2016 年 64 巻 6 号 p. 564-569
2016 年 64 巻 6 号 p. 564-569
Stomatitis induced by radiation therapy or cancer chemotherapy is a factor in sleep disorders and/or eating disorders, markedly decreasing patient quality of life. In recent years, disintegrating oral films that are easy to handle have been developed; therefore, we focused on the formulation of these films. We prepared an adhesive film for the oral cavity using xyloglucan (Xylo), which is a water-soluble macromolecule. We used loperamide, which has been reported to relieve pain caused by stomatitis effectively, as a model drug in this study. Films were prepared from Xylo solutions (3% (w/w)) and hypromellose (HPMC) solutions (1% (w/w)). Xylo and HPMC solutions were mixed at ratios of 1 : 1, 2 : 1, or 3 : 1 for each film, and films 2×2 cm weighing 3 g were prepared and dried at 37°C for 24 h. Physicochemical properties such as strength, adhesiveness, disintegration behavior, and dissolution of loperamide from films were evaluated. Films prepared from Xylo solution alone had sufficient strength and mucosal adhesion. On the other hand, films prepared from a mixture of Xylo and HPMC were inferior to those made from Xylo, but showed sufficient strength and mucosal adhesion and were flexible and easy to handle. The films prepared in this study are useful as adhesion films in the oral cavity.
Cancer patients undergoing cytotoxic chemotherapy and/or radiation therapy are susceptible to aphthous stomatitis. Aphthous stomatitis is a causative factor in eating and sleep disorders, which can lead to a significant decrease in patients’ QOL. The pathogenesis of stomatitis caused by cancer chemotherapy is thought to be due to the direct action of anticancer drugs on mucosal epithelial cells, and is induced by local infection, neutropenia, production of cytokines and DNA damage by active enzymes produced by anticancer agents. Although various treatments have been attempted, there is no established effective therapy, thus the main treatment option is symptomatic therapy using such formulations as mouth washes and/or oral ointments. Under such conditions, most patients are dissatisfied with the handling and usability, as oral mouthwashes need to be spit out, and oral ointments can lead to nausea due to finger insertion at the time of application.
Recently, oral film formulations have been developed and disseminated. It has been reported that films are easy of handling and convenient dosing to improve patient compliance.1) Furthermore it has been reported that films are suitable for pediatric and geriatric use.1) Therefore, it may be said that films are “patient-friendly formulation.” Oral film formulations are rapidly disintegrated and dissolved in the presence of the small amount of water, and directly applicable the therapeutic agents to the aphthous stomatitis.
For these reasons, in the present study, we focused on the use of oral film formulations as alternatives to mouthwashes and oral ointments.
Many kinds of polymers such as hydroxypropylcellulose, hydroxypropyl methylcellulose, pullulan have been studied as film base. Xyloglucan (Xylo) is water-soluble polysaccharide derived from tamarind seeds, and is composed of a (1→4)-β-D-glucan backbone chain, which has (1→6)-α-D-xylose branches that are partially substituted by (1→2)-β-D-galactoxylose.2) Xylo gelates by the intermolecular cross-linking by hydrogen bonding at the amorphous surface of different cellulose fibrils. The Xylo based gel indicates the elasticity and its gelation behavior, i.e., sol–gel transition is temperature- or concentration-dependent just like poloxamer.2) Furthermore, Xylo solution gelates at low concentration as compared with that of poloxamer.3) For these properties, feasibility of the pharmaceutical application of Xylo into the various sustained drug delivery formulations have been investigated.4–12) However, there are few reports that Xylo is used as film base.13) It is known that the Xylo film is hard and is inflexible.13) Therefore in this study, we attemted to prepare a flexible Xylo film without adding plasticizers by adding other polymers.
Opioids are the most powerful agents for the treatment of severe acute and chronic pain. However, their use is limited by side effects such as sedation, respiratory depression and drug dependence. There are 3 known opioid receptors (μ-, δ-, κ-), and these receptors are present in the central nervous and peripheral nervous systems.14)
Recently, loperamide (LP), an opioid agonist that does not readily cross the blood–brain barrier and is antagonized by peripherally restricted opiate antagonists, has been shown to produce analgesia in various models of pathological pain.14) It has been reported that the μ-receptor is involved in pain in the oral cavity, and LP is a μ-receptor agonist. LP, a piperidine derivative with structural similarities to meperidine, was developed to mimic the effects of morphine in the intestinal tract as an antidiarrheal agent that lacks any central morphinomimetic effects. LP has also been used as a painkiller for cancer treatment-induced oral mucositis.15)
The goal of this study was to develop and evaluate the physicochemical properties of the oral film formulation composed of Xylo and LP to remedy aphthous stomatitis as a “patient-friendly formulation.”
Xylo was generously supplied from DSP Food & Chemical Co., Ltd. (Osaka, Japan). Hypromellose (HPMC) was purchased from Sigma-Aldrich, Inc. (St. Louis, MO). LP (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) was used as a model drug (Fig. 1). Carmellose sodium (CMC-Na) was purchased from Maruishi Pharmaceutical Co., Ltd. (Osaka, Japan), D-sorbitol, KCl, NaCl, CaCl2, MgCl2 and K2HPO4 were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) were used.

All other reagents were of analytical grade.
Preparation of FilmsFilms were prepared by mixing 1% (w/w) HPMC solution and 3% (w/w) Xylo solution. Thus, 1% (w/w) HPMC solution and 3% (w/w) Xylo solution were mixed at various weight ratios (1 : 1, 2 : 1, 3 : 1, respectively). The solutions were poured into a 2×2 cm mold to give films. Films were then dried for 24 h at 37°C. Thickness of films were measured with using the slide gauge. Polymer contents and their thickness of each film are shown in Table 1.
| Xylo (%) | HPMC (%) | Thickness (mm) | |
|---|---|---|---|
| Xylo | 100 | 0 | 0.20±0.05 |
| Rp. 1 | 90 | 10 | 0.13±0.02 |
| Rp. 2 | 86 | 14 | 0.13±0.02 |
| Rp. 3 | 82 | 18 | 0.12±0.02 |
| Rp. 4 | 75 | 25 | 0.12±0.02 |
| HPMC | 0 | 100 | 0.08±0.01 |
Measurement of the breaking strength of films were carried out with using a creep meter (RHEONER II, RE2-33005C; Yamaden Co., Ltd., Tokyo, Japan). For strength measurement of the film, a plunger (2 mm in diameter) was lowered at a constant speed (0.5 mm/s), and the movement distance of the plunger and the maximum load up to breaking were recorded. From these results, the breaking strength (σ), distortion ratio (ε) and Young’s modulus (E) were calculated as following equation,
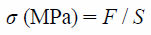 | (1) |
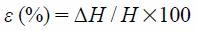 | (2) |
 | (3) |
Filter paper (55 mm in diameter) was impregnated with 2 mL of 10% mucin solution. Thereafter, filter papers were dried at room temperature (r.t.) (25±1°C) for 24 h, and were used as “mucin disks.”16)
Mucin derived from pig stomach was purchased from Sigma-Aldrich, Inc.
Artificial saliva, consisting of CMC-Na D-sorbitol, KCl, NaCl, CaCl2, MgCl2 and K2HPO4 were prepared.
The adhesive measurement of films were performed with a creep meter (RHEONER II; Yamaden Co., Ltd.).
Mucin disks of 20 mm diameter were attached to the movable sample stage, and film samples (20 mm in diameter) were attached to the plunger. Disks were then dipped on the 100 µL of artificial saliva for 60 s. Contact between mucin disks and film was induced with a force of 2 N for 30 s. In addition, the plunger and movable sample stage were pulled apart at a rate of 5 cm/min. The adhesion of the films was defined as the force acting between the mucin disk and film. The measurement was carried out in 6 films and the average and standard deviation were calculated.16)
Viscosity of SolutionsThe viscosity of various solutions was measured using a viscometer (TVE-35; Toki Sangyo Co., Ltd., Tokyo, Japan). A cone rotor (1°34′×R24; shear rate coefficient: 3.83) was used for measurements, which were performed at 25±1°C.
Measurement of Water Absorption Time of FilmsWater absorption of films was measured over time using a water absorption apparatus.17)
As for this apparatus, a glass tube is connected with a glass filter of a diameter of 1.5 cm. This glass tube was filled with purified water, after that it was adjusted to set the scale to zero. The measurement was started just after the sample film was placed on the membrane filter was made by cellulose acetate (0.8 µm of the pore size, ADVANTEC, Tokyo, Japan) it was put on the glass filter. When the sample film absorbed water through the glass filter, water in the glass tube decreased. The amount of water absorbed by film was measured as the change in the weight of water. The measurement was carried out in 6 films and the average and standard deviation were calculated.
Measurement of Disintegration Time of FilmsDisintegration time of films were measured with using a water level alarm that was created using an “Electroninc Block” (EX-150; Gakken Holdings Co., Ltd., Tokyo, Japan).18) Films were prepared a 1×1 cm aperture in the bottom of the polyethylene vessel, and after placing the film to touch the aperture. Two detection terminal boards were put on every 1 cm on the part of the aperture in the film upper part. Disintegration time of films were when a circuit turned on electricity by penetration of the water to a film inside. The measurement was carried out in 6 films and the average and standard deviation were calculated.
Dissolution Behavior of LP from Films (Dissolution Test)Dissolution of LP from the film in 35 mL of distilled water (pH 5.5) and phosphate buffer solution (PBS, pH 6.8) was measured using a Franz diffusion cell. LP concentration was then measured by absorption at 195 nm using high performance liquid chromatography (HPLC, LC-20AD; Shimadzu Corp., Kyoto, Japan). An aliquot of sample was injected onto a C18(2) column [Phenomenex Luna 5μ C18(2) 100A, 250×4.60 mm 5 micron, Shimadzu GLC Ltd., Kyoto, Japan] at r.t. (20–22°C). The flow rate was set at 1.0 mL/min for all separations using a mobile phase composed of acetonitrile–sodium phosphate buffer (pH 2.30)–20 mM di-ethylamine (40 : 60 : 0.08, v/v/v), and the elution time was 15–16 min.19)
The measurement was carried out in triplicate and the average and standard deviation were calculated.
The flexibility of film is important factor to evaluate the handling or texture of film. Xylo is well known that gelate with coexistence of sugar, sugar alcohols, and so on. In this study, though the gelation was not observed by adding of HPMC, the thin films were formed (thickness: 0.08–0.20 mm) (Table 1). Figure 2 depicts the stress rupture (N) of films and their strain (%) obtained from the stress–strain curves of films. In the case of this study, HPMC itself formed thin film in which has low stress rupture level and high strain. In contrast, with the addition of Xylo, the stress rupture and strain of the films increased at the amount not less than 75% of Xylo. Stress rupture of the film of Xylo itself formed unpliable film, the stress rupture was 57.25 N which is about 2.7 times higher than that of Rp.4. That is, HPMC might act as plasticizer and make the Xylo film flexible. The rate of increase in strains observed in Xylo, Rp. 1, Rp. 2 and Rp. 3 were lower than that of stess ruputure. The Young’s modulus is the constant of proportionality of stress and strain in the same axial direction. The Young’s modulus indicates the flexibility of the film; the lower the observed values are, the greater their flexibility are. Figures 2 and 3 show that HPMC films were soft and frail. When the rate of HPMC in the films were high, their flexibility increased (Figs. 2, 3). This was considered to be related to the high film-forming ability of HPMC.

○, Stress at rupture of films, ■, strain of films. Each point represents the mean±S.D. (n=6).
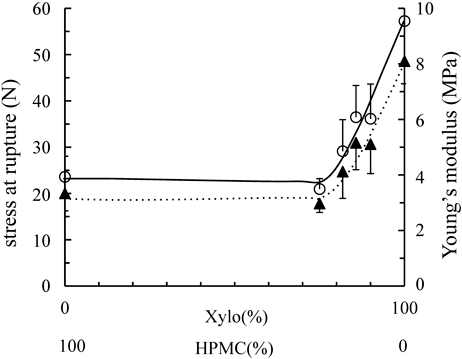
○, Stress at rupture of films, ▲, Young’s modulus of films. Each point represents the mean±S.D. (n=6).
Figure 2 shows the relationship between rupture strengths of films and their Young’s modulus. The addition of Xylo did not affect the film flexibility or strength. Meanwhile, the addition of HPMC affected in the film flexibility and strength. When the amount of HPMC increased, the films became stronger and more flexible.
It is known that Xylo form the intermolecular cross-linking by the hydrogen bonding at the amorphous surface of different cellulose fibrils.20) In this study, the coexistence of HPMC made the cross-linking network between Xylo-cellulose more developed, the flexibility and strength seemed to be improved.
Adhesion of the FilmsThe adhesiveness of films prepared from Xylo were similar to that of films prepared from HPMC. Adhesiveness of Xylo film was strengthened by mixing with HPMC (Fig. 4).
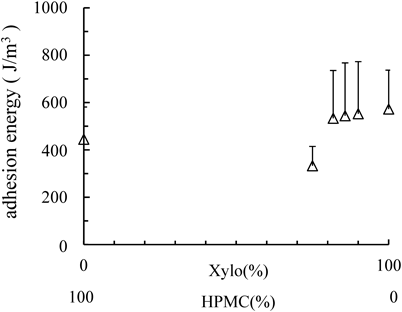
△, Adhesion energy of films. Each point represents the mean±S.D. (n=6).
Mucin is consisting of a thread-like petide backbone existing and densely packed carbohydrate side chains. Such unique structure makes mucin act as like-surfactants, i.e. they tend to adsorb to hydrophobic surfaces via protein–surface interactions while they hold water molecules via their hydrophilic oligosaccharide clusters.21) The mucoadhesiveness is brought about by the non-covalent bonding between mucin and substances.22,23) For example, it is reported that the mucoadhesion of the guar gum is caused by the interaction between its hydroxyl groups and the mucin’s carbonyl groups.23) In addition, though it is reported that HPMC has muco-adhesiveness to mucin due to the interaction between HPMC’s hydroxyl group and mucin, the extent of their interaction is weaker than that of carbopol, a known mucoadhesive polymer.24,25)
In this study, Xylo has many hydroxy groups as is the case with guar gum, and its muco-adhesive force is stronger than that of carbopol,4) Xylo’s muco-adhesive characteristics could be considered as the same as that of guar gum.
Disintegration Time of the FilmsFilm disintegration time was shortened by increasing the amount of HPMC in the film26) (Fig. 5). Rotta et al. investigated the wettability of chitosan/HPMC film by the measurement of contract angle, and demonstrated that the wettability of chitosan/HPMC films were improved with increase in the amount of HPMC, and it is due to an increment in the values of the polar surface free energy and the hydrophilic character when HPMC component was increased in the blend. Even in the case of this study, the higher the HPMC proportion in the films, the higher the solubility in water.
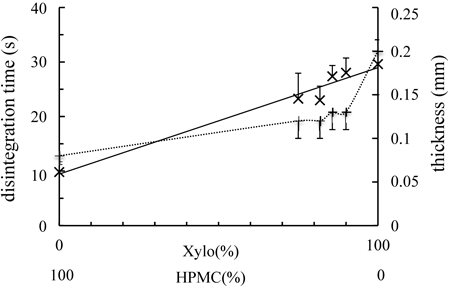
×, Disintegration time of films, +, thickness of films. Each point represents the mean±S.D. (n=6).
In the case of Xylo, it consists of cellulose chain with xylose and/or galacto-xylose side chain.27) Generally, cellulose is insoluble in water because its crystal structure involves many intra- and intermolecular hydrogen bonds. However, Xylo is water-soluble because steric hindrance of its side chains prevents this type of cellulose-like crystallization.28,29) However, the many water-soluble side saccharide chains make it possible to be water-soluble in whole.27) Furthermore, since Xylo has a high water retaining function, it gelates by absorbing water. When the disintegration test was performed on Xylo films, disintegration was not observed, instead gelation with water absorption was observed.
In the case of this study, as the disintegration time was detected as the energization between the electrodes by the water absorption, the disintegration time seemed to be affected by the amount of HPMC in films.
Water Absorption Behavior of the FilmFigure 6 shows the water absorption behavior of the films. In the period of the first 60 s, all films showed rapidly water absorption (Fig. 6). The amount of water absorbed increased with an increase in the amount of HPMC in films. As shown in the the results of disintegration tests, since HPMC has high water solubility, HPMC films dissolved completely at the same time of the water absorption. Meanwhile, Xylo films were not dissolved completely because of its high water-retention capacity, a formation of hydrogel-like structure was observed after absorbing water. From also these results, the amount of HPMC in films seems to be a limiting factor for the water absorption into films.
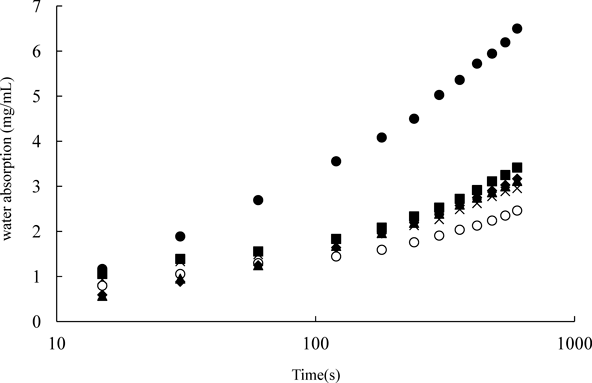
〇, Xylo, ×, Rp 1, ▲, Rp 2, ◆, Rp 3, ■, Rp 4, ●, HPMC. Each point represents the mean±S.D. (n=6).
Figure 7 shows the dissolution behavior of LP from the film in PBS (pH 6.8). Release of LP from films was accelerated with increase in an amount of HPMC contained in the films. As mentioned above, Xylo forms the hydrogel-like structure by absorbing water, the diffusion of LP in the film seemed to be regulated by the amount of absorbed water. Furtheremore, it is reported that Xylo is gelated by adding iodine or Congo red into Xylo.27,28) Xylo molecule has a rod-like structure due to its cellulose backbone.27,28) When the substance having flat structure is included among the structure, gelation occurs.27,28) It is suggested to interact between a rod-like structure and a flat structure. For example, Congo red can not only interact via hydrogen bonds between its amino or azo groups and the hydroxyl groups in the Xylo, but also through van der Waals’ force between its aromatic groups and the hydrophobic cellulose surface. These interaction allows Xylo chains to intercalate Congo red into and among its structure.28)
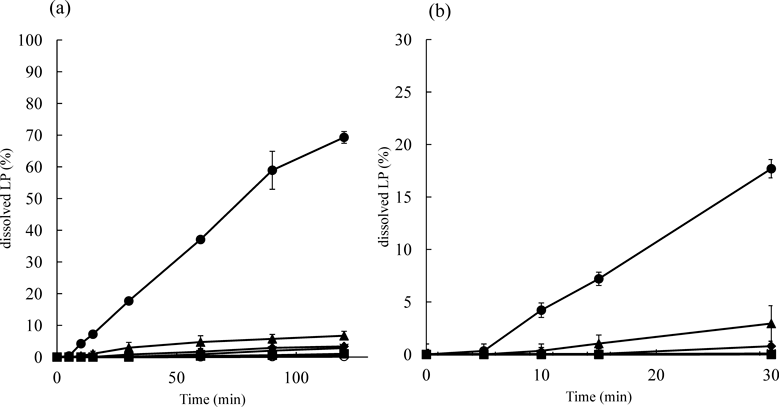
(a) The release profiles of LP in 120 min. (b) The release profile of LP in 30 min. 〇, Xylo, ×, Rp 1, ▲, Rp 2, ◆, Rp 3, ■, Rp 4, ●, HPMC. Each point represents the mean±S.D. (n=3).
LP is a class-IV molecule in the Biopharmaceutics Classification Scheme (BCS).29) LP has low solubility and low permeability. Furthermore, as shown in Fig. 1, LP molecule has also a flat structure, and has amino and hydroxyl groups (Fig. 1). In vitro drug release from Xylo gels has been reported to occur by diffusion through aqueous channels in the gel matrix.11)
In this study, it is considered that geletion was induced by the inclusion of LP molecules among the molecular chain of Xylo i.e., a part of the flat structure of LP molecule is included by Xylo. In Fig. 7, the release of the LP from an HPMC film was faster than Xylo films. That is, the addition of LP which has flat portion in its structure to Xylo and LP induced the gelation, it would appear that the diffusion rate of LP among the matrix and the release of LP was restricted.
In this study, the film-forming ability of Xylo was confirmed. However, the Xylo films consisting of solely Xylo were hard and relatively difficult to handle. The addition of HPMC as a plasticizer to Xylo could improve the film adhesion, strength and flexibility. It revealed that Xylo’s mucoadhesive force is stronger than that of carbopol which is known as mucoadhesive polymer. The adhesiveness of Xylo films increased with increase in the amount of HPMC added. This suggests that the degree of the interaction between hydroxyl group of Xylo and/or HPMC and the mucin was strengthened.
Though Xylo has cellulose which is water-insoluble as backbone, its side saccharide chains suppress the inter- and intra-molecular hydrogen bonding between cellulose chains, and therefore make Xylo water-soluble.27) At the same time, Xylo has a high water-retention capacity, Xylo gelates by absorbing water. The disintegration time of films were shortened with increasing the amount of HPMC in films due to HPMC’s high water solubility. In accordance with this, the release of LP from Xylo films were accelerated with increase in the amount of HPMC in film. In other words, it means that the drug release from Xylo films are controllable by changing the relative amount of Xylo and HPMC in films.
In an experimental animal model, there is a limitation for evaluating the pain caused by stomatitis. The efficacy of such LP-containing films for relieving stomatitis pain was not clear in this study. A gargle containing LP has been used as the hospital formulation for a pain of the stomatitis in Japan.30) Furthermore, we would like to apply the LP films as hospital formulations by collaborating with other hospitals, and validate the efficacy as the pain relief of stomatitis in our future study. It is known that Xylo is used for forming hydrogel. In this study, film formation ability of Xylo was newly confirmed. From these results, it appeared that Xylo is applicable to not only the film formulation but also the controlled release of drug formulations.
We would like to thank DSP Food & Chemical Co., Ltd. for providing xyloglucan.
The authors declare no conflict of interest.