2016 Volume 64 Issue 7 Pages 1009-1018
2016 Volume 64 Issue 7 Pages 1009-1018
A practical preparation of 4-(substituted benzyl)-3-(2,3,4,6-tetra-O-acyl-β-D-glucopyranosyloxy)-1H-pyrazole derivative 2 is described. O-Glycosylation of 4-(substituted benzyl)-1,2-dihydro-3H-pyrazol-3-one derivative 3 was facilitated by introduction of electron-withdrawing substituents, such as an acetyl group, at the N1-position of the pyrazole ring. 1-Acetyl-4-(substituted benzyl)-1,2-dihydro-3H-pyrazol-3-one 10 reacted with 2,3,4,6-tetra-O-acyl-α-D-glucopyranosyl bromide 5 in the presence of potassium carbonate in acetonitrile to provide the 1-acetyl-4-(substituted benzyl)-3-(2,3,4,6-tetra-O-acyl-β-D-glucopyranosyloxy)-1H-pyrazole derivative 11 in high yield. When 2,3,4,6-tetra-O-pivaloyl-α-D-glucopyranosyl bromide (5b) was used as a glycosyl donor, the resulting O-glycosylated product 11 was N1-deacetylated in the presence of potassium bicarbonate in methanol without unfavorable deprotection of the glycosyl moiety to provide 2 in excellent yield. The synthetic intermediate 2b of Remogliflozin etabonate (1b) was synthesized using this strategy.
Some glucopyranosyloxypyrazole derivatives such as 1a–d (Fig. 1) have been demonstrated to inhibit the low-affinity Na+-dependent glucose co-transporter SGLT2.1–3) Two types of SGLT are known, SGLT1 and SGLT2, both of which act as transmembrane glucose transporters. Although SGLT1 (high-affinity Na+-dependent glucose co-transporter) is expressed to some extent in the kidney and contributes to glucose reabsorption, it is mainly expressed in the small intestine, where it plays an important role in glucose absorption.4,5) SGLT2 is specifically expressed in the kidney and plays an important role in renal glucose reabsorption in the proximal tubule.6) Remogliflozin (1a), discovered at Kissei Pharmaceutical Co., Ltd., exhibits an inhibitory activity that is highly selective for SGLT2.7,8) Remogliflozin etabonate (1b), a prodrug of 1a, is metabolized to its active form 1a in the body, and may therefore be useful as a preventive or therapeutic agent for diseases attributable to hyperglycemia such as diabetes, complications related to diabetes, and obesity.9,10)

The synthetic strategy for 1b, given in Chart 1, shows that the 4-[(4-isopropoxyphenyl)methyl]-5-methyl-3-(2,3,4,6-tetra-O-acyl-β-D-glucopyranosyloxy)-1H-pyrazole derivative 2 is an important intermediate. O-Glycosylation of 4-[(4-isopropoxyphenyl)methyl]-5-methyl-1,2-dihydro-3H-pyrazol-3-one (3a) with a glycosyl donor (4 or 5) is therefore a key step in the production of 1b. Various O-glycosylation methods of 1,2-dihydro-3H-pyrazol-3-one derivatives 3 have been reported, including the Koenigs–Knorr reaction, which employs a phase-transfer catalyst, and the Mitsunobu reaction.1,2,11–13) Although we also evaluated these methods for the preparation of 2, no successful results were obtained. Therefore, developing an efficient O-glycosylation method is strongly desired to establish scalable synthesis of 1b. We report here a convenient and practical method for the O-glycosylation of 3 with 2,3,4,6-tetra-O-acyl-α-D-glucopyranosyl bromide 5 via N1-acetylation of the pyrazole ring.

4-(Substituted benzyl)-1,2-dihydro-3H-pyrazol-3-one derivatives 3a–c were prepared by a two-step sequence starting from benzyl halide derivatives 6a–c, as shown in Table 1. Compounds 6a–c were reacted with methyl acetoacetate (7) in the presence of lithium halide and N,N-diisopropylethylamine to yield the corresponding alkylated β-keto ester derivatives 8a–c.14) The crude products of 8a–c were treated with hydrazine monohydrate in toluene to provide 3a–c as white solids.15)
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Benzyl halide | R1 | R2 | R3 | X1 | Product | Yield (%) |
| 1 | 6a | Oi-Pr | H | H | Cl | 3a | 71 |
| 2 | 6b | OMe | F | H | Cl | 3b | 69 |
| 3 | 6c | OMe | H | F | Cl | 3c | 64 |
a) The formation of α-anomer was not observed.
The various precedent methods for direct O-glycosylation of the 5-methylpyrazole derivative 3a with glycosyl donors 4 and 5a, b were first evaluated. The results are summarized in Table 2. In the Mitsunobu reaction, the desired product was provided, but with moderate β-selectivity (β : α ratio of 92 : 8) and only 29% yield (Table 2, entry 1). The Koenigs–Knorr reaction was more effective than the Mitsunobu reaction. The desired product was provided with high β-selectivity (β : α ratio of 98 : 2) due to the anchimeric assistance via intermediacy of 9 in Fig. 2; however, a satisfactory yield was not provided (44%, entry 2). In addition to low yield, these two methods are known to have several other disadvantages in terms of scalability. It is difficult to remove triphenylphosphine oxide from the Mitsunobu reaction mixture, especially at larger scales. The Koenigs–Knorr reaction has a high likelihood of contamination from silver-related elemental impurities in the final drug substance and coating of reaction vessels by the silver mirror reaction. Entries 3–5 indicate that the desired reaction did not proceed completely, with recovery of significant amounts of 3a and side reactions that resulted in the N1-acetylated product 10a and its O-glycosylated product 11a, causing low yields. When the reaction was performed with 2,3,4,6-tetra-O-pivaloyl-α-D-glucopyranosyl bromide (5b), N1-acylation of 3a did not occur. However, a satisfactory yield was not provided with concurrent recovery of 3a (47%, entry 6). Formation of an α-anomer was not observed in entries 3–6.
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Glycosyl donor | R5 | Reagent | Solvent | Temp. (°C) | Products | Yield (%) |
| 1 | 4 | Ac | PPh3, DEAD | THF | rt | 2a | 29 (α-anomer: 2.5) |
| 2 | 5a | Ac | Ag2CO3 | THF | rt | 2a | 44 (α-anomer: 0.7) |
| 3 | 5a | Ac | NaOH, BnN(n-Bu)3Cl | CH2Cl2–H2O | rt | 2a | 25 (10a: 21, 11a: 19)a) |
| 4 | 5a | Ac | K2CO3, BnN(n-Bu)3Cl | CH2Cl2–H2O | rt | 2a | 32 (10a: 18, 11a: 23)a) |
| 5 | 5a | Ac | K2CO3 | MeCN | 60 | 2a | 36 (10a: 10, 11a: 26)a) |
| 6 | 5b | Piv | K2CO3 | MeCN | 60 | 2b | 47a) |
a) The formation of α-anomer was not observed.

To improve the yield of O-glycosylation, we attempted the O-glycosylation reaction of 1-substituted-1,2-dihydro-4-[(4-isopropoxyphenyl)methyl]-5-methyl-3H-pyrazol-3-one 10. 1-Acetyl-1,2-dihydro-4-[(4-isopropoxyphenyl)methyl]-5-methyl-3H-pyrazol-3-one (10a), which has an acetyl group as an electron-withdrawing group at the N1-position of the pyrazol ring, was prepared in an 81% yield by reacting 3a with acetic anhydride in the presence of potassium carbonate (K2CO3) in N,N-dimethylformamide (DMF) at 70°C, as shown in Chart 2. As a different type of pyrazole derivative, 1-methyl-1,2-dihydro-4-[(4-isopropoxyphenyl)methyl]-5-methyl-3H-pyrazol-3-one (10b), having a methyl group as an electron-donating group at the N1-position of the pyrazole ring, was prepared by a four-step sequence starting from 10a, as shown in Chart 3. 10a was reacted with benzyl bromide in the presence of K2CO3 in acetonitrile (MeCN) to provide 12 in a 69% yield. 12 was treated with potassium bicarbonate (KHCO3) in MeOH to provide 13. The crude product of 13 was reacted with iodomethane (MeI) in the presence of sodium hydride (NaH) to provide 14 in an 85% yield from 12. Then, 14 was debenzylated in the presence of 10% Pd/C in tetrahydrofuran (THF) under a H2 atmosphere to provide 10b in a 99% yield.


Reagents: (a) Benzyl bromide, K2CO3, MeCN; (b) KHCO3, MeOH; (c) MeI, NaH, N,N-dimethylacetamide (DMAc); (d) 10% Pd/C (wet), H2, THF.
The O-glycosylation of 10a with 5a in the presence of K2CO3 in MeCN was evaluated at 60°C. Under this condition, O-glycosylation proceeded efficiently and provided 11a with a 90% yield (Table 3, entry 1). However, under the same condition, O-glycosylation of 10b with 5a provided 11b in only 26% yield (entry 2). Formation of the α-anomer and glucoside-orthoester was not observed in either case. Based on these results, the suggested reaction mechanism is that O-glycosylation proceeds through a direct SN2-type displacement in the presence of K2CO3 in MeCN, and formation of the oxonium intermediate 9 does not occur. The reactive species in this reaction is the imidic acid of 10, and the electron-withdrawing acetyl group at the N1-position of the pyrazole ring in 10a increases the acidity of the 3-OH group of this imidic acid. Therefore, the formation of the imidic acid salt of 10a is facilitated under mildly basic conditions such as K2CO3, as shown in Chart 4.
 | ||||
|---|---|---|---|---|
| Entry | Aglycon | R6 | O-Glycosylation | |
| Product | Yield (%)a) | |||
| 1 | 10a | Ac | 11a | 90b) |
| 2 | 10b | Me | 11b | 26b) |
a) Isolated by column chromatography. b) The formation of α-anomer was not observed.

The acetyl group at the N1-position of the pyrazole ring was easily removed under N1-deacetylation conditions, that is, in the presence of KHCO3 in MeOH. Unfortunately, treatment of 11a under the above conditions provided 2a in a low yield (45%) because of the low selectivity of O- and N1-deacetylation in the presence of KHCO3 in MeOH, as shown in Chart 5.

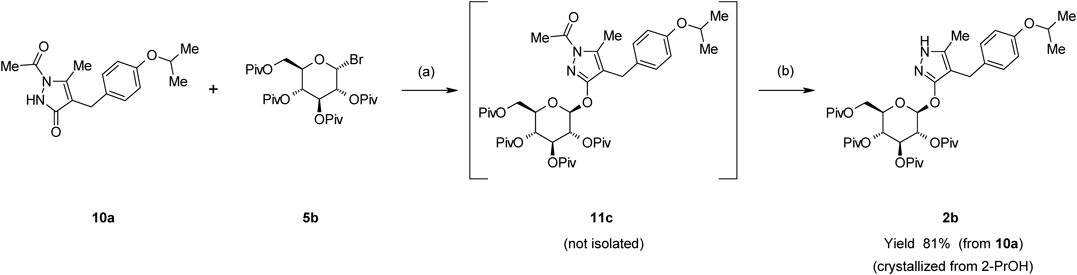
To prevent undesired O-deacetylation of the glucosyl moiety, we attempted to introduce a pivaloyl group as the O-protective group of the glucosyl moiety, which is more stable than the acetyl group under basic conditions. The results are summarized in Table 4. The O-glycosylation of 10a with 5b in the presence of K2CO3 in MeCN provided 11c with a 92% yield (entry 1). On the other hand, sodium carbonate (Na2CO3) and potassium t-butoxide (t-BuOK) as a base were less efficient (entries 2, 3). With regard to solvents, THF was less efficient (entry 4). The best overall condition for O-glycosylation was therefore observed using K2CO3 and MeCN. The O-glycosylation of 10a with 5b provided the desired product 11c in a high yield.
 | ||||
|---|---|---|---|---|
| Entry | Base | (mol%) | Solvent | Yield (%) |
| 1 | K2CO3 | (140) | MeCN | 92 |
| 2 | Na2CO3 | (140) | MeCN | 75 |
| 3 | t-BuOK | (110) | MeCN | 77 |
| 4 | K2CO3 | (140) | THF | 73 |
Furthermore, introducing a pivaloyl group as the O-protective group of the glucosyl moiety effectively led to N1-deacetylation with high selectivity without unfavorable deprotection of the glucosyl moiety, providing the desired product 2b in a 98% yield, as shown in Chart 6. 5b is easily prepared from D-glucose by a known synthetic method, and is commercially available and inexpensive.16,17) In addition, 5a is an unstable compound that requires storage below −20°C; 5b is stable and can be stored at room temperature without special caution. These characteristics make 5b the more suitable glycosyl donor for this method.

Implementation of the above results made it possible to proceed with O-glycosylation of 10a with 5b and N1-deacetylation in a high yield. Therefore, the crude product of 11c after O-glycosylation was treated with KHCO3 in MeOH, and then the resulting crude product of 2b was crystallized from 2-PrOH to provide 2b in an 81% yield from 10a, as shown in Chart 7. This method represents an easily scalable process for the efficient synthesis of 2b.
Reagents: (a) K2CO3, MeCN; (b) KHCO3, MeOH.
An even more efficient pathway to 2b was attempted in a one-pot synthesis without isolation of 10a and 11c. The results are summarized in Table 5. Compound 3a was acetylated by acetic anhydride in the presence of K2CO3 in DMF. MeCN, aqueous K2CO3, and 5b were then added to the reaction mixture and the reaction was allowed to proceed at 60°C. The resulting crude product of 11c was treated with KHCO3 in MeOH. In this way, the desired product 2b was provided, with a high yield of 86%, from 3a (entry 1).
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Aglycon | R1 | R2 | R3 | 5b (mol%) | Product | Yield (%) |
| 1 | 3a | Oi-Pr | H | H | (130) | 2b | 86 |
| 2 | 3b | OMe | F | H | (130) | 2c | 85 |
| 3 | 3c | OMe | H | F | (130) | 2d | 87 |
The same methodology was then applied to other 5-methylpyrazole derivatives 3b, c, and the corresponding products 2c, d were provided in high yields (entries 2, 3). These results demonstrate the applicability of this method for O-glycosylation of various 5-methylpyrazole derivatives.
Remogliflozin etabonate (1b) was prepared by a four-step sequence starting from 2b, as shown in Chart 8. Introduction of an isopropyl group to 2b with 2-iodopropane in the presence of NaH in 1,3-dimethyl-2-imidazolidinone (DMI) provided 4-[(4-isopropoxyphenyl)methyl]-1-isopropyl-5-methyl-3-(2,3,4,6-tetra-O-acyl-β-D-glucopyranosyloxy)-1H-pyrazole derivative (15) in an 86% yield. The depivaloylation of 15 in the presence of sodium methoxide (MeONa) in MeOH provided Remogliflozin (1a) in a 99% yield. The reaction of 1a with ethyl chloroformate in the presence of 2,6-lutidine and pyridine in MeCN provided 16 as an ethanol solvate of 1b in a 72% yield. 16 was crystallized from a mixed solvent of methyl t-butyl ether (MTBE) and n-heptane to provide 1b in a 98% yield.

Reagents: (a) 2-Iodopropane, NaH, DMI; (b) MeONa, MeOH; (c) Ethyl chloroformate, 2,3-lutidine, pyridine, MeCN; (d) MTBE, n-heptane.
In conclusion, an efficient and practical method for the synthesis of 2b, an important intermediate in the synthesis of 1b, was established. These results suggest that this O-glycosylation method could be applied in syntheses of additional glucopyranosyloxypyrazole derivatives exhibiting SGLT2 inhibitory activity such as 1c, d.
All melting points were determined on a Yanagimoto melting point apparatus and are uncorrected. Optical rotations were recorded on a JASCO P-2300 polarimeter. IR spectra were recorded on a Nicolet AVATAR 320 FT-IR spectrometer. 1H- and 13C-NMR spectra were recorded on a Bruker AV-400M (400 MHz) or DRX-500 (500 MHz) spectrometer using tetramethylsilane as the internal standard. High-resolution (HR)-MS were recorded on an Agilent Technologies Q-TOF 6520 mass spectrometer.
General Procedure for the Preparation of 4-(Substituted benzyl)-1,2-dihydro-5-methyl-3H-pyrazol-3-ones (3a–c)N,N-Diisopropylethylamine (12.9 g, 100 mmol) was added dropwise to a mixture of an appropriate benzyl chloride 6a–c (50 mmol), methyl acetoacetate (7) (6.39 g, 55 mmol), lithium chloride (2.33 g, 55 mmol), and potassium iodide (0.896 g, 5.4 mmol) in a mixed solvent of toluene (20 mL) and DMF (20 mL) at 50°C with stirring. The reaction mixture was stirred at 70°C for 12 h and then cooled to room temperature. After the addition of toluene (20 mL), the resulting mixture was washed successively with water (30 mL), 2 M HCl (30 mL), water (30 mL), saturated aqueous NaHCO3 (30 mL), and water (30 mL). The organic layer was concentrated under reduced pressure. Hydrazine monohydrate (3.25 g, 65 mmol) was added dropwise to the residual solution in toluene (30 mL) at room temperature. The reaction mixture was stirred for 12 h at 70°C and then cooled to room temperature. n-Hexane (30 mL) and 2-propanol (5 mL) were then added to the mixture in that order. The resulting slurry was stirred for 1 h at room temperature and for an additional 1 h at 0°C. The precipitate was filtered off and dried in vacuo to provide 3a–c.
1,2-Dihydro-4-[(4-isopropoxyphenyl)methyl]-5-methyl-3H-pyrazol-3-one (3a)White solid (71% from 6a). mp 227–234°C (decomp.). IR (KBr) cm−1: 2977, 1609, 1543, 1507, 1477, 1420, 1383, 1372. 1H-NMR (DMSO-d6) δ: 1.22 (6H, d, J=6.0 Hz), 2.00 (3H, s), 3.46 (3H, s), 4.46–4.55 (1H, m), 6.77 (2H, d, J=8.8 Hz), 7.04 (2H, d, J=8.8 Hz), 10.02–10.46 (2H, br s). 13C-NMR (DMSO-d6) δ: 9.86 (q), 21.77 (q×2), 26.28 (t), 68.87 (d), 100.30 (s), 115.26 (d×2), 128.83 (d×2), 133.60 (s), 136.65 (s), 155.22 (s), 159.55 (s). HR-MS (electrospray ionization (ESI)) m/z: 247.1448 [M+H]+ (Calcd for C14H19N2O2: 247.1441).
1,2-Dihydro-4-[(3-fluoro-4-methoxyphenyl)methyl]-5-methyl-3H-pyrazol-3-one (3b)White solid (69% from 6b). mp 216–220°C (decomp.). IR (KBr) cm−1: 1609, 1518, 1435, 1275. 1H-NMR (DMSO-d6) δ: 2.01 (3H, s), 3.49 (2H, s), 3.78 (3H, s), 6.90–6.97 (2H, m), 7.02 (1H, t, J=8.7 Hz), 10.39 (2H, br s). 13C-NMR (DMSO-d6) δ: 9.79 (q), 26.17 (t), 55.85 (q), 99.74 (s), 113.55 (d, JCF=1.5 Hz), 115.22 (d, JCF=17.6 Hz), 123.68 (d, JCF=2.9 Hz), 134.98 (d, JCF=5.8 Hz), 136.83 (s), 144.83 (d, JCF=10.3 Hz), 151.17 (d, JCF=244.3 Hz), 159.55 (s). HR-MS (ESI) m/z: 237.1030 [M+H]+ (Calcd for C12H14FN2O2: 237.1034).
1,2-Dihydro-4-[(2-fluoro-4-methoxyphenyl)methyl]-5-methyl-3H-pyrazol-3-one (3c)White solid (64% from 6c). mp 226–227°C (decomp.). IR (KBr) cm−1: 1628, 1598, 1512, 1466, 1446, 1435, 1409. 1H-NMR (DMSO-d6) δ: 2.00 (3H, s), 3.48 (2H, s), 3.71 (3H, s), 6.67 (1H, dd, J=2.4, 8.4 Hz), 6.74 (1H, dd, J=2.9, 12 Hz), 7.02 (1H, t, J=8.8 Hz), 10.38 (2H, br s). 13C-NMR (DMSO-d6) δ: 9.71 (q), 19.41 (d, JCF=2.9 Hz), 55.34 (q), 98.48 (s), 101.00 (d, JCF=25.7 Hz), 109.76 (d, JCF=2.9 Hz), 119.70 (d, JCF=16.1 Hz), 130.58 (d, JCF=7.4 Hz), 136.85 (s), 158.53 (d, JCF=11.0 Hz), 159.68 (s), 160.34 (d, JCF=242.9 Hz). HR-MS (ESI) m/z: 237.1031 [M+H]+ (Calcd for C12H14FN2O2: 237.1034).
1-Acetyl-1,2-dihydro-4-[(4-isopropoxyphenyl)methyl]-5-methyl-3H-pyrazol-3-one (10a)Acetic anhydride (3.27 g, 32 mmol) was added dropwise to a mixture of 3a (7.39 g, 30 mmol) and K2CO3 (4.56 g, 33 mmol) in DMF (27 mL) at 40°C. The reaction mixture was stirred for 30 min at 40°C and for 1 h at 70°C. The reaction mixture was cooled to 50°C and the inorganic salt was filtered off and washed with DMF (12 mL). Acetic acid (0.180 g, 3.0 mmol) in H2O (1.8 mL) was added to the combined filtrates and stirred for 30 min at 20°C. H2O (30 mL) was added to the resulting slurry and the mixture was stirred for 1 h at 20°C. The precipitate was filtered off and dried in vacuo at 60°C to provide 10a (7.01 g, 81%) as a white solid. mp 166–173°C (decomp.). IR (KBr) cm−1: 2978, 2924, 1725, 1626, 1609, 1539, 1507, 1396, 1377, 1318, 1307. 1H-NMR (DMSO-d6) δ: 1.22 (6H, d, J=6.0 Hz), 2.41 (3H, s), 2.45 (3H, s), 3.52 (2H, s), 4.47–4.56 (1H, m), 6.79 (2H, d, J=8.7 Hz), 7.05 (2H, d, J=8.7 Hz), 10.97 (1H, br s). 13C-NMR (DMSO-d6) δ: 12.84 (q), 21.77 (q×2), 23.16 (q), 25.75 (t), 68.93 (d), 111.25 (s), 115.46 (d×2), 128.93 (d×2), 131.70 (s), 139.97 (s), 155.62 (s), 161.62 (s), 169.88 (s). HR-MS (ESI) m/z: 289.1541 [M+H]+ (Calcd for C16H21N2O3: 289.1547).
1-Acetyl-3-benzyloxy-4-[(4-isopropoxyphenyl)methyl]-5-methyl-1H-pyrazole (12)A mixture of 10a (4.32 g, 15 mmol), benzyl bromide (3.08 g, 18 mmol), and K2CO3 (3.11 g, 22.5 mmol) in MeCN (20 mL) was stirred at 60°C for 2 h and the resulting inorganic salt was filtered off. The filtrate was concentrated under reduced pressure and the residue was purified by silica gel chromatography (eluent AcOEt–n-hexane, 1 : 3) to provide 12 (3.89 g, 69%) as a colorless oil. IR (KBr) cm−1: 2975, 2934, 1718, 1612, 1507, 1467, 1453, 1375, 1337. 1H-NMR (CDCl3) δ: 1.31 (6H, d, J=6.0 Hz), 2.84 (3H, s), 2.56 (3H, s), 3.56 (2H, s), 4.44–4.53 (1H, m), 5.26 (2H, s), 6.77 (2H, d, J=8.5 Hz), 7.06 (2H, d, J=8.5 Hz), 7.30–7.35 (5H, m). 13C-NMR (CDCl3) δ: 13.14 (q), 22.07 (q×2), 23.37 (q), 26.50 (t), 69.86 (d), 70.07 (t), 111.38 (s), 115.85 (d×2), 127.84 (d×2), 127.93 (d), 128.34 (d×2), 129.17 (d×2), 131.69 (s), 136.83 (s), 141.24 (s), 156.24 (s), 162.54 (s), 170.98 (s). HR-MS (ESI) m/z: 379.2018 [M+H]+ (Calcd for C23H27N2O3: 379.2016).
3-Benzyloxy-4-[(4-isopropoxyphenyl)methyl]-1,5-dimethyl-1H-pyrazole (14)A mixture of 12 (3.60 g, 9.51 mmol) and KHCO3 (0.286 g, 2.85 mmol) in MeOH (20 mL) was stirred at 50°C for 2 h and the reaction mixture was concentrated under reduced pressure. AcOEt (40 mL) was added to the residue and the mixture was washed with H2O (20 mL). The organic layer was dried over MgSO4 and the filtrate was concentrated under reduced pressure to provide an oil. The oil was dissolved in DMAc (10 mL) and added dropwise to a suspension of sodium hydride (0.571 g, 14.3 mmol, 60% oil dispersion) in DMAc (5 mL) at 0°C under a N2 atmosphere and the mixture was stirred for 15 min at 0°C. MeI (2.70 g, 19.0 mmol) was added and the mixture was stirred for 2 h. The reaction mixture was diluted with AcOEt (70 mL) and washed with H2O (30 mL×2). The resulting organic layer was dried over MgSO4 and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel chromatography (eluent AcOEt–n-hexane, 1 : 2) to provide 14 (2.84 g, 85% yield) as a colorless oil. IR (KBr) cm−1: 2974, 2934, 1738, 1611, 1580, 1506, 1464, 1453, 1383, 1365, 1333. 1H-NMR (CDCl3) δ: 1.30 (2H, d, J=6.0 Hz), 2.07 (3H, s), 3.60 (2H, s), 3.62 (3H, s), 4.43–4.52 (1H, m), 5.21 (2H, s), 6.74 (2H, d, J=8.6 Hz), 7.08 (2H, d, J=8.6 Hz), 7.26–7.39 (5H, m). 13C-NMR (CDCl3) δ: 9.88 (q), 22.10 (q×2), 27.21 (t), 35.56 (q), 69.86 (d), 69.97 (t), 102.40 (s), 115.76 (d×2), 127.45 (d×2), 127.55 (d), 128.28 (d×2), 129.10 (d×2), 133.52 (s), 137.45 (s), 137.77 (s), 155.93 (s), 160.17 (s). HR-MS (ESI) m/z: 351.2071 [M+H]+ (Calcd for C22H27N2O2: 351.2067).
1,2-Dihydro-4-[(4-isopropoxyphenyl)methyl]-1,5-dimethyl-3H-pyrazol-3-one (10b)A solution of 14 (2.70 g, 7.70 mmol) in THF (30 mL) was hydrogenated over 10% Pd/C (50% wet, 0.30 g) for 10 h at room temperature under atmospheric pressure. The Pd–C was filtered off and the filtrate was concentrated under reduced pressure to provide 10b (1.98 g, 99%) as a white solid. mp 160–162°C. IR (KBr) cm−1: 1613, 1540, 1511, 1372, 1300. 1H-NMR (DMSO-d6) δ: 1.22 (6H, d, J=6.0 Hz), 2.03 (3H, s), 3.45 (2H, s), 3.47 (3H, s), 4.46–4.55 (1H, m), 6.76 (2H, d, J=8.5 Hz), 7.03 (2H, d, J=8.5 Hz), 9.37 (1H, br s). 13C-NMR (DMSO-d6) δ: 9.31(q), 21.76 (q×2), 26.56 (t), 34.97 (q), 68.87 (d), 100.95 (s), 115.27 (d×2), 128.76 (d×2), 133.59 (s), 136.22 (s), 155.24 (s), 157.91 (s). HR-MS (ESI) m/z: 261.1609 [M+H]+ (Calcd for C15H21N2O2: 261.1598).
1-Acetyl-4-[(4-isopropoxyphenyl)methyl]-5-methyl-3-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyloxy)-1H-pyrazole (11a)5a (0.987 g, 2.4 mmol) was added to a mixture of 10a (0.577 g, 2.0 mmol) and K2CO3 (0.387 g, 2.8 mmol) in MeCN (5 mL), and the reaction mixture was stirred for 16 h at 60°C. The mixture was diluted with AcOEt (20 mL) and washed with H2O (5 mL). The resulting organic layer was concentrated under reduced pressure. The residue was purified by silica gel chromatography (eluent AcOEt–n-hexane, 1 : 3) to provide 11a (1.11 g, 90% yield). An analytical sample of 11a was obtained as a white solid by recrystallization from Et2O. mp 68–72°C. [α]D20 −19.3 (c=1.0, dimethyl sulfoxide (DMSO)). IR (KBr) cm−1: 3436, 2977, 2934, 1759, 1611, 1509, 1472, 1431, 1373, 1336. 1H-NMR (CDCl3) δ: 1.29 (3H, dd, J=3.8, 6.2 Hz), 1.86 (3H, s), 2.02 (3H, s), 2.04 (3H, s), 2.06 (3H, s), 2.50 (3H, s), 2.55 (3H, s), 3.54 (2H, dd, J=15.6, 19.9 Hz), 3.86–3.91 (1H, m), 4.16 (1H, dd, J=2.3, 12.5 Hz), 4.27 (1H, dd, J=4.8, 12.2 Hz), 4.43–4.52 (1H, m), 5.18 (1H, t, J=9.6 Hz), 5.24–5.33 (2H, m), 5.71 (1H, d, J=7.8 Hz), 6.75 (2H, d, J=8.5 Hz), 7.01 (2H, d, J=8.5 Hz). 13C-NMR (CDCl3) δ: 13.12 (q), 20.41 (q), 20.60 (q×2), 20.71 (q), 22.03 (q), 22.09 (q), 23.19 (q), 26.25 (t), 61.82 (t), 68.17 (d), 69.87 (d), 70.62 (d), 72.48 (d), 72.83 (d), 96.67 (d), 111.39 (s), 115.93 (d×2), 129.13 (d×2), 131.30 (s), 141.76 (s), 156.31 (s), 160.45 (s), 169.15 (s), 169.42 (s), 170.23 (s), 170.61 (s), 170.85 (s). HR-MS (ESI) m/z: 619.2473 [M+H]+ (Calcd for C30H39N2O12: 619.2498).
4-[(4-Isopropoxyphenyl)methyl]-1,5-dimethyl-3-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyloxy)-1H-pyrazole (11b)5a (0.987 g, 2.4 mmol) was added to a mixture of 10b (0.521 g, 2.0 mmol) and K2CO3 (0.387 g, 2.8 mmol) in MeCN (5 mL) and the reaction mixture was stirred for 16 h at 60°C. The reaction mixture was diluted with AcOEt (20 mL) and washed with H2O (5 mL). The resulting organic layer was concentrated under reduced pressure. The residue was purified by silica gel chromatography (eluent AcOEt–n-hexane, 1 : 1) to provide 11b (0.311 g, 26%). An analytical sample of 11b was obtained as a white solid by recrystallization from Et2O-n-hexane. mp 65–69°C. [α]D20 −11.2 (c=1.0, DMSO). IR (KBr) cm−1: 2977, 1759, 1751, 1508, 1496, 1371. 1H-NMR (CDCl3) δ: 1.29 (6H, dd, J=1.9, 6.3 Hz), 1.90 (3H, s), 2.01 (3H, s), 2.03 (3H, s), 2.06 (3H, s), 2.07 (3H, s), 3.54 (2H, dd, J=16.0, 14.7 Hz), 3.60 (3H, s), 3.82–3.86 (1H, m), 4.09–4.16 (1H, m), 4.30 (1H, dd, J=4.3, 12.5 Hz), 4.42–4.51 (1H, m), 5.17–5.29 (3H, m), 5.53–5.55 (1H, m), 6.74 (2H, d, J=8.7 Hz), 7.02 (2H, d, J=8.7 Hz). 13C-NMR (CDCl3) δ: 9.92 (q), 20.53 (q), 20.62 (q), 20.65 (q), 20.75 (q), 22.07 (q), 22.10 (q), 26.88 (t), 35.77 (q), 61.71 (t), 68.18 (d), 69.85 (d), 70.98 (d), 71.94 (d), 72.96 (d), 97.76 (d), 103.62 (s), 115.78 (d×2), 129.06 (d×2), 133.09 (s), 137.63 (s), 155.97 (s), 157.67 (s), 169.34 (s), 169.45 (s), 170.27 (s), 170.77 (s). HR-MS (ESI) m/z: 591.2554 [M+H]+ (Calcd for C29H39N2O11: 591.2548).
4-[(4-Isopropoxyphenyl)methyl]-5-methyl-3-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyloxy)-1H-pyrazole (2a)A mixture of 11a (0.618 g, 1.0 mmol) and KHCO3 (0.030 g, 0.30 mmol) in MeOH (3 mL) was stirred at 50°C for 1 h; AcOH (0.018 g, 0.30 mmol) was then added at room temperature. After the reaction mixture was concentrated under reduced pressure, the residue was purified by silica gel chromatography (eluent AcOEt–n-hexane, 1 : 2) to provide 2a (0.259 g, 45% yield). An analytical sample of 2a was obtained as a white solid by recrystallization from Et2O. mp 155–156°C. [α]D20 −7.9 (c=1.0, DMSO). IR (KBr) cm−1: 3393, 3222, 2978, 2943, 1751, 1735, 1612, 1509, 1474, 1434, 1374. 1H-NMR (CDCl3) δ: 1.30 (6H, dd, J=2.3, 5.9 Hz), 1.89 (3H, s), 2.01 (3H, s), 2.03 (3H, s), 2.05 (3H, s), 2.10 (3H, s), 3.55 (2H, dd, J=15.7, 11.7 Hz), 3.83–3.87 (1H, m), 4.11 (1H, dd, J=2.3, 12.5 Hz), 4.31 (1H, dd, J=4.1, 12.3 Hz), 4.43–4.52 (1H, m), 5.18–5.30 (3H, m), 5.58 (1H, d, J=6.8 Hz), 6.75 (2H, d, J=8.8 Hz), 7.02 (2H, d, J=8.8 Hz), 9.09 (1H, br s). 13C-NMR (CDCl3) δ: 10.36 (q), 20.48 (q), 20.61 (q), 20.63 (q), 20.72 (q), 22.07 (q), 22.10 (q), 26.50 (t), 61.64 (t), 68.07 (d), 69.89 (d), 70.93 (d), 72.06 (d), 72.92 (d), 97.67 (d), 103.54 (s), 115.84 (d×2), 129.12 (d×2), 132.69 (s), 138.21 (s), 156.05 (s), 159.89 (s), 169.31 (s), 169.44 (s), 170.30 (s), 170.76 (s). HR-MS (ESI) m/z: 577.2380 [M+H]+ (Calcd for C28H37N2O11: 577.2392).
1-Acetyl-4-[(4-isopropoxyphenyl)methyl]-5-methyl-3-(2,3,4,6-tetra-O-pivaloyl-β-D-glucopyranosyloxy)-1H-pyrazole (11c)5b (1.39 g, 2.4 mmol) was added to a mixture of 10a (0.577 g, 2.0 mmol) and K2CO3 (0.387 g, 2.8 mmol) in MeCN (5 mL), and the mixture was stirred for 16 h at 60°C. The reaction mixture was diluted with AcOEt (20 mL) and washed with H2O (5 mL). The resulting organic layer was concentrated under reduced pressure. The obtained residue was purified by silica gel chromatography (eluent AcOEt–n-hexane, 1 : 3) to provide 11c (1.44 g, 92% yield). An analytical sample of 10c was obtained as a white solid by recrystallization from n-hexane. mp 137–140°C. [α]D20 −2.0 (c=1.0, DMSO). IR (KBr) cm−1: 3469, 2975, 2936, 2875, 1747, 1612, 1509, 1480, 1470, 1398, 1370, 1333. 1H-NMR (CDCl3) δ: 1.01 (9H, s), 1.13 (9H, s), 1.16 (9H, s), 1.18 (9H, s), 1.29 (6H, dd, J=2.4, 6.1 Hz), 2.47 (3H, s), 2.54 (3H, s), 3.53 (2H, s), 3.88–3.92 (1H, m), 4.12–4.20 (2H, m), 4.42–4.51 (1H, m), 5.23 (1H, t, J=9.7 Hz), 5.28–5.33 (1H, m), 5.43 (1H, t, J=9.4 Hz), 5.84 (1H, d, J=8.2 Hz), 6.74–6.78 (2H, m), 7.01–7.04 (2H, m). 13C-NMR (CDCl3) δ: 13.19 (q), 22.05 (q), 22.10 (q), 23.22 (q), 26.23 (t), 26.88 (q×3), 27.03 (q×6), 27.15 (q×3), 38.66 (s), 38.74 (s), 38.77 (s), 38.83 (s), 61.61 (t), 67.64 (d), 69.83 (d), 70.71 (d), 72.21 (d), 72.89 (d), 97.00 (d), 111.52 (s), 115.96 (d×2), 129.17 (d×2), 131.23 (s), 141.82 (s), 156.30 (s), 160.57 (s), 170.88 (s), 176.38 (s), 176.40 (s), 177.20 (s), 178.03 (s). HR-MS (ESI) m/z: 787.4373 [M+H]+ (Calcd for C42H63N2O12: 787.4376).
4-[(4-Isopropoxyphenyl)methyl]-5-methyl-3-(2,3,4,6-tetra-O-pivaloyl-β-D-glucopyranosyloxy)-1H-pyrazole (2b)A mixture of 11c (0.787 g, 1.0 mmol) and KHCO3 (0.030 g, 0.30 mmol) in MeOH (3 mL) was stirred at 50°C for 1 h; AcOH (0.018 g, 0.30 mmol) was then added at room temperature. After the reaction mixture had been concentrated under reduced pressure, the residue was purified by silica gel chromatography (eluent AcOEt–n-hexane, 1 : 2) to provide 2b (0.731 g, 98% yield). An analytical sample of 2b was obtained as a white solid by recrystallization from 2-PrOH. mp 159–163°C. [α]D20 −10.8 (c=1.0, DMSO). IR (KBr) cm−1: 3383, 2975, 2936, 2874, 1746, 1729, 1507, 1482. 1H-NMR (CDCl3) δ: 1.05 (9H, s), 1.12 (9H, s), 1.15 (9H, s), 1.18 (9H, s), 1.30 (6H, dd, J=1.2, 6.0 Hz), 2.06 (3H, s), 3.54 (2H, s), 3.84–3.88 (1H, m), 4.11–4.20 (2H, m), 4.41–4.53 (2H, m), 5.23–5.32 (2H, m), 5.38 (1H, t, J=9.2 Hz), 5.68 (2H, d, J=8.0 Hz), 6.75 (2H, d, J=8.8 Hz), 7.04 (2H, d, J=8.8 Hz), 8.82 (1H, br s). 13C-NMR (CDCl3) δ: 10.42 (q), 22.07 (q), 22.11 (q), 22.6 (t), 26.95 (q×3), 27.04 (q×3), 27.06 (q×3), 27.14 (q×3), 38.68 (s), 38.72 (s), 38.74 (s), 38.83 (s), 61.53 (t), 67.57 (d), 69.84 (d), 70.94 (d), 72.41 (d), 72.45 (d), 97.72 (d), 103.62 (s), 115.80 (d×2), 129.23 (d×2), 132.68 (s), 138.00 (s), 156.00 (s), 159.83 (s), 176.36 (s), 176.55 (s), 177.24 (s), 178.12 (s). HR-MS (ESI) m/z: 745.4262 [M+H]+ (Calcd for C40H61N2O11: 745.4270).
General Procedure for the Preparation of 4-(Substituted benzyl)-5-methyl-3-(2,3,4,6-tetra-O-pivaloyl-β-D-glucopyranosyloxy)-1H-pyrazoles (2b–d)Acetic anhydride (0.325 g, 3.18 mmol) was added dropwise to a mixture of an appropriate 1,2-dihydro-4-(substituted benzyl)-5-methyl-3H-pyrazol-3-one (3a–c) (3.0 mmol), K2CO3 (0.456 g, 3.3 mmol) in DMF (2 mL) at 40°C. The mixture was stirred for 30 min at 40°C and for 1 h at 70°C. The reaction mixture was cooled to 40°C and MeCN (25 mL), and an aqueous solution of 25% K2CO3 (3.33 g, 6.0 mmol) and 5b (2.26 g, 3.9 mmol) were added successively to the mixture. The mixture was stirred for 6 h at 60°C and cooled to room temperature. AcOEt (25 mL) and H2O (5 mL) were added to the mixture with stirring and the aqueous layer was removed. The organic layer was concentrated under reduced pressure. KHCO3 (0.090 g, 0.90 mmol) was added to a solution of the residue in MeOH (10 mL) and the mixture was stirred for 2 h at 60°C. After the addition of AcOH (0.054 g, 0.90 mmol), the reaction mixture was concentrated under reduced pressure. The residue was purified by silica gel chromatography (eluent AcOEt–n-hexane, 1 : 3) to provide 2b–d.
4-[(3-Fluoro-4-methoxyphenyl)methyl]-5-methyl-3-(2,3,4,6-tetra-O-pivaloyl-β-D-glucopyranosyloxy)-1H-pyrazole (2c)An analytical sample of 2c was obtained as a white solid by recrystallization from 2-PrOH–n-hexane. mp 189–192°C. [α]D20 −11.1 (c=1.0, DMSO). IR (KBr) cm−1: 3373, 2970, 2909, 2874, 1747, 1729, 1603, 1516, 1482, 1396, 1365. 1H-NMR (CDCl3) δ: 1.05 (9H, s), 1.12 (9H, s), 1.15 (9H, s), 1.18 (9H, s), 2.07 (3H, s), 3.54 (2H, s), 3.84 (3H, s), 3.84–3.89 (1H, m), 4.11–4.21 (2H, m), 5.23–5.31 (2H, m), 5.39 (1H, t, J=9.5 Hz), 5.69 (1H, d, J=8.0 Hz), 6.81–6.89 (3H, m), 8.93 (1H, br s). 13C-NMR (CDCl3) δ: 10.36 (q), 26.49 (t), 26.92 (q×3), 27.03 (q×6), 27.14 (q×3), 38.68 (s), 38.72 (s), 38.74 (s), 38.83 (s), 56.32 (q), 61.50 (t), 67.56 (d), 70.93 (d), 72.35 (d), 72.48 (d), 97.76 (d), 102.97 (s), 113.36 (d, JCF=2.2 Hz), 115.88 (d, JCF=18.3 Hz), 123.76 (d, JCF=2.9 Hz), 133.89 (d, JCF=5.9 Hz), 138.04 (s), 145.65 (d, JCF=10.3 Hz), 152.23 (d, JCF=244.9 Hz), 159.75 (s), 176.37 (s), 176.59 (s), 177.25 (s), 178.11 (s). HR-MS (ESI) m/z: 735.3844 [M+H]+ (Calcd for C38H56FN2O11: 735.3863).
4-[(2-Fluoro-4-methoxyphenyl)methyl]-5-methyl-3-(2,3,4,6-tetra-O-pivaloyl-β-D-glucopyranosyloxy)-1H-pyrazole (2d)An analytical sample of 2d was obtained as a white solid by recrystallization from 2-PrOH–n-hexane. mp 167–169°C. [α]D20 −7.1 (c=1.0, DMSO). IR (KBr) cm−1: 3381, 2976, 2934, 2910, 1747, 1729, 1626, 1587, 1506, 1482, 1443, 1431, 1394, 1364. 1H-NMR (CDCl3) δ: 1.05 (9H, s), 1.13 (9H, s), 1.15 (9H, s), 1.18 (9H, s), 2.10 (3H, s), 3.54 (2H, dd, J=15.6, 20.7 Hz), 3.75 (3H, s), 3.84–3.88 (1H, m), 4.12 (1H, dd, J=4.5, 12.3 Hz), 4.17 (1H, dd, J=1.8, 12.3 Hz), 5.23–5.32 (2H, m), 5.38 (1H, t, J=9.5 Hz), 5.68 (1H, d, J=8.0 Hz), 6.52–6.59 (2H, m), 7.07 (1H, t, J=8.7 Hz), 8.93 (1H, br s). 13C-NMR (CDCl3) δ: 10.14 (q), 19.70 (d, JCF=3.3 Hz), 26.94 (q×3), 27.04 (q×6), 27.16 (q×3), 38.69 (s), 38.73 (s), 38.75 (s), 38.84 (s), 55.47 (q), 61.52 (t), 67.60 (d), 70.98 (d), 72.43 (d), 72.47 (d), 97.72 (d), 101.28 (d, JCF=25.9 Hz), 102.34 (s), 109.65 (d, JCF=2.8 Hz), 119.27 (d, JCF=16.2 Hz), 131.17 (d, JCF=6.5 Hz), 138.08 (s), 159.00 (s), 159.09 (s), 160.98 (d, JCF=244.3 Hz), 176.37 (s), 176.58 (s), 177.25 (s), 178.10 (s). HR-MS (ESI) m/z: 735.3856 [M+H]+ (Calcd for C38H56FN2O11: 735.3863).
4-[(4-Isopropoxyphenyl)methyl]-1-isopropyl-5-methyl-3-(2,3,4,6-tetra-O-pivaloyl-β-D-glucopyranosyloxy)-1H-pyrazole (15)A solution of 2b (50 g, 0.0671 mol) and 2-iodopropane (45.7 g, 0.269 mol) in DMI (105 g) was added dropwise to a suspension of NaH (8.05 g, 0.201 mol, 60% oil dispersion) in DMI (150 g) while maintaining the internal temperature between 10 and 20°C. The reaction mixture was stirred for 0.5–1 h at this temperature. The reaction mixture was added dropwise to a mixture of H2O (200 g), glacial acetic acid (8.05 g, 0.134 mol) and toluene (200 g) at 0–30°C, and the layers were separated. The organic layer was washed twice with 1% brine (300 g) and concentrated under reduced pressure. The residue was dissolved in 2-PrOH (250 g) and the solution was concentrated under reduced pressure. The residue was dissolved in 2-PrOH and adjusted to a final weight of 250 g. The 2-propanol solution was stirred at 20°C for 2 h and the resulting slurry was stirred at −8~−2°C for an additional 2 h. The slurry was filtered and the wet cake washed with 2-PrOH (50 g), which was cooled to 0°C. The precipitate was dried in vacuo at 60°C to give 45.4 g (86% yield) of 15 as a white solid. mp 137–138°C. [α]D20 −2.2 (c=1.0, DMSO). IR (KBr) cm−1: 2979, 2940, 1747, 1508, 1483, 1398, 1384, 1370. 1H-NMR (CDCl3) δ: 1.04 (9H, s), 1.13 (9H, s), 1.15 (9H, s), 1.19 (9H, s), 1.29 (6H, d, J=6.3 Hz), 1.33 (6H, dd, J=6.8, 9.3 Hz), 2.04 (3H, s), 3.52 (2H, dd, J=15.8, 19.6 Hz), 3.81–3.85 (1H, m), 4.10–4.19 (2H, m), 4.18–4.28 (1H, m), 4.41–4.51 (1H, m), 5.21–5.30 (2H, m), 5.41 (1H, t, J=9.6 Hz), 5.74 (1H, d, J=8.3 Hz), 6.74 (2H, d, J=8.7 Hz), 7.04 (2H, d, J=8.7 Hz). 13C-NMR (CDCl3) δ: 9.67 (q), 22.10 (q), 22.12 (q), 22.23 (q), 22.24 (q), 26.90 (t), 26.94 (q×3), 27.05 (q×3), 27.09 (q×3), 27.16 (q×3), 38.68 (s), 38.72 (s), 38.74 (s), 38.84 (s), 49.16 (d), 61.72 (t), 67.72 (d), 69.81 (d), 71.13 (d), 72.49(d), 72.51 (d), 98.02 (d), 102.89 (s), 115.71 (d×2), 129.18 (d×2), 133.43 (s), 135.72 (s), 155.88 (s), 157.69 (s), 176.38 (s), 176.58 (s), 177.27 (s), 178.18 (s). HR-MS (ESI) m/z: 787.4740 [M+H]+ (Calcd for C43H67N2O11: 787.4739).
3-(β-D-Glucopyranosyloxy)-4-[(4-isopropoxyphenyl)methyl]-1-isopropyl-5-methyl-1H-pyrazole (1a)A methanolic solution of 28% MeONa (1.93 g, 10 mmol) was added to a suspension of 15 (7.87 g, 10 mmol) in MeOH (75 mL) at room temperature. The mixture was then heated to 55°C and stirred for 3 h at this temperature. After cooling to 40°C, acetic acid (0.601 g, 10 mmol) was added dropwise to the reaction mixture. The reaction mixture was concentrated under reduced pressure to evaporate the methyl pivalate contained in the mixture. The residue was purified by silica gel chromatography (eluent dichloromethane–MeOH, 10 : 1) to provide 1a (4.45 g, 99% yield) as a pale yellowish oil. [α]D20 −8.1 (c=1.0, DMSO). IR (KBr) cm−1: 3407, 2975, 2931, 1506, 1466, 1384. 1H-NMR (CD3OD) δ: 1.26 (6H, d, J=6.0 Hz), 1.36 (6H, dd, J=3.8, 6.8 Hz), 2.09 (3H, s), 3.21–3.26 (1H, m), 3.33–3.43 (3H, m), 3.62–3.72 (3H, m), 3.77 (1H, dd, J=2.5, 12.1 Hz), 4.36–4.46 (1H, m), 4.46–4.55 (1H, m), 5.00–5.05 (1H, m), 6.76 (2H, d, J=8.7 Hz), 7.07 (2H, d, J=8.7 Hz). 13C-NMR (CD3OD) δ: 8.93 (q), 21.61 (q×2), 21.62 (q), 21.65 (q), 26.79 (t), 49.77 (d), 61.85 (t), 70.25 (d), 70.48 (d), 74.32 (d), 77.24 (d), 77.49 (d), 102.41 (d), 104.50 (s), 116.18 (d×2), 129.39 (d×2), 134.00 (s), 137.53 (s), 156.55 (s), 159.47 (s). HR-MS (ESI) m/z: 451.2444 [M+H]+ (Calcd for C23H35N2O7: 451.2439).
5-Methyl-4-[4-(1-methylethoxy)benzyl]-1-(1-methylethyl)-1H-pyrazol-3-yl-6-O-(ethoxycarbonyl)-β-D-glucopyranoside Ethanolate (16)A solution of ethyl chloroformate (522 mg, 4.81 mmol) in MeCN (1 mL) was added dropwise to a mixture of 1a (1.89 g, 4.19 mmol), 2,6-lutidine (672 mg, 6.28 mmol) and pyridine (13 mg, 0.17 mmol) in MeCN (10 mL) while maintaining the temperature between −3 and 3°C. The reaction mixture was stirred at 0°C for 2 h. After addition of glacial acetic acid (113 mg, 1.88 mmol), the reaction mixture was allowed to warm to room temperature. The reaction mixture was diluted with MTBE (10 mL) and 10% brine (5 mL), and then the layers were separated. The organic layer was washed twice with brine (5 mL), dried over anhydrous MgSO4 (2 g) and concentrated under reduced pressure. The residue was dissolved in EtOH (17 mL) and concentrated again under reduced pressure. EtOH was added to the residue, and the weight was adjusted to 9.3 g. To the EtOH solution, n-heptane (6 mL) was added and heated to 60°C to dissolve solids. The mixture was cooled to 45°C and stirred for 1 h at this temperature for an additional 1 h at 0–5°C. The slurry was filtered and the wet cake washed successively with a mixed solvent of EtOH (1.2 mL) and n-heptane (2.8 mL), which was cooled to 0°C, and then n-heptane (2.8 mL). The precipitate was dried in vacuo at room temperature to give 1.72 g (72% yield) of 16 as a white solid. mp 70–74°C. [α]D20 −17.7 (c=1.0, DMSO). IR (KBr) cm−1: 3353, 2980, 2926, 1753, 1731, 1508, 1477, 1467, 1449, 1386, 1371. 1H-NMR (CDCl3) δ: 1.23 (3H, t, J=7.0 Hz), 1.28 (3H, t, J=7.0 Hz), 1.30 (6H, d, J=6.0 Hz), 1.38 (6H, dd, J=2.3, 6.6 Hz), 2.06 (3H, s), 3.47–3.63 (6H, m), 3.71 (2H, q, J=7.0 Hz), 4.17 (2H, q, J=7.0 Hz), 4.24–4.31 (1H, m), 4.32–4.39 (2H, m), 4.43–4.52 (1H, m), 4.98 (1H, d, J=7.6 Hz), 6.77 (2H, d, J=8.6 Hz), 7.05 (2H, d, J=8.6 Hz). 13C-NMR (CDCl3) δ: 9.72, 14.21, 18.35, 22.09, 22.21, 22.25, 26.87, 49.44, 58.35, 64.23, 66.48, 69.49, 69.86, 73.65, 74.24, 76.44, 102.32, 104.67, 115.78, 129.10, 133.15, 136.55, 155.46, 155.96, 158.07. HR-MS (ESI) m/z: 523.2648 [M+H]+ (Calcd for C26H39N2O9: 523.2650).
5-Methyl-4-[4-(1-methylethoxy)benzyl]-1-(1-methylethyl)-1H-pyrazol-3-yl-6-O-(ethoxycarbonyl)-β-D-glucopyranoside (1b)16 (1.50 g, 2.64 mmol) was dissolved in MTBE (10 mL) at 45°C The solution was concentrated under reduced pressure to evaporate EtOH. MTBE was added to the residue, and the weight was adjusted to 9.0 g. H2O (0.015 mL) and n-heptane (3.6 g) were added to the solution at 40°C and the solution was cooled to 25°C. The solution was seeded with 1a and stirred at 25°C for 3 h. The resulting slurry was warmed to 40°C, and then a mixed solvent of MTBE (0.44 g) and n-heptane (2.4 g) was added dropwise to the slurry while maintaining the temperature between 37 and 43°C. The slurry was stirred at 40°C for 1 h and for an additional 3 h at 10°C. The slurry was filtered and the wet cake washed successively with a mixed solvent of MTBE (1.5 g) and n-heptane (1.5 g) followed by n-heptane (3.0 g). The product was dried in vacuo at room temperature to give 1.35 g (98% yield) of 1a as a white solid. mp 80–83°C. [α]D20 −19.3 (c=1.0, DMSO). IR (KBr) cm−1: 3414, 2979, 1747, 1506, 1477, 1474, 1466, 1458, 1449, 1382, 1370, 1317. 1H-NMR (CD3OD) δ: 1.23 (3H, t, J=7.2 Hz), 1.26 (6H, d, J=6.1 Hz), 1.37 (6H, dd, J=2.3, 6.7 Hz), 2.07 (3H, s), 3.34–3.42 (4H, m), 3.61–3.69 (2H, m), 4.12 (2H, q, J=7.2 Hz), 4.21 (1H, dd, J=5.4, 11.5 Hz), 4.35 (1H, dd, J=1.7, 11.6 Hz), 4.35–4.45 (1H, m), 4.45–4.54 (1H, m), 5.04–5.06 (1H, m), 6.75 (2H, d, J=8.6 Hz), 7.06 (2H, d, J=8.6 Hz). 13C-NMR (CD3OD) δ: 9.70, 14.60, 22.43, 22.49, 22.54, 27.63, 50.53, 65.07, 67.67, 71.07, 71.21, 75.02, 75.56, 77.84, 103.25, 105.62, 116.98, 130.21, 134.81, 138.21, 156.65, 157.33, 159.99. HR-MS (ESI) m/z: 523.2651 [M+H]+ (Calcd for C26H39N2O9: 523.2650).
Masahiro Kobayashi, Hidetoshi Isawa, Junichi Sonehara, Minoru Kubota and Tetsuji Ozawa are employees of Kissei Pharmaceutical Co., Ltd.