2016 Volume 64 Issue 6 Pages 602-608
2016 Volume 64 Issue 6 Pages 602-608
A toxic mushroom, Russula subnigricans, causes fatal poisoning by mistaken ingestion. In spite of the potent bioactivity, the responsible toxin had not been identified for about 50 years since its first documentation. Recently, we isolated an unstable toxin and determined the structure. The slow elucidation was partly due to the instability of the toxin and also due to misidentification of R. subnigricans for similar mushrooms. To discriminate genuine Russula subnigricans from similar unidentified Russula species, we searched for a unique chemical marker contained in the mushroom. Cyclopropylacetyl-(R)-carnitine specific to R. subnigricans was identified as a novel compound whose 1H-NMR signals appearing in the upfield region were easily recognizable among the complicated signals of the crude extract.
Mushroom poisonings attributable to the Russulaceae mushroom Russula subnigricans were first documented in 1954 in Japan.1) In the past 50 years, the following seven poisonings have occurred: [deaths/cases (year, place where poisoning occurred)] unknown/unknown (1954, Kyoto); 2/4 (1958, Osaka); 1/3 (1958, Osaka); 0/2 (1970, Toyama); 2/2 (2005, Aichi); 1/1 (2006, Miyazaki); 1/3 (2007, Osaka). In addition, nine individuals in Taiwan were identified with symptoms of R. subnigricans poisoning in 1998.2) Typical symptoms following ingestion of R. subnigricans are vomiting and diarrhea, which first appear approximately 30 min after ingestion, followed by stiff shoulders, back ache, and bloody urine that is reddish-brown in color due to high levels of myoglobin, not hemoglobin. Myoglobin is an oxygen-binding protein pigment of striated muscles, such as skeletal and cardiac muscles, and is discharged following the breakdown of myocells, a condition termed rhabdomyolysis. In severe cases of R. subnigricans poisoning, further symptoms develop, including speech impediment, chronic convulsion, contraction of pupils, loss of consciousness, and weakening of the heart, resulting in death.3)
In spite of the strong toxicity of R. subnigricans, the responsible toxin remained unknown until recently, when we isolated and identified a small, unstable compound, cycloprop-2-ene carboxylic acid (1; Fig. 1), as the fatal toxin of this mushroom.4) The toxicity of this compound was demonstrated on administration to mice, which displayed severe rhabdomyolysis. It appears that the difficulty in identifying this compound as the responsible toxin was due in part to its instability; concentration of solutions of this toxin by drying, a common technique in chemical separation and isolation steps, promotes its polymerization. Polymerized cycloprop-2-ene carboxylic acid lacks toxicity.
In addition to inactivation, the long period required for the determination of cycloprop-2-ene carboxylic acid as the responsible toxin is attributable to difficulties discriminating R. subnigricans from similar species, which is the primary cause of accidental poisonings. The three representative Russula species identified to date in Japan are R. subnigricans, R. nigricans, and R. densifolia; the latter two are considered to be edible after cooking. The most useful characteristic feature to discriminate R. subnigricans from the other two species is the color change that occurs after scratching fruiting body. All three species have whitish flesh that are tinged reddish brown on scratching. After that, the colors of R. nigricans and R. densifolia turn black, whereas the color of R. subnigricans is persistent. However, classifying these species based on only this color change is difficult and unreliable. Although discrimination of R. subnigricans from R. densifolia is rather easy, as the latter has crowded gills compared to the former, the classification of Russula species becomes more complicated due to the existence of several similar unclassified species in addition to the three aforementioned Russula species. Unclassified Russula spp. have distant gills and fruiting bodies that undergo color changes similar to those of R. subnigricans.
Here, to discriminate genuine R. subnigricans from similar unclassified Russula species, we searched for a compound that could serve as a unique chemical marker.
Prior to our identification of cycloprop-2-ene carboxylic acid as the compound responsible for R. subnigricans toxicity,4) several candidate molecules had been reported. Using fruiting bodies collected in Miyagi Prefecture, Japan, 3-hydroxybaikiain (2),5) russuphelins A–F (3A–F),6,7) and a related compound, russuphelol,8) were isolated (Fig. 1). Among these compounds, russuphelins A–D showed cytotoxic acitivity. To confirm the results of these experiments, we also collected R. subnigricans candidate in Miyagi Prefecture in a broadleaf forest including the Japanese oak, Quercus serrata (one of the generally proposed host trees of R. subnigricans; Japanese name: konara), and subjected fruiting bodies to methanol extraction. According to a previous report,5) we isolated 3-hydroxybaikiain (2) from the aqueous layer of the methanol extract, which partitioned between water (H2O) and ethyl acetate (EtOAc), while russuphelins A and D (3A, D)6,7) were isolated from the organic layer. Thus, we confirmed that the Russula sp. collected in Miyagi Prefecture was the mushroom materials previously analyzed and reported in refs. 5–8. Notably, in addition to these compounds, we isolated a new russuphelin congener which we named russuphelin G (3G). On comparison of the spectroscopic data (see Experimental) with those of other russuphelins, the structure of 3G was determined to be a demethyl congener of russuphelin E or F (3E, F). In addition, the position of the methoxy group was determined by nuclear Overhauser effect (NOE) analysis (see Experimental).
We also collected two additional R. subnigricans candidate specimens in Kyoto and Saitama Prefectures for analysis, because the first poisoning of R. subnigricans occurred in Kyoto, and Saitama Prefecture is located between Miyagi and Kyoto Prefectures with respect to latitude. In Kyoto Prefecture, mushrooms were collected in a chinquapin forest including Castanopsis cuspidate (Japanese name: tsuburajii or kojii), whereas in Saitama Prefecture, mushrooms similar to those of Miyagi Prefecture were collected in a broadleaf forest including Quercus serrata, similar forest to that of Miyagi Prefecture. Our analysis revealed that neither of these two candidate specimens contained either russuphelins or 3-hydroxybaikiain (2). Among the three candidate R. subnigricans specimens obtained from Miyagi, Kyoto, and Saitama Prefectures, only the Kyoto candidate exhibited fatal toxicity to mice on oral administration of the water extract. Following further separation, we isolated the responsible lethal toxin, cycloprop-2-ene carboxylic acid (1), from the Kyoto specimen, but could not isolate the toxin in the other two R. subnigricans candidates. Taken together, these results indicated that the Kyoto specimen was genuine R. subnigricans, whereas the other two specimens collected in Miyagi and Saitama were not.4) Thus, the existence of two unidentifiable mushroom species that shared nearly identical features with R. subnigricans prompted us to search for a unique chemical marker among these three species. The photos of R. subnigricans and unidentified two Russula spp. collected in Miyagi or Saitama are cited in Fig. 2.

(a) R. subnigricans collected in Kyoto, (b) Russula sp. collected in Miyagi, (c) Russula sp. collected in Saitama.
Genuine R. subnigricans contains cycloprop-2-ene carboxylic acid (1), which could potentially serve as a chemical marker to distinguish the fatally toxic mushroom from other similar species by chemical analysis. However, as mentioned, toxic compound 1 is easily polymerized under concentrated conditions, limiting the usefulness of this toxin as a unique marker of genuine R. subnigricans. We next compared the 1H-NMR spectra of the crude water extracts of R. subnigricans and the two unidentifiable species. In the 1H-NMR spectrum of the R. subnigricans (Kyoto) extract, characteristic signals of a cyclopropane unit were observed in the upfield region (0.15, 0.52 ppm). As this compound was not found in the 1H-NMR spectra of the extracts from the two examined species, we attempted to isolate the compound indicated by the 1H-NMR measurement.
The fruiting bodies of genuine R. subnigricans were cut into small pieces (approximately 5 mm) and extracted with 0.3% acetic acid (AcOH). After filtration and dialysis, the extracts were concentrated in vacuo, and the resulting residue was dissolved in 1% AcOH–methanol (MeOH). The soluble fraction was chromatographed on alumina using 1% AcOH–MeOH as the eluent, and the resulting eluate was chromatographed on an ODS (octadecyl group-bonded silica) column by stepwise elution with H2O and 50% MeOH–H2O. The 50% MeOH fraction was concentrated in vacuo, and the residue was found to be partitioned between H2O–EtOAc. The aqueous layer was concentrated in vacuo and the residue was chromatographed on an ODS column, involving stepwise elution with 20 and 50% MeOH–H2O. The 20% MeOH eluate was further purified by ODS TLC (20% CH3CN–H2O) followed by HPLC (ODS, gradient elution with CH3CN–H2O), yielding colorless syrup.
The molecular mass of the obtained compound was estimated to be 243 by FAB-MS. The NMR data of the purified cyclopropane-containing compound are listed in Table 1. The 1H-NMR signals indicated the existence of 21 protons, including a monosubstituted cyclopropane unit and a trimethylammonium group, while 10 signals were observed in the 13C-NMR spectrum, including two carbonyl carbons and one overlapped carbon corresponding to the trimethylammonium group. Taking these data into account with the heteronuclear multiple bond correlation (HMBC) spectra, we inferred the structure to be carnitine ester 4, as shown above.
 | |||
|---|---|---|---|
| Position | 1H-NMR (300 MHz, D2O) HOD=4.79 | 13C-NMR (75 MHz, D2O) DSSa)=−2.04 | HMBC correlation |
| 1 | — | 177.1 | C2-Ha, Hb |
| 2 | a: 2.49 (1H, dd, J=8.0, 16.0 Hz) | 40.9 | C3-H, C4-Ha |
| b: 2.63 (1H, dd, J=5.6, 16.0 Hz) | |||
| 3 | 5.63 (1H, m) | 67.5 | C2-Ha, Hb, C4-Hb |
| 4 | a: 3.60 (1H, d, J=14.0 Hz) | 68.9 | C2-Ha, Hb, C3-H, NMe |
| b: 3.86 (1H, dd, J=9.0, 14.0 Hz) | |||
| NMe3 | 3.18 (9H, s) | 54.5 | C4-Ha, Hb |
| 1′ | — | 175.6 | C2′-Ha, Hb |
| 2′ | a: 2.27 (1H, dd, J=7.0, 16.0 Hz) | 39.7 | C4′-Ha, C5′-Ha |
| b: 2.36 (1H, dd, J=7.4, 16.0 Hz) | |||
| 3′ | 0.98 (1H, m) | 6.7 | C4′-Ha, C5′-Ha, C2′-Ha, Hb |
| 4′ | a: 0.15 (1H, m) | 4.2 | C2′-Ha, Hb |
| b: 0.52 (1H, m) | |||
| 5′ | a: 0.15 (1H, m) | 4.4 | C2′-Ha, Hb |
| b: 0.52 (1H, m) | |||
a) DSS=sodium 2,2-dimethyl-2-silapentane-5-sulfonate.
To confirm the structure of 4, the identical carnitine ester was synthesized by condensation of (R)-carnitine and cyclopropylacetic acid using an acid chloride method (see Experimental). The 1H- and 13C-NMR data of the natural and synthetic samples were identical, and the absolute configuration was also determined to be R by comparing the specific rotation of the synthetic compound and that of the natural one. Thus, the isolated cyclopropane-containing new compound was confirmed to be cyclopropylacetyl-(R)-carnitine (4). Interestingly, carnitine esters, including a cyclopropylcarboxylic acid ester, were also isolated from a Boletaceae mushroom.9) We examined the toxicity of 4 in mouse by an intraperitoneal route; however, no toxicity was detected.
Cyclopropylacetyl-(R)-carnitine is specific to genuine R. subnigricans and sufficiently stable under ordinary experimental conditions. In addition, the upfield signals in the 1H-NMR spectrum corresponding to the cyclopropane core are easily recognizable in the 1H-NMR spectrum of crude mixtures of fruiting bodies; therefore, it would be a useful chemical marker for the identification of genuine R. subnigricans (Fig. 3).
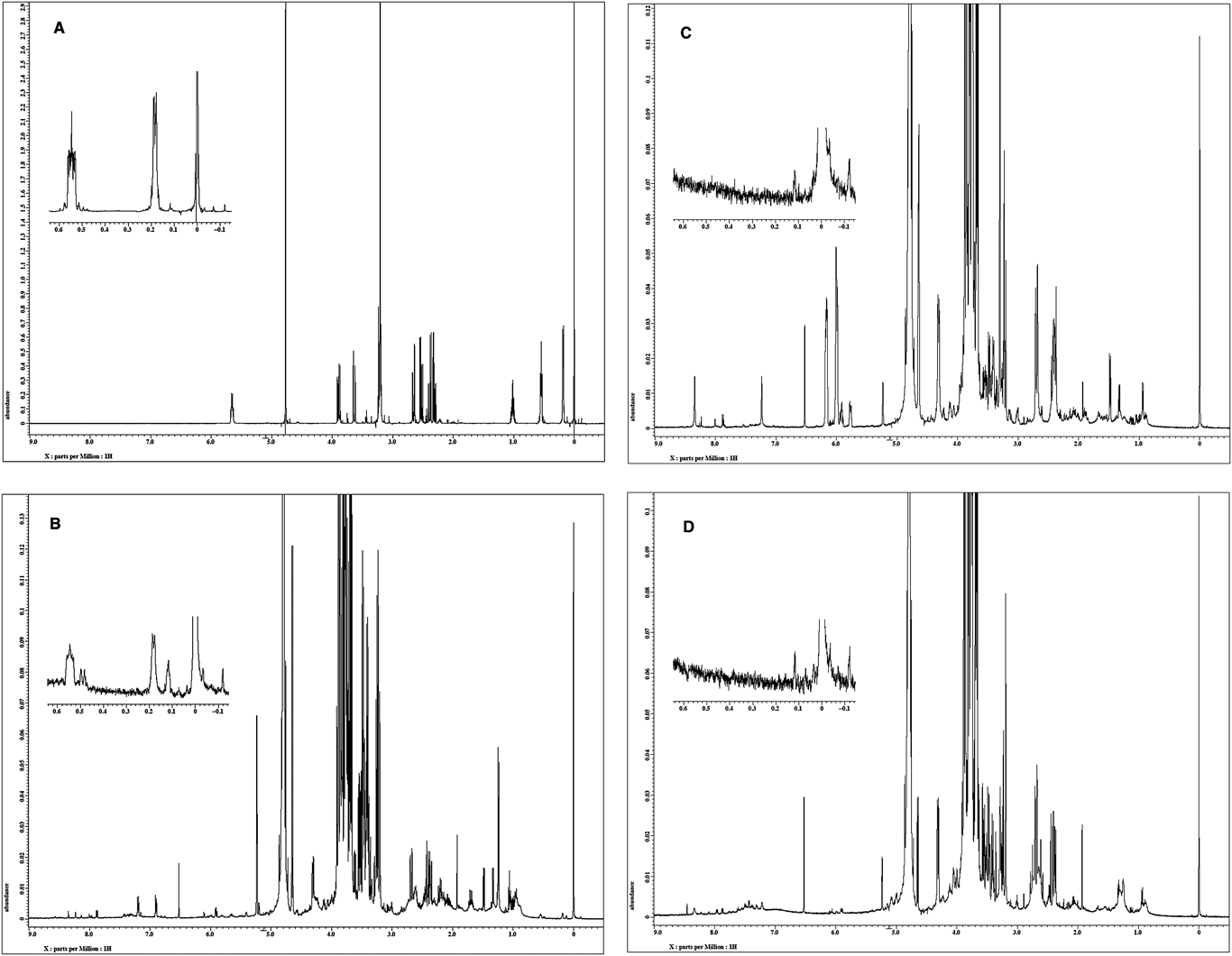
(A) Authentic sample of cyclopropylacetyl-(R)-carnitine (4). (B) Crude water extract of R. subnigricans collected in Kyoto. (C) Crude water extract of Russula sp. collected in Miyagi. (D) Crude water extract of Russula sp. collected in Saitama. a) TSP=3-(trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt.
A number of identification manuals for mushrooms, including several local manuals of exceptional species and traits, have been published in Japan. In these manuals, it is often ambiguously described that R. subnigricans is found in summer and fall in broadleaf forests where chinquapin or oak trees grow. We have examined several Russula species during the past ten years, and have concluded that descriptions of genuine R. subnigricans and unclassified Russula spp. are often unclear and/or contradictory. Presently, we can locate genuine R. subnigricans only in chinquapin forests, specifically forests of Castanopsis cuspidate. This tree is distributed in warm, western areas of Japan, and the fruiting bodies of R. subnigricans appear only in the summer months. In contrast, the two unclassified Russula spp., which were collected in Miyagi and Saitama, are found in rainy seasons during the summer and fall months in broadleaf forests including the Japanese oak Quercus serrata.
Japanese oak grows in nearly all areas of Japan, including forests of Kyoto, where the two unclassified Russula spp. independently grow during the identical season. Since the kinds of mushroom species that flourish and their respective colony sizes depend on location and climate, it is expected, as in the cases of Miyagi and Saitama, that only a single kind of Russula spp. would be found in each forest during the identical season. Although more than one species may grow in these locations, the exclusive appearance of apparently only one type of species misled local mushroom collectors on the correct identification of Russula spp. This situation led to differing and unclear descriptions of R. subnigricans in numerous identification manuals, and also hampered the accuracy of previous chemical studies of this toxic mushroom.5–8)
In addition to physical appearance, it is also possible that R. subnigricans can be discriminated from the two unclassified Russula spp. based on the proposed kind of host tree. However, it is difficult to distinguish the host tree with which the mushroom symbiotically lives in a mixed broadleaf forest. Therefore, our chemical method described herein can impart useful information to aid in the accurate identification of R. subnigricans.
The comparative data of the two unclassified Russula species with those of genuine R. subnigricans are summarized in Table 2. Detailed data together with the dried materials of the mushrooms used in this study were deposited in The Royal Botanical Gardens, Kew, U.K. and classification of the two Russula spp. collected in Miyagi and Saitama is currently in progress.
| Russula sp. Collected in Miyagi | Russula sp. Collected in Saitama | Russula subnigricans Collected in Kyoto | |
|---|---|---|---|
| Location of the spot where each mushroom was collected | E 140°49′N 38°15′ (Aoba-yama) | E 139°27′N 35°46′ (Hachikoku-yama) | E 135°47′N 35°00′ (Koudaiji-san) |
| Toxicity (p.o.)a) of the water extract | Non toxic | Non toxic | Toxic |
| Cycloprop-2-ene carboxylic acid (1) | Absent | Absent | Present |
| 3-Hydroxybaikiain (2) | Present | Absent | Absent |
| Russuphelins (3) | Present | Absent | Absent |
| Cyclopropylacetyl-(R)-carnitine (4) | Absent | Absent | Present |
| Color change of fresh | White to reddish brownb) | White to reddish brownc) | White to reddish brownd) |
| Odor | Pungent like halogens | Not special | Not special |
| Gills | Distant | Distant | Distant |
| Proposed host treese) | Quercus serrata | Quercus serrata | Castanopsis cuspidata |
| Growth | Rainy seasons in summer and fall | Rainy seasons in summer and fall | Summer |
a) p.o.: per os (oral administration). b) Faint and extremely slow coloration to reddish brown on scratching, and mainly aging-dependent coloration. c) Fast and intense coloration on scratching: Red to reddish brown or reddish gray, and then to black. d) Through reddish brown on scratching, finally to gray during about 1 h. e) The major tree in the forest where each mushroom was collected.
Melting points were determined on a micro hot-stage Yanaco MP-S3 and were uncorrected. Optical rotations were measured on a JASCO DIP-360 polarimeter. IR spectra were recorded on a JASCO FT-IR-200 spectrometer as a KBr pellet. 1H- and 13C-NMR spectra were recorded on JEOL Lambda-300, JEOL ECA-500, or Varian MERCURY plus 300 at ambient temperature and their chemical shifts were reported as values in ppm downfield or upfield from internal standard (noted before data). High and low-resolution electron ionization (HR-EI and LR-EI) and FAB mass spectra were recorded on JEOL GC mate mass spectrometer.
(R)-Carnitine was purchased from TCI (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) and cyclopropylacetic acid from Wako (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and these were used without further purification.
Mushroom MaterialThe mushrooms were collected in Kyoto in 2004–2007, Saitama in 2002–2003, and Miyagi in 2007 and stored in freezer at −30°C and used for the chemical study. Exact locations for all collections were summarized in Table 2.
The Russulaceae are a family of fungi in the order Agaricales. The Russulaceae mushrooms have common characteristic appearances which are easily recognizable as the species should be classified into the family; however, further identification of each species is troublesome. Typical characteristics of the Russulaceae are the followings; friable fruit bodies, whitish to ocher spore print, spores with amyloid fibrils, no clamp connection in the hyphae of the fruit bodies. Friable flesh is due to its heteromerous structure which is constructed from filamentous hyphae and many spherical cells. The Russulaceae family is classified into two genera, Russula and Lactarius. The former has no latex; on the other hand, the latter has it.10)
All of the Russula mushrooms used in this study meet the above requirements. Identification of each mushroom was performed by Shigeo Morimoto (Kansai Mycological Club). Detailed data together with the dried materials of the mushrooms collected in 2011 were deposited in The Royal Botanical Gardens. The reference numbers are as follows: K(M) 173268 for Russula subnigricans, K(M) 173269 for Russula sp1 (collected in Miyagi), and K(M) 173270 for Russula sp2 (collected in Saitama).
3-Hydroxybaikiain (2)Isolation of 3-Hydroxybaikiain (2) from a Russula sp. Collected in MiyagiThe frozen fresh fruiting bodies of the mushroom collected in Miyagi Prefecture (22.2 g) were cut in pieces and extracted with MeOH (200 mL). The mixture was filtered and the filtrate was concentrated in vacuo. The residue was partitioned between EtOAc (50 mL×3) and H2O and each layer was concentrated to dryness to give the EtOAc extract (255 mg) as a red syrup and the water extract (173 mg) as a brown syrup.
According to the Nozoe’s procedure,5) 3-hydroxybaikiain (2) was isolated from the water extract through two-step ion exchange chromatography (Amberlite IRC-50, eluted with H2O, and then Amberlite IR-120-B, eluted with 4% aqueous NH4OH) to give a mixture (33.8 mg) containing 2. Finally, recrystallization from H2O–MeOH gave pure 2 (14.1 mg) as colorless crystals. mp >300°C (lit.5) 300–302°C; HR-EI-MS m/z 143.0601 [M]+; Calcd for C6H9NO3, 143.0582; [α]D36 −322.7 (H2O, c=1.19) (lit5): [α]D20 −332.7 (H2O, c=0.3)). 1H-NMR, 13C-NMR, and IR spectra of 2 were identical to those of the reported data.5)
Detection of 3-Hydroxybaikiain (2) in Other Two Russula spp. Collected in Saitama and KyotoUsing the same method as above, detection of 3-hydroxybaikiain (2) was tried on the other two Russula spp. by 1H-NMR. The MeOH extract of the Russula sp. collected in Saitama (fruiting bodies; 350 g/MeOH; 1.2 L×2) was partitioned to give the EtOAc extract (1.43 g) and the water extract (6.00 g). On the other hand, the MeOH extract of the Russula sp. collected in Kyoto (fruiting bodies; 720 g/MeOH; 1.0 L×2) was partitioned to give the EtOAc extract (2.68 g) and the water extract (9.67 g). Each water extract was separated, respectively, as mentioned in the section of “Isolation of 3-hydroxybaikiain (2) from a Russula sp. collected in Miyagi” and the resulting aqueous ammonia eluate was checked by 1H-NMR. No 3-hydroxybaikiain (2) was detected in either extracts (5–10 mg in D2O (0.75 mL)).
Russuphelins (3)Isolation of Russuphelins (3) from a Russula sp. Collected in MiyagiThe EtOAc extract (see Experimental of “Isolation of 3-hydroxybaikiain (2) from a Russula sp. collected in Miyagi”) was separated according to the Nozoe’s procedure6,7) using silica gel column chromatography (successive 4 : 1 hexane–EtOAc, 9 : 1 CHCl3–EtOAc, and 4 : 1 CHCl3–EtOAc), and the chemical components of each fraction were checked by TLC and 1H-NMR spectra. Russuphelin-containing fractions (fr. 1: eluted with 9 : 1 CHCl3–EtOAc, 12.8 mg; fr. 2: eluted with 9 : 1 CHCl3–EtOAc, 6.0 mg; fr. 3: eluted with 4 : 1 CHCl3–EtOAc, 3.8 mg) were further separated by preparative TLC as follows.
4,4′-[(2,5-Dimethoxy-1,3-phenylene)bis(oxy)]bis[3,5-dichlorophenol] (Russuphelin A, 3A)Above fr. 3 (3.8 mg) was separated by preparative TLC (silica gel, 19 : 1 CHCl3–MeOH) to give russuphelin A (3A, 1.3 mg) as a colorless powder. mp 294–297°C (not recrystallized) (lit.6) 293–294°C); HR-EI-MS m/z 489.9523 [M]+; Calcd for C20H14O6Cl4, 489.9544. 1H-NMR, 13C-NMR, and IR spectra of 3A were identical to those of the reported data.6,7)
4-(3-Chloro-2,5-dimethoxyphenoxy)-3,5-dichlorophenol (Russuphelin D, 3D)Above fr. 1 (12.8 mg) was separated by preparative TLC (silica gel, 19 : 1 CHCl3–MeOH) followed by further purification by preparative TLC (silica gel, 4 : 1 CHCl3–EtOAc) to give russuphelin D (3D, 2.3 mg) as colorless solids. mp 127–128°C (not recrystallized) (lit.7) 136–138°C); HR-EI-MS m/z 347.9720 [M]+; Calcd for C14H11O4Cl3, 347.9723. 1H-NMR, 13C-NMR, and IR spectra of 3D were identical to those of the reported data.6,7)
2-Chloro-6-(2,6-dichloro-4-methoxyphenoxy)-1,4-hydroquinone (Russuphelin G, 3G)Above fr. 2 (6.0 mg) was separated by preparative TLC (silica gel, 19 : 1 CHCl3–MeOH) to give 2-chloro-6-(2,6-dichloro-4-methoxyphenoxy)-1,4-hydroquinone (3G, 2.9 mg) as colorless solids. mp 184–185°C (not recrystallized); Rf=0.39 (1 : 19 MeOH–CHCl3); IR νmax (KBr): 3419, 1610, 1504, 1470, 1219 cm−1; 1H-NMR (CDCl3, TMS=0.00) δ: 3.82 (3H, s, 4′-OMe), 5.92 (1H, d, J=3.0 Hz, H-5), 6.56 (1H, d, J=3.0 Hz, H-3), 6.95 (2H, s, H-3′, 5′); 1H-NMR (CD3OD, solvent peak=3.31) δ: 3.84 (3H, s, 4′-OMe), 5.77 (1H, d, J=3.0 Hz, H-5), 6.44 (1H, d, J=3.0 Hz, H-3), 7.10 (2H, s, H-3′, 5′); NOE (increased by 5.1%) was observed at H-3′ and H-5′ overlapped signals by irradiation of 4′-OMe; 13C-NMR (CD3OD, solvent peak=49.00) δ: 56.8 (4′-OMe), 101.5 (C5), 110.8 (C3), 116.0 (C3′, 5′), 122.8 (C2), 131.0 (C2′, 6′), 137.0 (C1), 141.9 (C1′), 147.9 (C6), 151.2 (C4), 158.9 (C4′); HR-EI-MS m/z 333.9547 [M]+; Calcd for C13H9O4Cl3, 333.9567.
Detection of Russuphelins (3) in Other Two Russula spp. Collected in Saitama and KyotoEach EtOAc extract (see Experimental of “Detection of 3-hydroxybaikiain (2) in other two Russula spp. collected in Saitama and Kyoto”) was dissolved in CDCl3 and checked by 1H-NMR. No russuphelins (3) were detected in either extracts (5–10 mg in CDCl3 (0.75 mL)).
Cyclopropylacetyl-(R)-carnitine (4)Isolation of Cyclopropylacetyl-(R)-carnitine (4) from the Russula subnigricans (Collected in Kyoto)The fruiting bodies (500 g) of Russula subnigricans (collected in Kyoto) were cut into pieces and soaked in aqueous 0.3% AcOH (1.5 L) at 4°C overnight. The extract was filtered through filter paper under suction and then the filtrate was concentrated to about 100 mL under reduced pressure. The concentrated solution was dialyzed (relative molecular mass (Mr) 14000) against aqueous 0.3% AcOH (2.0 L×2) overnight. The dialyzate was concentrated to dryness and lyophilized to give a crude extract (27.1 g). A part (4.8 g) of the crude extract was dissolved in 1% AcOH in MeOH (48 mL), and then the soluble part was applied to an alumina column (aluminium oxide 90 standardized, Merck, 32 g), which was eluted with 1% AcOH in MeOH (300 mL). The eluate was concentrated to 5 mL, and this was diluted with aqueous 0.3% AcOH (10 mL) and chromatographed on ODS (Cosmosil 140C18 OPN, 16 g) which was eluted with H2O (300 mL) and 50% aqueous MeOH (100 mL). After removal of MeOH from the 50% MeOH fraction, the aqueous solution was washed with EtOAc (100 mL×3). The aqueous layer was concentrated in vacuo and then chromatographed on ODS by elution with 20% MeOH. The obtained fractions which contained a cyclopropane derivative were concentrated (16.2 mg) and purified by preparative TLC (ODS, 20% CH3CN) followed by HPLC (ODS ϕ6×250 mm, eluted with H2O for 10 min and then linear gradient from H2O to 20% CH3CN for 50 min at a flow rate of 1.5 mL/min with monitoring at 210 nm) to give 4 (3.4 mg, tR=20.9 min) as colorless syrup. Rf=0.31 (ODS, 1 : 4 MeOH–H2O); IR νmax (KBr): 3735, 3468, 1732, 1594, 1398, 1188, 1054 cm−1; LR-FAB-MS m/z 244 [M+H]+, 162 [M−C5H6O]+, HR-FAB-MS m/z 244.1572 [M+H]+; Calcd for C12H22NO4, 244.1549; [α]D31 −14.5 (H2O, c=0.96).
Detection of Cyclopropylacetyl-(R)-carnitine (4) in Three Russula spp. Collected in Kyoto, Miyagi, and SaitamaThe water extract of R. subnigricans collected in Kyoto (fruiting bodies; 7.42 g/H2O; 22.3 mL) was concentrated to dryness to give the crude extract (369 mg). A partial crude extract (10 mg) was dissolved into D2O (0.75 mL) containing TSP (0.77 mM) as an internal standard. Carnitine ester 4 was detected in 1H-NMR spectrum of the crude extract and its content was estimated to be ca. 133 mg/kg fruiting bodies. Similarly, Saitama species (fruiting bodies; 6.96 g/H2O; 20.9 mL) and Miyagi species (fruiting bodies; 11.4 g/H2O; 34.2 mL) were extracted with H2O to give the crude extracts (83.0, 257 mg, respectively). A part of each water extract was dissolved in D2O and this was checked by 1H-NMR. No cyclopropylacetyl-(R)-carnitine (4) was detected in either extracts (10 mg in D2O (0.75 mL)).
Synthesis of Cyclopropylacetyl-(R)-carnitine (4)To a stirred cyclopropylacetic acid (0.098 mL, 1.05 mmol) was added thionyl chloride (0.080 mL, 1.10 mmol) and the mixture was stirred at room temperature (rt) for 1 h. To the crude acid chloride was directly added (R)-carnitine (86.0 mg, 0.533 mmol) and the mixture was stirred at rt for 1.5 h. After evaporation, the residue was dissolved into H2O and the aqueous layer was washed with EtOAc. The aqueous layer was applied to ODS column chromatography (H2O). The eluate was neutralized by saturated aqueous NaHCO3 solution and then concentrated to dryness. The resulting residue was extracted with MeOH and it was filtered through Celite. The filtrate and washings were concentrated in vacuo to afford cyclopropylacetyl-(R)-carnitine (4) (60.4 mg, 47% yield) as colorless foam. mp 180°C (decomp.); Rf=0.31 (ODS, 1 : 4 MeOH–H2O); IR νmax (KBr): 3735, 3433, 1734, 1592, 1392, 1179, 1034 cm−1; 1H-NMR (D2O, HOD=4.79) δ: 0.15 (2H, m, H-4′, 5′), 0.52 (2H, m, H-4′, 5′), 0.99 (1H, m, H-3′), 2.28, (1H, dd, J=7.0, 16.0 Hz, H-2′), 2.36 (1H, dd, J=7.4, 16.0 Hz, H-2′), 2.49 (1H, dd, J=8.0, 16.0 Hz, H-2), 2.63 (1H, dd, J=5.6, 16.0 Hz, H-2), 3.18 (9H, s, 4-N+Me3), 3.61 (1H, d, J=14.0 Hz, H-4), 3.86 (1H, dd, J=9.0, 14.0 Hz, H-4), 5.63 (1H, m, H-3); 13C-NMR (D2O, DSS=–2.04) δ: 4.2 (C4′, 5′), 4.4 (C4′, 5′), 6.7 (C3′), 39.7 (C2′), 40.9 (C2), 54.5 (4-N+Me3), 67.5 (C3), 68.9 (C4), 175.7 (C1′), 177.2 (C1); LR-FAB-MS m/z 244 [M+H]+, 162 [M−C5H6O]+, HR-FAB-MS m/z 244.1555 [M+H]+; Calcd for C12H22NO4, 244.1549; [α]D34 −16.6 (H2O, c=0.67).
Toxicity on MiceOral InjectionThe water extract (200 mg) of the mushroom was dissolved in H2O (0.2 mL) and the solution was orally afforded to a mouse (ddy, female, 19–21 g body weight) using a catheter.
Intraperitoneal InjectionSynthetic carnitine ester (4, 10 mg) was dissolved in saline (0.2 mL) and the solution was intraperitoneally injected to a mouse (ddy, female, 9.5–10.5 g body weight).
Within 24 h, when the mouse died, the extract was regarded as toxic.
All animal experiments were performed with the approval of the Keio University School of Medicine Laboratory Animal Care and Use Committee.
The animals were maintained under constant environmental conditions and free access to food and water.
Incidents of mushroom poisoning attributed to R. subnigricans have recently increased in Japan; however, accurate identification of the fungus is difficult due to the existence of several similar, unidentified Russula species. Here, cyclopropylacetyl-(R)-carnitine (4) was isolated as a new compound and turned out to be a useful marker specific to genuine R. subnigricans. In the 1H-NMR spectrum of a crude water extract, the cyclopropane unit of the marker compound is easily recognizable, since the signals appear in the upfield region (0.15, 0.52 ppm), where other signals are rarely observed. It is expected that this unique marker compound will aid in future classification of this toxic fungus.
We are grateful to Messrs. Shigeo Morimoto and the late Toshiho Ueda (Kansai Mycological Club), Takashi Suda (Gunma Mushroom Club), and Prof. Emeritus Kazumasa Yokoyama (Shiga University) for the help in collecting and identifying the Russula species.
The authors declare no conflict of interest.