t)According to the same procedure used for 2d, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 6-nitrobenzo[d]thiazol-2-amine (215 mg, 1.10 mmol), 3t (339 mg, 88%) was obtained as yellow powder. 1H-NMR (DMSO-d6) δ: 7.40 (1H, d, J=4.0 Hz), 7.88 (1H, d, J=8.8 Hz), 8.09 (1H, d, J=4.0 Hz), 8.28 (1H, dd, J=8.8, 2.4 Hz), 8.99 (1H, d, J=2.4 Hz). 13C-NMR (DMSO-d6) δ: 118.6, 119.6, 120.2, 121.5, 131.7, 131.9, 132.5, 138.3, 143.0, 152.1, 160.1, 164.0. HPLC purity: 96%, ESI-MS m/z 382 [M−H]−.
5-Bromo-N-(6-(trifluoromethyl)benzo[d]thiazol-2-yl)thiophene-2-carboxamide (3u)A mixture of 5-bromothiophene-2-carboxylic acid (208 mg, 1.00 mmol), 6-(trifluoromethyl)benzo[d]thiazol-2-amine (241 mg, 1.10 mmol), DMAP (184 mg, 1.50 mmol) and EDC·HCl (288 mg, 1.50 mmol) in CH2Cl2 (2 mL) was stirred overnight at rt. CH2Cl2 and 2 N HCl were added, the precipitate was washed several times with CH2Cl2 and MeOH. The residue was purified by preparative layer chromatography (eluent: CHCl3/MeOH=30 : 1) to give 3u (184 mg, 70%) as white powder. 1H-NMR (DMSO-d6) δ: 7.44 (1H, d, J=4.0 Hz), 7.77 (1H, dd, J=8.0, 1.6 Hz), 7.92 (1H, d, J=8.0 Hz), 8.12 (1H, s), 8.51 (1H, s). 13C-NMR (DMSO-d6) δ: 120.3 (d, J=4.1 Hz), 120.4, 120.6, 123.2 (d, J=4.1 Hz), 123.9 (q, J=32.2 Hz), 125.9, 128.6, 131.9, 132.4, 132.5, 132.7, 138.7, 165.2. HPLC purity: >99%, ESI-MS m/z 407 [M+H]+.
5-Bromo-N-(6-(trifluoromethoxy)benzo[d]thiazol-2-yl)thiophene-2-carboxamide (3v)According to the same procedure used for 2a, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 6-(trifluoromethoxyl)benzo[d]thiazol-2-amine (258 mg, 1.10 mmol), 3v (22 mg, 5%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 7.43–7.47 (2H, m), 7.84 (1H, dt, J=8.8 Hz), 8.11 (1H, d, J=3.2 Hz), 8.15 (1H, d, J=2.4 Hz). 13C-NMR (DMSO-d6) δ: 115.1, 120.0, 120.2 (q, J=256.2 Hz), 120.3, 121.1, 132.3, 132.4, 132.5 (d, J=5.7 Hz), 138.8 (d, J=6.6 Hz), 144.2, 144.9, 160.0, 160.3. HPLC purity: >99%, ESI-MS m/z 423 [M+H]+.
Ethyl 2-(5-Bromothiophene-2-carboxamido)benzo[d]thiazole-6-carboxylate (3w)According to the same procedure used for 2a, starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and ethyl 2-aminobenzo[d]thiazole-6-carboxylate (244 mg, 1.10 mmol), 3w (184 mg, 45%) was obtained as white powder. 1H-NMR (DMSO-d6) δ: 1.36 (3H, t, J=3.2 Hz), 4.35 (2H, q, J=3.2 Hz), 7.43 (1H, d, J=4.0 Hz), 7.82 (1H, d, J=8.0 Hz), 8.03 (1H, dd, J=8.0, 1.6 Hz), 8.10 (1H, s), 8.65 (1H, s). 13C-NMR (DMSO-d6) δ: 14.2, 60.7, 119.7, 120.4, 120.5, 123.9, 125.0, 127.2, 131.3, 132.3, 132.4, 138.7, 138.8, 159.8, 165.4. HPLC purity: >99%, ESI-MS m/z 411 [M+H]+.
5-Bromo-N-(6-(tert-butyl)benzo[d]thiazol-2-yl)thiophene-2-carboxamide (3x)According to the same procedure used for 3u (eluent : CHCl3/MeOH=40 : 1), starting from 5-bromothiophene-2-carboxylic acid (207 mg, 1.00 mmol) and 6-(tert-butyl)benzo[d]thiazol-2-amine (227 mg, 1.10 mmol), 3x (225 mg, 57%) was obtained as light yellow powder. 1H-NMR (DMSO-d6) δ: 1.35 (9H, s), 7.42 (1H, d, J=3.6 Hz), 7.52 (1H, d, J=8.8 Hz), 7.64 (1H, s), 8.00 (1H, s), 8.05 (1H, s). 13C-NMR (DMSO-d6) δ: 31.4, 34.7, 118.0, 119.1, 119.8, 124.1, 131.0, 131.9, 132.2, 139.5, 146.7, 147.0, 159.7, 160.4. HPLC purity: 97%, ESI-MS m/z 395 [M+H]+.
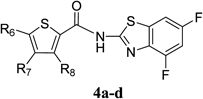
5-Chloro-N-(4,6-difluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (4a)According to the same procedure used for 2f, starting from 5-chlorothiophene-2-carboxylic acid (163 mg, 1.00 mmol) and 4,6-difluorobenzo[d]thiazol-2-amine (205 mg, 1.10 mmol), 4a (192 mg, 58%) was obtained as white powder. mp: 247–249°C. 1H-NMR (DMSO-d6) δ: 7.35 (1H, d, J=4.0 Hz), 7.40 (1H, dt, J=10.2, 2.4 Hz), 7.83 (1H, dd, J=8.4, 2.4 Hz), 8.21 (1H, d, J=4.0 Hz). 13C-NMR (DMSO-d6) δ: 102.4 (dd, J=28.9, 22.3 Hz), 104.5 (dd, J=26.4, 4.1 Hz), 129.0, 131.8, 133.8, 133.9 (dd, J=13.3, 4.1 Hz), 134.9 (dd, J=13.3, 4.1 Hz), 135.5, 136.5, 153.3 (d, J=250.4, 15.4 Hz), 158.2 (dd, J=242.2, 9.8 Hz), 158.6. HPLC purity: >99%, ESI-MS m/z 331 [M+H]+.
3,5-Dibromo-N-(4,6-difluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (4b)According to the same procedure used for 2f, starting from 3,5-dibromothiophene-2-carboxylic acid (286 mg, 1.00 mmol) and 4,6-difluorobenzo[d]thiazol-2-amine (204 mg, 1.10 mmol), 4b (270 mg, 60%) was obtained as white powder. mp: 245–246°C. 1H-NMR (DMSO-d6) δ: 7.43 (1H, dt, J=10.2, 2.0 Hz), 7.56 (1H, s), 7.83 (1H, dd, J=8.4, 2.0 Hz). 13C-NMR (DMSO-d6) δ: 102.2 (dd, J=28.0, 23.1 Hz), 104.7 (dd, J=26.4, 3.3 Hz), 114.3, 118.4, 131.4 (d, J=7.4 Hz), 134.3 (d, J=10.7 Hz), 134.9, 135.2, 152.7 (d, J=241.2, 20.7 Hz), 158.3 (dd, J=242.2, 10.7 Hz), 159.0, 159.7. HPLC purity: >99%, ESI-MS m/z 453 [M+H]+.
3,5-Dichloro-N-(4,6-difluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (4c)According to the same procedure used for 3a (eluent : CHCl3/MeOH=20 : 1 to 4 : 1), starting from 3,5-dichlorothiophene-2-carboxylic acid (197 mg, 1.00 mmol) and 4,6-difluorobenzo[d]thiazol-2-amine (205 mg, 1.10 mmol), 4c (165 mg, 45%) was obtained as white powder. mp: 224–225°C. 1H-NMR (DMSO-d6) δ: 7.29 (1H, dt, J=10.2, 2.4 Hz), 7.75 (1H, ddd, J=8.8, 2.4, 1.2 Hz), 8.26 (1H, s). 13C-NMR (DMSO-d6) δ: 101.5 (dd, J=28.1, 21.5 Hz), 103.9 (dd, J=26.4, 4.9 Hz), 123.8, 130.4, 130.8, 133.5 (dd, J=12.4, 4.2 Hz), 133.7, 134.7 (dd, J=12.4, 4.2 Hz), 153.0 (dd, J=254.6, 14.1 Hz), 158.0 (dd, J=243.0, 10.7 Hz), 158.3, 158.6. HPLC purity: 96%, ESI-MS m/z 365 [M+H]+.
4,5-Dichloro-N-(4,6-difluorobenzo[d]thiazol-2-yl)thiophene-2-carboxamide (4d)According to the same procedure used for 2d, starting from 4,5-dichlorothiophene-2-carboxylic acid (198 mg, 1.00 mmol) and 4,6-difluorobenzo[d]thiazol-2-amine (205 mg, 1.10 mmol), 4d (268 mg, 73%) was obtained as white powder. mp: 225–226°C. 1H-NMR (DMSO-d6) δ: 7.29 (1H, t, J=10.2 Hz), 7.75 (1H, d, J=8.0 Hz), 8.26 (1H, s). 13C-NMR (DMSO-d6) δ: 101.6 (dd, J=28.9, 22.3 Hz), 104.0 (dd, J=26.5, 4.9 Hz), 123.8, 130.4, 130.9, 133.4 (dd, J=13.3, 4.9 Hz), 133.6, 134.7 (dd, J=13.3, 4.9 Hz), 153.1 (dd, J=253.7, 13.3 Hz), 158.0 (dd, J=243.0, 10.7 Hz), 158.3, 158.6. HPLC purity: >99%, ESI-MS m/z 365 [M+H]+.
Biological AssayMaterialStock solutions (10 and 100 mM) of each compound were prepared in DMSO and kept at −20°C. Appropriate dilutions were freshly prepared just prior to each assay. MNV (S7 strain, kindly provided by Prof. Yukinobu Tohya, Department of Veterinary Medicine, Nihon University, Kanagawa, Japan) was propagated in RAW 264.7 cells (ATC C TIB-71; American Type Culture Collection, Manassas, VA, U.S.A.) cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (growth medium) or 2% (maintenance medium) heat-inactivated fetal bovine serum, antibiotics (100 U/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL amphotericin B), 4500 mg/L D-glucose, 4 mM L-glutamine, 25 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES), 1 mM sodium pyruvate, and 15 mg/L phenol red at 37°C in a humidified atmosphere of 5% CO2. The virus was titrated in RAW264.7 cells with a conventional assay as described previously.16) After infection for 3 d, the cells were observed for CPE, and the TCID50 was calculated by the Kärber formula. Cell debris was removed by centrifugation at 10000×g for 1 h, and the supernatant was stored at −80°C until use. RAW264.7 cells (5.0×103 cells/well) were seeded in a 96-well plate in growth medium. After incubation for 24 h, cells were used for antiviral or cytotoxicity assay.
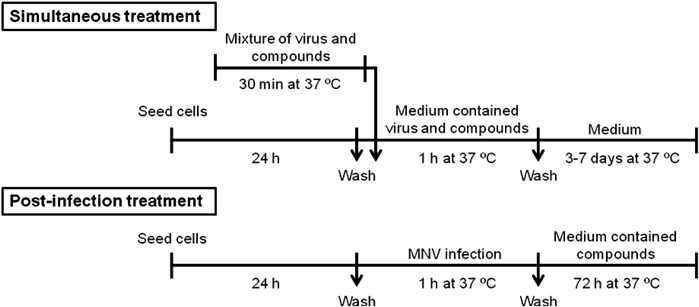
Measurement of Anti-norovirus ActivityMethod A: Screening and CPE Reduction AssayThe antiviral activity of the compounds was determined by using a water-soluble tetrazolium salt (WST)-8 CPE reduction assay. 120 µL of the mixture containing 280 TCID50 of MNV and a dilution series of compounds (0.0061–100 µM) with fetal bovine serum-free medium was incubated for 30 min. RAW264.7 cells were exposed to the mixture. After incubation for 1 h, the cells were washed, replaced with the maintenance medium and incubated for 3 d (i.e., until complete CPE was observed in infected untreated cells). To quantify cell viability, WST-8 solution was added and the plates were incubated for 1 h. The absorbance was measured at 450 nm. The EC50 was defined as the compound concentration that protected 50% of the cells from virus-induced CPE.
Antiviral activity of 4b was evaluated further at different time points. After infection with MNV for 1 h, RAW264.7 cells were treated with a dilution series of 4b, 2′-CMC, and GC376 for 72 h.
The methods for screening and measuring the antiviral activity of the compounds were similar. However, for screening, 100 TCID50/50 µL of MNV and 25 µM of the compounds were used and CPE was observed by microscope without measuring the absorbance.
Method B: TCID50 Assay with FiltrationA total volume of 50 µL of the mixture containing 225 µM compound (4b, 2′-CMC, and GC376) in fetal bovine serum-free medium and 3150 TCID50 of MNV was incubated at room temperature in a centrifugal filter tube (Amicon Ultra-0.5 (100 K), Merck Millipore). In an experiment of 0.02% (v/v) sodium hypochlorite, commercial breach in distilled water was used instead of compound solution. After 1 h, 450 µL of fetal bovine serum-free medium was added and spun at 20000×g for 1 min to eliminate compound. The wash step was repeated once more. MNV was recovered from the filter according to the manufacturer’s instructions and was 5-fold serially diluted with fetal bovine serum-free medium. RAW264.7 cells in a 96-well plate were infected with the diluted virus solution. After 3 d, the cells were observed for CPE, and TCID50 was calculated.
Cytotoxicity AssayThe cytotoxicity of compounds was evaluated by the WST-8 assay in triplicate. RAW264.7 cells were exposed to 3–100 µM of each compound for 72 h. Control cells were treated with the maximum concentration of DMSO (0.1%) and the same incubation time. Cell viability was evaluated by the WST-8 method described in the previous section. CC50 was defined as the compound concentration that reduces the number of viable cells by 50%.