2016 Volume 64 Issue 7 Pages 778-784
2016 Volume 64 Issue 7 Pages 778-784
The deprotection of the methoxyphenylmethyl (MPM) ether and ester derivatives can be generally achieved by the combinatorial use of a catalytic Lewis acid and stoichiometric nucleophile. The deprotections of 2,4-dimethoxyphenylmethyl (DMPM)-protected alcohols and carboxylic acids were found to be effectively catalyzed by iron(III) chloride without any additional nucleophile to form the deprotected mother alcohols and carboxylic acids in excellent yields. Since the present deprotection proceeds via the self-assembling mechanism of the 2,4-DMPM protective group itself to give the hardly-soluble resorcinarene derivative as a precipitate, the rigorous purification process by silica-gel column chromatography was unnecessary and the sufficiently-pure alcohols and carboxylic acids were easily obtained in satisfactory yields after simple filtration.
The protection/deprotection method is important and the deprotection under mild conditions associated with perfect tolerance of the non-target functional groups can be a powerful tool to effectively construct the target molecules in organic reactions.1) The 4-methoxyphenylmethyl (MPM) group and its derivatives are widely utilized as the protecting group of alcohols, and the various deprotection methods have been developed.1–21) 4-MPM ethers are generally deprotected by the use of a stoichiometric amount of oxidants [2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ)2,3) and ceric ammonium nitrate (CAN)4,5)], Pd/C-catalyzed hydrogenation under atmospheric hydrogen gas1) or the combination of a stoichiometric amount of nucleophiles and the catalytic or stoichiometric Lewis acids6–17) [Chart 1, (a)–(d)]. However, the stoichiometric byproducts derived from 4-MPM protective groups (e.g., anisaldehydes, and toluene derivatives) and the acidic residues derived from reagents when using DDQ and CAN should be removed from the reaction mixture by the purification process using silica-gel column chromatography. In addition, a stoichiometric amount of Ph3C·BF418,19) and the chlorosulfonyl isocyanate (CSI)–NaOH combination20) has also been used for the deprotection of the 4-MPM ether. Although a catalytic method using ZrCl4 in CH3CN has been developed for the deprotection of the 4-MPM ether, silica-gel column chromatography purification is necessary to remove the undefined side-products.21) We have found that the widely used iron(III) chloride (FeCl3) could effectively activate the benzylic position and achieved various reactions accompanied by the cleavage of the benzylic C–O bond to construct unique skeletons.22–29) Furthermore, we have recently developed the FeCl3-catalyzed self-cleaving deprotection method of various types of MPM ethers without the addition of nucleophiles30) [Chart 1, (e)]. We now report the extensive results of the FeCl3-catalyzed deprotection method of 2,4-dimethylphenylmethyl (DMPM)-protected alcohols without purification using silica-gel column chromatography together with a novel application for the deprotection of 2,4-DMPM-protected carboxylic acids. Because 2,4-DMPM-protected alcohols were found to be preferentially deprotected in comparison with 4-MPM-protected alcohols in the reported methods (DDQ,1,31) the combination of CeCl3 and NaI10) or Ph3C·BF419)), the synthetic route via the 2,4-DMPM-protected alcohol could be adapted in the efficient synthesis of target molecules (e.g., total syntheses of natural products).19,31,32) However, the problems of side-products derived from 2,4-DMPM group, which should be separated by silica-gel column chromatography, have been unsolved.

The 4-MPM-protected 1-decanol (1a) effectively underwent deprotection in the presence of 5 mol% of FeCl3 without any additional nucleophile in CH2Cl2 at room temperature to produce the mother alcohol (2a) and many side-products derived from the 4-MPM moiety, which should be separated by silica-gel column chromatography (Eq. 1). Since benzyl (Bn) ether was tolerant under the FeCl3-catalyzed conditions, the chemoselective deprotection of the 4-MPM protective group of 1b could be accomplished (Eq. 2). Meanwhile, the deprotection of 3,4-DMPM also efficiently proceeded to form the corresponding deprotected alcohol (2a) and an inseparable mixture of the corresponding cyclic trimer, tetramer, pentamer and hexamer (m/z; 478.1934, 623.2623, 773.3293, 923.3951 [M+Na]+)33–35) of the 3,4-DMPM protective group, which were detected and determined by an electrospray ionization (ESI)/MS spectral analysis (Eq. 3, the chart is in the Supplementary Materials). It is noteworthy that the FeCl3-catalyzed deprotection of the 2,4-DMPM ether (1d) was completed within 5 min and only the sufficiently-pure 1-decanol (2a) was isolated without the purification process using silica-gel column chromatography (Eq. 4, Chart 2, middle 1H-NMR spectrum). Peaks derived from the 2,4-DMPM group of the substrate (1d, Chart 2, top) were never observed after the filtration and subsequent extraction of the reaction mixture of the deprotection reaction of 1d (Chart 2, middle 1H-NMR spectrum), and sufficiently-pure 1-decanol (2a) could be obtained in comparison to the spectrum of the commercially available 1-decanol as a standard sample (2a, Chart 2, bottom). During the deprotection of 1d, the resorcinarene derivative (3d) was produced as a hardly-soluble precipitate, which was slightly soluble in CH2Cl2, but hardly dissolved in Et2O. Therefore, the residual mixture containing the deprotected 2a and 3d was treated with Et2O after removal of CH2Cl2 as the solvent under reduced pressure, and the insoluble 3d was simply removed by filtration. The subsequent extraction (Et2O washing) of the filtrate gave the sufficiently-pure 1-decanol (2a). In a similar fashion, the FeCl3-catalyzed deprotection of 2,4-dibutoxyphenylmethyl ether (1e) also efficiently proceeded to produce lipophilic resorcin[4]arene octabutyl ether (3e: m/z; 959.6376 [M+Na]+ see Supplementary Materials) together with the deprotected 2a (Eq. 5), which strongly supported that the hardly-soluble tetramer of 2,4-DMPM was obtained as nearly the only side-product from the deprotection of 1d (Eq. 4).






(Top) substrate 1d; (Middle) deprotection product; (Bottom) standard sample 2a.
The present deprotection of 2,4-DMPM ether (1d) should proceed via the FeCl3-catalyzed self-assembling mechanism (Chart 3). The electron-rich aromatic ring of 1d attacked the benzylic position of another substrate (1d) activated by FeCl3 to give the deprotected alcohol (2a) and a corresponding dimer (4). The repeated similar intermolecular nucleophilic attacks continue until the formation of the tetramer (5), which undergoes the intramolecular annulation to give the hardly-soluble 3d accompanied by the complete deprotection. Alternatively, the homo-coupling of the dimer (4) can also directly produce the tetramer (5).

The green and self-cleaving deprotection method of the 2,4-DMPM ether (1d) without the silica-gel column chromatography purification could be accomplished using other Lewis acids (e.g., AuCl3,36–38) trimethylsilyl trifluoromethanesulfonate (TMSOTf), BF3·Et2O) instead of FeCl3 in CH2Cl2 to produce the deprotected 1-decanol (2a) in high yields within 5 min (Table 1, entries 1–4). On the other hand, Brønsted acids, such as trifluoroacetic acid (TFA) and AcOH, were insufficient and most of the starting material (1d) remained unchanged. CH2Cl2 or CH3CN was found to be an adequate solvent (Table 1, entries 1, 10), while the use of n-hexane, THF or toluene gave the desired deprotected alcohol (2a) in a relatively low yield (entries 7–9).
 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Solvent | Time | Yield (%) |
| 1 | FeCl3 | CH2Cl2 | 5 min | 99 |
| 2 | AuCl3 | CH2Cl2 | 5 min | 99 |
| 3 | TMSOTf | CH2Cl2 | 5 min | 99 |
| 4 | BF3·Et2O | CH2Cl2 | 5 min | 97 |
| 5 | TFA | CH2Cl2 | 3 h | 11 (70)a) |
| 6 | AcOH | CH2Cl2 | 3 h | 0 (100)a) |
| 7 | FeCl3 | n-Hexane | 3 h | 38 (59)a) |
| 8 | FeCl3 | THF | 3 h | 25 (55)a) |
| 9 | FeCl3 | Toluene | 30 min | 75 |
| 10 | FeCl3 | CH3CN | 5 min | 92 |
a) The yield in parenthesis indicates the recovered starting material (1d).
Furthermore, the deprotection reactions using 2,4-dibutoxyphenylmethyl ether (1e) as a protected alcohol produced the resorcin[4]arene octabutyl ether (3e) as nearly the only tetramer side-product even when using AuCl3, FeBr3 and ZnCl2 as the Lewis acid catalysts instead of FeCl3 (Eq. 6).

MPM-type protecting groups are also utilized for the protection of carboxylic acids.1,39,40) Similarly, DDQ or the nucleophile in the presence of a catalytic Lewis acid is used for the deprotection of MPM-protected carboxylic acids. The 2,4-DMPM-protected palmitic acid (6a) was also effectively deprotected using FeCl3 and AuCl3 in a short time to give the corresponding deprotected carboxylic acid (7a) in excellent yields (Table 2, entries 1, 2). Although TMSOTf and BF3·Et2O were also effective (entries 3, 4), Brønsted acids, such as TFA and AcOH, were insufficient (entries 5, 6). While the FeCl3-catalyzed deprotection could smoothly proceed in CH2Cl2, toluene and CH3CN as the solvent, n-hexane and THF were found to be inadequate solvents (entries 1, 9, 10 vs. 7, 8). From the viewpoint of cost performance and reaction efficiency, the use of a catalytic amount (5 mol%) of FeCl3 in CH2Cl2 was chosen as the optimum reaction conditions for the deprotection of the 2,4-DMPM-protected carboxylic acids.
 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Solvent | Time | Yield (%) |
| 1 | FeCl3 | CH2Cl2 | 5 min | 99 |
| 2 | AuCl3 | CH2Cl2 | 20 min | 96 |
| 3 | TMSOTf | CH2Cl2 | 5 min | 88 |
| 4 | BF3·Et2O | CH2Cl2 | 5 min | 86 |
| 5 | TFA | CH2Cl2 | 3 h | 34 (46)a) |
| 6 | AcOH | CH2Cl2 | 3 h | 0 (99)a) |
| 7 | FeCl3 | n-Hexane | 3 h | 75 (25)a) |
| 8 | FeCl3 | THF | 3 h | 46 (50)a) |
| 9 | FeCl3 | Toluene | 45 min | 98 |
| 10 | FeCl3 | CH3CN | 5 min | 96 |
a) The yield in parenthesis indicates the recovered starting material (6a).
For the FeCl3-catalyzed deprotection of 2,4-DMPM-protected carboxylic acid, the rigorous purification process to remove the side-products derived from the 2,4-DMPM protective group was also unnecessary (Chart 4). After the filtration of the resulting precipitate derived from 2,4-DMPM using a small amount of a silica-gel pad, the 1H-NMR peaks resulting from 2,4-DMPM moiety were hardly observed (top vs. middle) and the sufficiently-pure palmitic acid (7a) was obtained (middle vs. bottom). Due to the highly polar property of the deprotective carboxylic acid (7), the use of water should be avoided during work-up to avoid any undesired loss. Therefore, the reaction mixtures including the deprotected carboxylic acid (7) and the hardly-soluble resorcinarene derivative (3d) were directly filtered with a small amount of a siliga-gel pad. Alternatively, the use of the celite pad for the filtration could also produce the highly pure carboxylic acid (7a). Since resorcin[4]arene octabutyl ether (3e) was also obtained in 95% yield as a tetramer side-product by the FeCl3-catalyzed deprotection of the 2,4-dibutoxyphenylmethyl ester (6b) (Eq. 7), the formation and structure of the hardly-soluble 3d was equally probable.

(Top) substrate 1d; (middle) deprotection product; (Bottom) standard sample 2a.

The versatility of the substrate applicability was next investigated regarding the FeCl3-catalyzed deprotection of 2,4-DMPM-protected alcohols and carboxylic acids (Table 3). The 2,4-DMPM ethers prepared from primary and secondary alcohols were effectively deprotected into the corresponding mother alcohols within 5 min in nearly quantitative yields without purification by the silica-gel column chromatography (entries 1, 2). Because benzyl ether was stable under the FeCl3-catalyzed conditions, benzyl 2,4-DMPM ether was perfectly converted into the corresponding benzyl alcohol by the chemoselective cleavage at the electron-rich benzylic position (entry 3) and the 2,4-DMPM ether coexisting benzyl ether within the same molecule was selectively deprotected without the deprotection of the benzyl ether moiety (entry 4). The tert-butyldimethylsilyl (TBS) ether and acetoxy (AcO) group could be tolerant under the present deprotection conditions (entries 5, 6), while the ketal as a protective group of the ketone was partially deprotected along with the complete deprotection of the 2,4-DMPM ether protective group to form a mixture of the ketal and ketone (entry 7). The acidic proton on the hydroxyl group never influenced the deprotection (entry 8) and the 2,4-DMPM-protected phenol was also efficiently deprotected into mother phenol (entry 9).
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Product | Time | Yield (%) |
| 1 |  |  | 5 min | 99 |
| 2 |  |  | 5 min | 99 |
| 3 |  |  | 5 min | 92 |
| 4 |  |  | 5 min | 99 |
| 5 |  |  | 5 min | Quant |
| 6 |  |  | 5 min | Quant |
| 7 |  |  | 5 min | 69 |
 | 31 | |||
| 8 |  |  | 15 min | 99 |
| 9 |  |  | 5 min | 80b) |
| 10 |  | 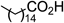 | 5 min | 96 |
| 11 |  |  | 5 min | 78c) |
| 12 |  |  | 6 h | No reaction |
| 13 |  |  | 5 min | 96 |
| 14 |  |  | 5 min | 99 |
| 15 | 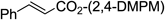 |  | 5 min | 94 |
| 16 |  |  | 5 min | 96 |
| 17 |  | 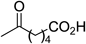 | 5 min | 75 |
| 18 |  |  | 5 min | 84 |
| 19 |  |  | 5 min | 99 |
| 20 |  |  | 5 min | 87 |
| 21 |  |  | 5 min | 86 |
a) The reactions were carried out according to the general procedures shown in Experimental unless otherwise noted. Spectral data without the rigorous purification process are shown in the Supplementary Materials. b) The product was purified by silica-gel column chromatography due to the contamination of unidentified byproducts. c) The yield after the purification using silica-gel column chromatography.
While the sufficiently-pure deprotected carboxylic acid could be obtained after a simple filtration of the 2,4-DMPM deprotection mixture through a small amount of a silica-gel pad (entry 10), the deprotection of the 4-MPM ester required the normal silica-gel column chromatography process to obtain the pure carboxylic acid (entry 11). Meanwhile, the benzyl ester was never deprotected under the FeCl3-catalyzed conditions (entry 12). The 2,4-DMPM esters derived from branched aliphatic carboxylic acids were also effectively deprotected into the corresponding carboxylic acids in nearly quantitative yields (entries 13, 14). The alkene, alkyne, ketone, tetrahydropyranyl (THP) ether and TBS ether were all tolerable under the deprotection conditions and only the deprotection of 2,4-DMPM could be achieved (entries 15–19). The 2,4-DMPM-protected benzoic acid derivatives were also adaptable to the present deprotection (entries 20, 21).
In conclusion, the 2,4-DMPM ethers and esters were effectively deprotected under inexpensive iron(III) chloride-catalyzed conditions without any additional nucleophile. The deprotections proceeded via the self-assembling mechanism by the nucleophilic attack of the 2,4-DMPM protective group itself to produce the hardly-soluble resorcinarene derivative as a precipitate, which was easily removed by adequate filtration. The present deprotection method generating less residues is useful and environmentally friendly in process chemistry.
To a solution of the 2,4-DMPM ether (0.2 mmol) in CH2Cl2 (1 mL) was added FeCl3 (0.01 mmol), then stirred at room temperature under argon. After being stirred until the reaction was completed, the reaction mixture was quenched with water (3 mL) and the organic layer (CH2Cl2) was evaporated in vacuo. Et2O was added to the aqueous residue and the resulting precipitate was filtered using a Celite pad and washed with Et2O. The combined organic layers were dried over Na2SO4, and concentrated in vacuo to give the sufficiently-pure alcohol.
Deprotection of 2,4-DMPM-Protected Ester (6)To a solution of the 2,4-DMPM ester (0.2 mmol) in CH2Cl2 (1 mL) was added FeCl3 (0.01 mmol), then stirred at room temperature under argon. After being stirred until the reaction was completed, the reaction mixture was directly filtrated through a small amount of a silica-gel pad and washed with Et2O. The filtrate was concentrated in vacuo to give the sufficiently-pure carboxylic acid. When some peaks derived from undetermined side-products were observed in the 1H-NMR spectra, the analytically-pure deprotected carboxylic acid was obtained after the normal silica-gel column chromatography (Table 3).
This work was supported by Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (JSPS).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.