2016 Volume 64 Issue 7 Pages 982-987
2016 Volume 64 Issue 7 Pages 982-987
Syntheses of androprostamine A (1), and resormycin (3), anti-prostate cancer peptidyl natural products produced by microorganisms, were completed. The characteristic enamide structures of these compounds were installed using the Horner-Wadsworth-Emmons reaction from the corresponding phosphonates in reasonable Z-selectivity.
Secondary metabolites of microorganisms are rich sources of biologically active natural products from which extremely important medicines for public health can be developed.1) Fascinated by the structural variation and diverse biological activities demonstrated by natural products, we have been involved in exploratory research for anticancer seeds of microbial origin with novel modes of action.
Prostate cancer is one of the leading causes of male mortality, especially in developed countries.2) In the early stage of prostate cancer before metastasis occurs, surgical removal of the prostate or radiation therapy are effective. Even after metastasis is observed, androgen ablation therapy is applicable as long as the cancer remains at an androgen-dependent stage. In many cases, however, successful androgen ablation therapy is followed by relapse of the cancer with exacerbated malignancy at the androgen-independent stage. The cancer in this state is called castration-resistant prostate cancer (CRPC),3) and the choice of treatment for curation is extremely limited. The molecular background of CRPC development has been actively studied, and it is now widely known that the androgen receptor (AR) retains its function even at this advanced level of the disease.4) In fact, AR knockdown inhibits CRPC growth.5) Although conventional antiandrogens such as bicalutamide and flutamide are not effective for the progressed prostate cancer,6) a novel type of inhibitor could potentially affect the AR signaling pathway of CRPC.
Androprostamines A (1) and B (2) produced by Streptomyces sp. MK932-CF8 were discovered as inhibitors of AR functions7); these natural products inhibit the androgen-dependent proliferation of human prostate cancer LNCaP and VCaP cells (Fig. 1). Interestingly, androprostamines inhibit growth without cytotoxicity and reduce the androgen-induced expression of AR-regulated genes.7) To explore the potential of these natural products as leads for chemotherapeutics against prostate cancer, we aimed to establish synthetic routes to androprostamines and related compounds with which extensive structure–activity relationship (SAR) studies could be performed.
Androprostamines are peptide compounds sharing a part of their structure with that of resormycin8,9) (3) containing the three non-proteinogenic amino acids: a characteristic dehydroamino acid at C-terminus, hydroxyvaline, and β-homolysine. Herein we disclose the first synthesis of natural products of this class, androprostamine A (1) and resormycin (3). Key to the success of the synthetic study was the preparation of the dehydroamino acid moiety by taking advantage of the Horner–Wadsworth–Emmons (HWE) reaction.

As resormycin is a core structure of androprostamines, we first undertook the synthesis of resormycin to establish a method of installing the enamide portion of these natural products at the C-terminus. The amino phosphonate is a well-known precursor of a wide range of dehydroamino acid derivatives that can be formed by the HWE reaction. The synthetic procedure of the counterpart of this reaction, aldehyde 8, is depicted in Chart 1. The protocol is rather straightforward: a commercially available resorcinol derivative 4 was esterified to give 5, which was followed by tert-butyldimethylsilyl (TBS)-protection, and conversion of an ester moiety to a formyl group by a reduction–oxidation sequence to afford 8.
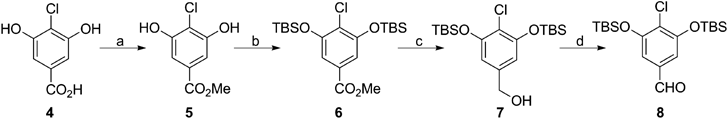
Reagents and conditions: (a) H2SO4, MeOH, r.t., 3.5 h, 89%; (b) TBSCl, imidazole, CH2Cl2, r.t., 4 h, 95%; (c) DIBAL-H, CH2Cl2, −78°C, 1 h, quant; (d) PCC, silica gel, CH2Cl2, r.t., 1 h, 83%.
Then, a literature-known hydroxyvaline derivative protected with an 9-fluorenylmethyloxycarbonyl (Fmoc) group was subjected to condensation with 10 using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM)10) as the coupling reagent to give a dipeptide intermediate 11 (Chart 2). Further elongation of the peptide was achieved using an Fmoc-protocol: removal of the Fmoc group from the N-terminus by a secondary amine (piperidine), followed by the formation of an amide bond with the protected homolysine 12. In this case also, DMT-MM was used as the coupling reagent. The resultant tripeptide compound 13 was used in the subsequent HWE reaction with resorcinol-derived aldehyde 8 using 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as the base. The desired Wittig-type reaction proceeded smoothly, but partial removal of the TBS group was observed, and purification at this stage was troublesome. The crude mixture was therefore treated with tetra-n-butylammonium fluoride (TBAF) to afford 26% (2 steps) of the desilylated product of the desired Z-isomer 14 as the main product. Concomitant formation of a small amount of the E-isomer (7% in 2 steps) was also observed. The final total deprotection was carried out under acidic conditions to complete the synthesis of resormycin (3) in the form of trifluoroacetic acid (TFA) salt. The 1H- and 13C-NMR data of 3 showed good agreement to those of the HCl salt of resormycin reported previously.9)

Reagents and conditions: (a) 10, DMT-MM, THF, r.t., 5 h, 57%; (b) piperidine, DMF, r.t., 30 min; 12, DMT-MM, MeOH, r.t., 3 h, 40%; (c) 8, DBU, CH2Cl2, r.t., 3 h; TBAF, THF, CH2Cl2, r.t., 40 min, 26% (with 7% of E-isomer); (d) TFA, CH2Cl2, r.t., 30 min, 52%.
Androprostamine A was synthesized from a β-homolysine derivative 15 in the direction of the C-terminus. Then, the γ-glutamyl portions were attached, and the HWE reaction was performed (Chart 3). At the outset, the benzyloxycarbonyl (Cbz) group of the N-terminal amino functionality of 15 was exchanged with an Fmoc group to afford 16, which, coupled with dipeptide 17 that was easily obtained from 11, afforded the tripeptide intermediate with a phosphonate moiety (18). The N-terminal primary amino group of 18 was unveiled, and was followed by amide bond formation with the commercially available glutamic acid derivative to result in 19. Next, each phosphonate was separately subjected to the HWE reaction with 8, and silyl protecting groups were detached by TBAF-treatment in the same manner as that for resormycin to afford 20. Again, the desired Z-isomer predominated in this reaction sequence (ca. 2 : 1 as the isolated yield). The final acidic deprotection accomplished the synthesis of androprostamine A (1) in 25% yield after HPLC purification. The 1H- and 13C-NMR data of the synthetic 1 was identical to that of the natural one reported previously.7)

Reagents and conditions: (a) H2, 10% Pd/C, MeOH, r.t., 5 h; Fmoc-Cl, NaHCO3, CH3CN, H2O, r.t., 1.5 h, 69%; (b) piperidine, DMF, r.t., 10 min; 11, DMT-MM, THF, r.t., 5 h, 76%; (c) piperidine, DMF, r.t., 30 min; Boc-L-Glu-OtBu, DMT-MM, THF, r.t., 18 h, 81%; (d) 8, DBU, CH2Cl2, r.t., 1.5 h; TBAF, THF, CH2Cl2, r.t., 40 min, 42% (with 21% of E-isomer); (e) TFA, CH2Cl2, r.t., 30 min, 25%.
Syntheses of androprostamines A (1), and resormycin (3), peptidyl anti-androgen natural products, were completed. The characteristic enamide structure at the C-terminus of these compounds was installed using the HWE reaction with the corresponding phosphonates in reasonable Z-selectivity. SAR studies using the synthetic protocol described here aimed at developing leads for clinical anti-prostate cancer agents are ongoing.
The reactions were performed in an oven-dried test tube or round bottom flask with a Teflon-coated magnetic stirring bar unless otherwise noted. All work-up and purification procedures were carried out with reagent-grade solvents under ambient atmosphere. Infrared (IR) spectra were recorded on a JASCO FT/IR 4100 Fourier transform infrared spectrophotometer. NMR was recorded on JEOL ECS-400 (1H-NMR: 400 MHz, 13C-NMR: 100 MHz) or on Bruker AVANCE 500 (1H-NMR: 500 MHz, 13C-NMR: 125 MHz) spectrometers. Chemical shifts for proton are reported in parts per million downfield from tetramethylsilane and are referenced to residual protium in the NMR solvent (CDCl3: δ 7.26 ppm, CD3OD: δ 3.30 ppm, DMSO-d6: 2.49 ppm). For 13C-NMR, chemical shifts were reported in the scale relative to NMR solvent (CDCl3: δ 77.0 ppm, CD3OD: δ 49.0 ppm, DMSO-d6: 39.7 ppm) as an internal reference, or calibrated based on independently recorded peak of 3-trimethylsilylpropanoic acid (TSP) at 0 ppm in D2O. NMR data are reported as follows: chemical shifts, multiplicity (s: singlet, d: doublet, dd: doublet of doublets, t: triplet, m: multiplet, br: broad signal), coupling constant (Hz), and integration. Optical rotation was measured using a 2 mL cell with a 1.0 dm path length on a JASCO polarimeter P-1030. High-resolution (HR)-mass spectra (ESI-Orbitrap) were measured on Thermo Fisher Scientific LTQ Orbitrap XL. Unless otherwise noted, materials were purchased from commercial suppliers and were used without purification. For reaction, tetrahydrofuran (THF), N,N-dimethylformamide (DMF), CH3CN, and CH2Cl2 were purified by passing through a solvent purification system (Glass Contour). Dry MeOH was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and used as received.
Methyl 4-Chloro-3,5-dihydroxybenzoate (5)To a solution of 4 (3.00 g, 15.9 mmol) in 15 mL of MeOH was added dropwise 0.3 mL of H2SO4 at 0°C. The mixture was stirred at room temperature (r.t.) for 3.5 h, and half of MeOH was removed under reduced pressure and followed by addition of saturated NaHCO3 until the pH value of the mixture reached to around 7. The resultant white solid was collected on funnel and washed thoroughly with H2O, then n-hexane to give 5 as a white powder (2.86 g, 14.1 mmol) in 89% yield; mp 145–146°C; IR (MeOH) ν 3419, 3338, 2360, 1705, 1429, 1376, 1041, 764 cm−1; HR-MS electrospray ionization (ESI) Anal. Calcd for C8H6ClO4 m/z 200.9955 [M−H]−; Found 200.9964; 1H-NMR (500 MHz, CD3OD) δ: 7.06 (2H, s), 3.85 (3H, s); 13C-NMR (125 MHz, CD3OD) δ: 168.1, 155.7, 130.2, 114.6, 109.1, 52.7
Methyl 3,5-Bis((tert-butyldimethylsilyl)oxy)-4-chlorobenzoate (6)To a solution of 5 (2.86 g, 14.1 mmol) in 70 mL of CH2Cl2 was added imidazole (4.23 g, 62.1 mmol), and TBSCl (4.68 g, 31.1 mmol), successively at 0°C. The mixture was stirred at r.t. for 4 h. The reaction mixture was diluted with CHCl3, and washed with saturated NH4Cl, and brine successively. The combined organic layers were dried over Na2SO4, and was concentrated to dryness. The residue was purified with silica gel column chromatography (n-hexane/AcOEt=6/1) to give 6 as a white powder (5.80 g, 13.5 mmol) in 95% yield; mp 95–96°C; IR (CHCl3) ν 2930, 2859, 1724, 1434, 1361, 1253, 841, 783 cm−1; HR-MS (ESI) Anal. Calcd for C20H36ClO4Si2 m/z 431.1841 [M+H]+; Found 431.1822; 1H-NMR (500 MHz, CD3OD) δ: 7.21 (2H, s), 3.89 (3H, s), 1.06 (18H, s), 0.25 (12H, s); 13C-NMR (125 MHz, CD3OD) δ: 167.5, 154.3, 130.0, 124.3, 115.3, 53.0, 26.2, 19.3, −4.3.
(3,5-Bis((tert-butyldimethylsilyl)oxy)-4-chlorophenyl)methanol (7)To a solution of 6 (5.80 g, 13.5 mmol) in 90 mL of CH2Cl2 was added DIBAL-H (diisobutylalminium hydride, 1.0 M in n-hexane, 26.9 mL, 26.9 mmol) at −78°C, and the mixture was stirred for 1 h at the same temperature. Then, 90 mL of aqueous solution of potassium sodium tartrate was added, and extracted with CHCl3. The organic layer was dried over Na2SO4 and concentrated in vacuo to afford 7, as a colorless oil (5.40 g, 13.4 mmol) in quantitative yield; IR (KBr) ν 3327, 2931, 2860, 1577, 1432, 1254, 1098, 829 cm−1; HR-MS (ESI) Anal. Calcd for C19H36ClO3Si2 m/z 403.1892 [M+H]+; Found 403.1882; 1H-NMR (500 MHz, CD3OD) δ: 6.60 (2H, s), 4.48 (2H, s), 1.05 (18H, s), 0.23 (12H, s); 13C-NMR (125 MHz, CD3OD) δ: 154.1, 142.4, 117.2, 113.0, 64.4, 26.2, 19.3, −4.2.
3,5-Bis((tert-butyldimethylsilyl)oxy)-4-chlorobenzaldehyde (8)To a solution of 7 (1.00 g, 2.48 mmol) in 12.4 mL of CH2Cl2 was added PCC (pyridinium chlorochromate, 588 mg, 2.73 mmol), and silica gel (588 mg) at 0°C, and the mixture was stirred for 1 h at r.t. Then, PCC (294 mg, 1.36 mmol) was added and stirred for additional 1 h. The reaction mixture was poured onto the pad of Celite, washed with CHCl3 thoroughly, and the filtrate was concentrated in vacuo. The organic layer was died over Na2SO4, and was concentrated to dryness. The residue was purified with silica gel column chromatography (n-hexane/AcOEt=8/1) to give 8 as a colorless oil (830 mg, 2.07 mmol) in 83% yield; IR (KBr) ν 2931, 2860, 1703, 1574, 1433, 1350, 827 cm−1; HR-MS (ESI) Anal. Calcd for C19H34ClO3Si2 m/z 401.1735 [M+H]+; Found 401.1728; 1H-NMR (400 MHz, CDCl3) δ: 9.83 (1H, s), 7.01 (2H, s), 1.05 (18H, s), 0.26 (12H, s); 13C-NMR (100 MHz, CDCl3) δ: 191.1, 153.8, 135.0, 125.5, 114.0, 113.3, 25.8, 18.5, −4.2.
tert-Butyl 2-((S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-hydroxy-3-methylbutanamido)-2-(dimethoxyphosphoryl)acetate (11)To a mixture of 9 (1.09 g, 3.06 mmol), and 10 (1.10 g, 4.60 mmol) in 10 mL of THF was added DMT-MM (1.27 g, 4.60 mmol), and the solution was stirred at r.t. for 5 h. The reaction was quenched with H2O, AcOEt was added to the mixture for extraction, and the combined organic layers were concentrated to dryness. The residue was suspended in toluene and concentrated, which was repeated 5 times. The residue was purified with silica gel column chromatography (CH2Cl2/AcOEt=1/1) to give 11 as a colorless oil (1.01 g, 1.75 mmol) in 57% yield as a colorless oil; IR (KBr) ν 3301, 2979, 1731, 1666, 1525, 1248, 1154, 1041, 758 cm−1; HR-MS (ESI) Anal. Calcd for C28H37N2NaO9P m/z 599.2134 [M+Na]+; Found 599.2123; 1H-NMR (500 MHz, CD3OD) δ: 7.79 (2H, d, J=7.6 Hz), 7.67 (2H, m), 7.38 (2H, m), 7.30 (2H, m), 4.43–4.36 (2H, m), 4.24–4.22 (2H, m), 3.85–3.74 (6H, m), 1.49 (4.5 H, s), 1.48 (4.5 H, s), 1.27 (1.5H, s), 1.27 (1.5H, s), 1.22 (1.5H, s), 1.22 (1.5H, s); 13C-NMR (125 MHz, CD3OD) δ: 172.6 (d, J=6.3 Hz), 172.4 (d, J=6.3 Hz), 166.3, 166.2, 158.7, 145.4, 145.2, 142.3, 128.8, 128.2, 126.3, 121.0, 84.9, 79.5, 72.8, 68.1, 63.72, 63.67, 55.0–54.7 (m, overlap), 48.6 (overlapped with solvent peaks), 28.2, 28.1, 27.7, 27.6, 26.1.
tert-Butyl (10S,14S)-10-((tert-Butoxycarbonyl)amino)-17-(dimethoxyphosphoryl)-14-(2-hydroxypropan-2-yl)-2,2-dimethyl-4,12,15-trioxo-3-oxa-5,13,16-triazaoctadecan-18-oate (13)To a solution of 11 (91.0 mg, 0.158 mmol) in 39 mL of DMF was added 17 µL of piperidine (0.174 mmol), and the mixture was stirred at r.t. for 30 min. Then, the solution was concentrated to dryness. The resultant residue was dissolved in 1.5 mL of MeOH, to which were added 12 (62.7 mg, 0.174 mmol), DMT-MM (52.4 mg, 0.189 mmol), and the resultant mixture was stirred at r.t. for 3 h. The solution was diluted with AcOEt, washed with 1 M hydrochloric acid, and the organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was purified with silica gel column chromatography (CHCl3/MeOH=20/1) to give 13 (44.0 mg, 40%) as a colorless oil (mixture of diastereomer); IR (KBr) ν 3324, 2979, 1688, 1522, 1251, 1171, 1038, 755 cm−1; HR-MS (ESI) Anal. Calcd for C30H57N4NaO12P m/z 719.3608 [M+Na]+; Found 719.3584; 1H-NMR (400 MHz, CDCl3) δ: 7.29 (1H, br), 6.74 (0.5H, br), 6.69 (0.5H, d, J=8.6 Hz), 5.12–4.99 (1H, m), 4.70 (1H, br), 4.39 (1H, dd, J=8.3, 4.2 Hz), 3.84–3.80 (6H, m), 3.80–3.69 (2H, m), 3.10 (1H, br), 2.49 (2H, m), 1.85–1.37 (6H, m), 1.50 (4.5H, s), 1.49 (4.5H, s), 1.47 (9H, s), 1.43 (4.5H, s), 1.42 (4.5H, s), 1.34 (1.5H, s), 1.33 (1.5H, s), 1.22 (1.5H, s), 1.21 (1.5H, s); 13C-NMR (125 MHz, CDCl3) δ: 171.7, 170.8 (d, J=6.3 Hz), 170.7 (d, J=5.0 Hz), 165.1, 164.9, 156.2, 155.7, 84.2, 84.0, 79.3, 79.0, 71.7, 59.5, 59.2, 58.5, 54.1 (d, J=6.3 Hz), 54.0 (d, J=6.3 Hz), 51.0 (d, J=144 Hz), 50.8 (d, J=145 Hz), 48.5, 40.8, 40.2, 34.0, 29.5, 28.4, 27.8, 27.3, 25.7, 25.4, 23.3, 18.4.
tert-Butyl (10S,14S)-10-((tert-Butoxycarbonyl)amino)-17-((Z)-4-chloro-3,5-dihydroxybenzylidene)-14-(2-hydroxypropan-2-yl)-2,2-dimethyl-4,12,15-trioxo-3-oxa-5,13,16-triazaoctadecan-18-oate (14)To a solution of 13 (150 mg, 0.215 mmol) in 1 mL of CH2Cl2 was added DBU (36.0 µL, 0.235 mmol), and 8 (78.0 mg, 0.195 mmol) at r.t. successively. The mixture was stirred at the same temperature for 3 h, diluted with CH2Cl2, washed with brine, dried over Na2SO4, and concentrated. The resultant residue was partially purified with silica gel column chromatography (CHCl3/MeOH=20/1) to give coupling product as a mixture of cis- and trans-isomers, and partially desilylated compounds. Then, this material was dissolved in 4 mL of CH2Cl2, to which was added 1.0 M TBAF in THF (0.47 mL, 0.470 mmol) at r.t. The solution was stirred for 40 min at the same temperature, diluted with CHCl3, washed with saturated NH4Cl and brine, successively. The organic layer was dried over Na2SO4, and concentrated to dryness. The resultant residue was partially purified with silica gel column chromatography (CHCl3/MeOH=20/1) to give 14 as a colorless oil (50.4 mg, 67.9 µmol) in 26% yield over 2 steps with concomitant formation of E-isomer (13.2 mg, 17.8 µmol, 7%) as a colorless oil; [α]D24 +78.6 (c 0.71, MeOH); IR (KBr) ν 3306, 1977, 1686, 1510, 1367, 1280, 1254, 1165 cm−1; HR-MS (ESI) Anal. Calcd for C35H55N4NaO11P m/z 765.3454 [M+Na]+; Found 765.3442; 1H-NMR (500 MHz, CDCl3) δ: 7.97 (2H, br), 7.18 (1H, s), 6.80 (2H, br), 4.90 (1H, br), 4.69 (1H, m), 4.28 (1H, m), 3.73 (1H, br), 3.03 (2H, m), 2.60–2.40 (2H, m), 1.52 (9H, s), 1.45–1.16 (6H, m), 1.44 (9H, s), 1.37 (9H, s), 1.37 (3H, s), 1.30 (3H, s); 13C-NMR (125 MHz, CDCl3) δ: 173.5, 170.5, 163.9, 156.4, 156.1, 153.0, 132.3, 132.1, 126.0, 109.6, 109.4, 82.5, 80.1, 79.4, 71.2, 61.8, 48.8, 42.4, 40.1, 34.7, 29.7, 28.5, 28.3, 28.1, 27.4, 26.4, 23.0.
Resormycin (3)To a solution of 14 (22.3 mg, 30.0 µmol) in 0.5 mL of CH2Cl2 was added TFA (trifluoroacetic acid, 0.3 mL) at 0°C. The mixture was stirred at r.t. for 30 min. Then the mixture was concentrated to dryness to give 3 as a white powder (11.3 mg, 15.7 µmol, 52%); [α]D24 +127.7 (c 0.44, MeOH), lit. for HCl salt, [α]D23 +146.8 (c 1, MeOH)8); IR (KBr) ν 3026, 1648, 1584, 1428, 1391, 1205, 1004 cm−1; HR-MS (ESI) Anal. Calcd for C21H32N4O7 m/z 487.1960 [M+H]+; Found 487.1941; 1H-NMR (500 MHz, DMSO-d6) δ: 8.51 (1H, s), 8.50 (1H, s), 8.03 (3H, s), 7.87 (3H, s), 7.00 (1H, s), 6.77 (2H, m), 5.06 (1H, br), 4.53 (1H, d, J=9.0 Hz), 3.39 (1H, m), 2.75–2.57 (3H, m), 1.62–1.36 (6H, m), 1.23 (3H, s), 1.22 (3H, s); 13C-NMR (125 MHz, DMSO-d6) δ: 169.9, 169.6, 166.1, 154.0, 132.0, 130.9, 126.7, 108.6, 108.5, 71.4, 60.4, 47.9, 38.4, 37.3, 31.4, 27.1, 26.4, 26.1, 21.3.
(S)-7-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-((tert-butoxycarbonyl)amino)heptanoic Acid (16)To a solution of 15 (49.2 mg, 0.123 mmol) in 0.6 mL of MeOH was added 10% palladium carbon (Pd/C), and the resulting suspension was stirred under atmospheric pressure of H2 at r.t. for 5 h. After removal of the catalyst by filtration on a pad of Celite, the filtrate was concentrated to dryness to give Boc-L-β-homolysine. This material was dissolved in 1/1 mixture of CH3CN and H2O, to which were added NaHCO3 (21.0 mg, 0.246 mmol), and Fmoc-Cl (9-fluorenylmethyl chloroformate, 35.0 mg, 0.135 mmol), successively. The mixture was stirred at r.t. for 1.5 h. Then, the solution was quenched with 1 M hydrochloric acid (until pH was adjusted to around 3.5), extracted with AcOEt. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified with preparative TLC (CHCl3/MeOH=15/1) to give 16 as a colorless amorphous (40.9 mg, 84.8 µmol) in 69% yield; [α]D24 −2.47 (c 1.8, MeOH); IR (KBr) ν 1694, 1523, 1450, 1250, 1169, 741 cm−1; HR-MS (ESI) Anal. Calcd for C27H34N2NaO6 m/z 505.2315 [M+Na]+; Found 505.2301; 1H-NMR (500 MHz, CD3OD) δ: 7.79 (1H, d, J=7.5 Hz), 7.65 (1H, d, J=7.5 Hz), 7.40 (1H, dd, J=7.5, 7.5 Hz), 7.32 (1H, dd, J=7.5, 7.5 Hz), 4.34 (2H, d, J=6.9 Hz), 4.19 (1H, t, J=6.9 Hz), 3.85 (1H, br), 3.11 (2H, t, J=6.8 Hz), 2.38 (2H, m), 1.59–1.30 (6H, m), 1.42 (9H, s); 13C-NMR (125 MHz, CD3OD) δ: 177.4, 158.9, 157.9, 145.4, 142.6, 128.8, 128.2, 126.2, 121.0, 79.8, 67.6, 42.3, 41.7, 35.6, 30.7, 28.8, 24.4.
tert-Butyl (9S,13S)-9-((tert-Butoxycarbonyl)amino)-16-(dimethoxyphosphoryl)-1-(9H-fluoren-9-yl)-13-(2-hydroxypropan-2-yl)-3,11,14-trioxo-2-oxa-4,12,15-triazaheptadecan-17-oate (18)To a solution of 11 (2.26 g, 3.93 mmol) in 39 mL of DMF was added 428 µL of piperidine (4.32 mmol), and the mixture was stirred at r.t. for 10 min. Then, the solution was concentrated to dryness. The resultant residue (17) was dissolved in 10 mL of THF, to which were added 16 (948 mg, 1.97 mmol), DMT-MM (1.09 g, 3.93 mmol), and the resultant mixture was stirred at r.t. for 5 h. The solution was diluted with AcOEt, washed with 1 M hydrochloric acid, and the organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was purified with silica gel column chromatography (n-hexane/AcOEt=2/1, CHCl3 only, CHCl3/MeOH=20/1) to give 18 (1.22g, 76%) as a white powder (mixture of diastereomer); IR (KBr) ν 3308, 2975, 2934, 2360, 1693, 1522, 1250, 1156, 1035 cm−1; HR-MS (ESI) Anal. Calcd for C40H59N4NaO12P m/z 841.3765 [M+Na]+; Found 841.3750; 1H-NMR (500 MHz, CD3OD) δ: 7.79 (1H, d, J=7.5 Hz), 7.64 (1H, d, J=7.5 Hz), 7.39 (1H, dd, J=7.5, 7.5 Hz), 7.30 (1H, dd, J=7.5, 7.5 Hz), 4.53 (1H, s), 4.34 (2H, d, J=6.6 Hz), 4.19 (1H, t, J=6.6 Hz), 3.86 (1H, br), 3.83–3.79 (6H, m), 3.10 (2H, br), 2.45–2.40 (2H, m), 1.58–1.20 (6H, m), 1.48 (9H, s), 1.41 (9H, br), 1.28 (1.5H, s), 1.28 (1.5H, s), 1.23 (1.5H, s), 1.23 (1.5H, s); 13C-NMR (125 MHz, CD3OD) δ: 173.6, 172.2 (d, J=6.3 Hz), 172.0 (d, J=6.3 Hz), 166.4, 166.2, 145.4, 142.7, 128.8, 128.2, 126.2, 121.0, 84.9, 80.1, 79.5, 72.9, 72.8, 67.6, 61.6, 55.0 (d, J=7.5 Hz), 54.84 (d, J=6.3 Hz), 54.76 (d, J=7.5 Hz), 49.6 (overlapped with solvent peaks), 42.6, 42.5, 41.6, 35.5, 30.7, 28.9, 28.2, 27.8, 27.7, 26.2, 26.1, 24.2.
tert-Butyl N5-((5S)-7-(((2S)-1-((2-(tert-Butoxy)-1-(dimethoxyphosphoryl)-2-oxoethyl)amino)-3-hydroxy-3-methyl-1-oxobutan-2-yl)amino)-5-((tert-butoxycarbonyl)amino)-7-oxoheptyl)-N2-(tert-butoxycarbonyl)-L-glutaminate (19)To a solution of 18 (700 mg, 0.855 mmol) in 7 mL of DMF was added 93 µL of piperidine (0.940 mmol), and the mixture was stirred at r.t. for 30 min. Then, the solution was concentrated to dryness. The resultant residue was dissolved in 2 mL of THF, to which were added Boc-L-Glu-OtBu (130 mg, 0.427 mmol), DMT-MM (237 mg, 0.855 mmol), and the resultant mixture was stirred at r.t. for 18 h. The solution was diluted with AcOEt, washed with 1 M hydrochloric acid, and the organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was purified with silica gel column chromatography (CHCl3/MeOH=10/1) to give 19 (307 mg, 81%) as a colorless oil (mixture of diastereomer); IR (KBr) ν 3309, 2978, 1691, 1654, 1525, 1367, 1253, 1156, 1037 cm−1; HR-MS (ESI) Anal. Calcd for C39H72N5NaO15P m/z 904.4660 [M+Na]+; Found 904.4662; 1H-NMR (500 MHz, CD3OD) δ: 4.53 (1H, s), 3.95 (1H, m), 3.88 (1H, m), 3.84–3.81 (6H, m), 3.15 (2H, t, J=6.8 Hz), 2.43 (2H, d, J=6.4 Hz), 2.26 (2H, t, J=6.4 Hz), 2.06 (1H, m), 1.85 (1H, m), 1.54–1.34 (4H, m), 1.49 (9H, s), 1.46 (9H, s), 1.44 (9H, s), 1.42 (9H, br), 1.28 (3H, s), 1.23 (1.5H, s), 1.23 (1.5H, s); 13C-NMR (125 MHz, CD3OD) δ: 174.8, 173.5, 173.3, 172.2 (d, J=6.3 Hz), 171.9 (d, J=6.3 Hz), 166.4, 166.2, 158.1, 157.9, 84.9, 61.6, 61.5, 55.0 (d, J=7.5 Hz), 55.0–54.7 (m, overlap), 49.7 (overlapped with solvent peaks), 42.5, 42.4, 40.4, 35.5, 33.4, 30.2, 28.9, 28.8, 28.3, 28.2, 27.8, 27.7, 26.2, 26.1, 24.4.
tert-Butyl N5-((S)-7-(((S)-1-(((Z)-3-(tert-Butoxy)-1-(4-chloro-3,5-dihydroxyphenyl)-3-oxoprop-1-en-2-yl)amino)-3-methyl-1-oxobutan-2-yl)amino)-5-((tert-butoxycarbonyl)amino)-7-oxoheptyl)-N2-(tert-butoxycarbonyl)-L-glutaminate (20)To a solution of 19 (15.8 mg, 17.9 µmol) in 0.2 mL of CH2Cl2 was added DBU (4.0 µL, 21.5 µmol), and 8 (7.0 mg, 16.3 µmol) at r.t. successively. The mixture was stirred at the same temperature for 1.5 h, diluted with CH2Cl2, washed with brine, dried over Na2SO4, and concentrated. The resultant residue was partially purified with silica gel column chromatography (CHCl3/MeOH=15/1) to give coupling product as a mixture of cis- and trans-isomers, and partially desilylated compounds. Then, this material was dissolved in 0.25 mL of CH2Cl2, to which was added 1.0 M TBAF in THF (28.0 µL, 28.0 μmol) at r.t. The solution was stirred for 40 min at the same temperature, diluted with CHCl3, washed with saturated NH4Cl and brine, successively. The organic layer was dried over Na2SO4, and concentrated to dryness. The resultant residue was partially purified with silica gel column chromatography (CHCl3/MeOH=15/1) to give 20 as a colorless oil (6.4 mg, 6.89 µmol) in 42% yield over 2 steps with concomitant formation of E-isomer (3.2 mg, 3.45 µmol, 21%) as a colorless oil; [α]D25 –48.4 (c 0.24, MeOH); IR (KBr) ν 3310, 2977, 1697, 1653, 1507, 1367, 1279, 1254, 1158, 1053 cm−1; HR-MS (ESI) Anal. Calcd for C44H71ClN5O14 m/z 928.4686 [M+H]+; Found 928.4664; 1H-NMR (400 MHz, CD3OD) δ: 7.11 (1H, s), 6.74 (2H, s), 4.49 (1H, s), 3.97–3.81 (2H, m), 3.11 (2H, m), 2.48 (2H, d, J=7.6 Hz), 2.25 (2H, d, J=9.2 Hz), 2.20–2.01 (1H, m), 1.90–1.80 (1H, m), 1.52 (9H, s), 1.50–1.25 (6H, m), 1.46 (9H, s), 1.44 (9H, s), 1.40 (9H, s), 1.38 (3H, s), 1.33 (3H, s); 13C-NMR (125 MHz, CD3OD) δ: 174.8, 174.3, 173.3, 172.4, 165.6, 158.1, 158.0, 155.5, 133.6, 133.5, 127.9, 110.8, 110.1, 83.2, 82.8, 80.6, 80.2, 72.4, 62.6, 55.6, 42.8, 40.3, 35.4, 33.4, 30.0, 28.9, 28.8, 28.4, 28.3, 27.5, 27.0, 24.3.
Androprostamine A (1)To a solution of 20 (11.6 mg, 12.5 µmol) in 0.25 mL of CH2Cl2 was added TFA (125 µL) at 0°C. The mixture was stirred at r.t. for 30 min. Then the mixture was concentrated to dryness to give a crude material containing 1, which was purified with LH-20 (MeOH) and quenched with saturated NaHCO3. The 1H-NMR of the resultant sample was identical to that of natural one. The sample was further purified with HPLC (Capcell Pak C18 UG, Shiseido, Tokyo, Japan, 20×250 mm; eluent, 10% CH3CN in H2O containing 0.1% of TFA; detection, UV at 300 nm; flow rate, 10 mL/min) to give androprostamine A TFA salt as a colorless amorphous. The material was dissolved in H2O, to which 1 M NH4OH was added until the pH reached to around 9. The solution was concentrated and the resulting crude material was purified by LH-20 (MeOH) to give 1 (1.9 mg, 2.05 µmol, 25%); [α]D27 +42.4 (c 0.085, MeOH), (natural sample: [α]D27 +43.2 (c 0.085, MeOH)); IR (KBr) ν 3100, 1671, 1397, 1202, 1136 cm−1; HR-MS (ESI) Anal. Calcd for C26H39ClN5O10 m/z 616.2385 [M+H]+; Found 616.2377; 1H-NMR (500 MHz, D2O) δ: 7.20 (1H, s), 6.75 (2H, s), 4.49 (1H, s), 3.75 (1H, t, J=6.5 Hz), 3.60 (1H, m), 3.06 (2H, m), 2.78 (1H, dd, J=14.4, 5.0 Hz), 2.74 (1H, dd, J=14.4, 6.0 Hz), 2.39 (1H, m), 2.12 (1H, m), 1.60 (1H, m), 1.53 (1H, m), 1.35 (3H, s), 1.36–1.32 (4H, m), 1.33 (3H, s); 13C-NMR (125 MHz, D2O) δ: 177.2, 176.7, 174.9, 174.1, 173.2, 155.4, 136.7, 133.4, 132.5, 111.7, 111.3, 74.5, 64.3, 56.9, 51.6, 41.7, 38.7, 34.3, 33.9, 30.6, 29.3, 28.5, 27.9, 24.7.
We thank Dr. R. Sawa, Ms. Y. Kubota, and Ms. Y. Takahashi (BIKAKEN) for collection of spectral data, and Dr. M. Igarashi for valuable discussion.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.