2016 Volume 64 Issue 7 Pages 880-885
2016 Volume 64 Issue 7 Pages 880-885
A methanol extract of the flowers of Mammea siamensis (Calophyllaceae) was found to inhibit enzymatic activity against aromatase (IC50=16.5 µg/mL). From the extract, two new geranylated coumarins, mammeasins C (1) and D (2), were isolated together with seven coumarins: 8-hydroxy-5-methyl-7-(3,7-dimethyl-octa-2,6-dienyl)-9-(2-methyl-1-oxobutyl)-4,5-dihydropyrano[4,3,2-de]chromen-2-one (9), 8-hydroxy-5-methyl-7-(3,7-dimethyl-octa-2,6-dienyl)-9-(3-methyl-1-oxobutyl)-4,5-dihydropyrano[4,3,2-de]chromen-2-one (10), mammeas A/AA (14), A/AB (15), A/AA cyclo D (18), E/BA (23), and E/BC cyclo D (25). The structures of 1 and 2 were elucidated on the basis of spectroscopic evidence. Among the isolates including 17 previously reported coumarins, 1 (IC50=2.7 µM), 2 (3.6 µM), and mammea B/AB cyclo D (21, 3.1 µM) showed relatively strong inhibitory activities comparable to the activity of the synthetic nonsteroidal aromatase inhibitor aminoglutethimide (2.0 µM).
Mammea siamensis (MIQ.) T. ANDERS. is a species of flowering plant in the Calophyllaceae family and is widely distributed in Thailand, Laos, Cambodia, Vietnam, and Myanmar. The flowers of this plant have been used for preparing a heart tonic in Thai traditional medicine (“Sarapi” in Thai).1–10) Several coumarins,1–7) xanthones,8,9) triterpenoids,10) and steroids10) have been isolated from the flowers,1,2,6,7,10) seeds,3,9) twigs,4,8) and bark5) of this plant. In the course of our characterization studies on bioactive constituents in Thai natural medicine,1,11–25) we reported that the methanol extract of the flowers of M. siamensis and its coumarin constituents showed inhibitory effects on nitric oxide production in lipopolysaccharide-activated RAW264.7 cells.1) Further studies revealed that the methanol extract inhibited enzymatic activity against aromatase. Separation of the active constituents in the extract allowed us to isolate two new geranylated coumarins, mammeasins C (1) and D (2). This paper describes the isolation and structure elucidation of these new coumarins (1, 2) and the inhibitory effects of the coumarin constituents (1–26) on aromatase.26)
Breast cancer is one of the most common reasons for mortality in women.26,27) Estrogens and estrogen receptors are well known to play an important role in the development and progression of hormone-dependent breast cancer; for this reason, estrogens and estrogen receptors are widely studied molecular targets.28–30) The presence of high concentrations of estrogen in breast tissue increases the risk of developing breast cancer and the ability of immature breast tissue cells to strongly bind to carcinogens, decreasing their DNA repair capacity.31,32) Aromatase, a CYP19 enzyme, is the rate-limiting enzyme in the conversion of testosterone and androstenedione to the estrogens, estrone and estradiol.26–30,32–34) It is involved in the final step of the estrogen biosynthetic pathway and its selective inhibition will not affect the production of other steroids in the pathway.32,35–37) The source of estrogen production in breast cancer tissues is intratumoral aromatase, and thus, inhibition of aromatase may inhibit the growth stimulation effect of estrogens in breast cancer tissues. Therefore, aromatase is considered a useful therapeutic target in the treatment and prevention of estrogen-dependent breast cancer.32)
The dried flowers of M. siamensis (collected from Nakhonsithammarat Province, Thailand) were extracted with methanol under reflux (25.66% from the dried flowers). The methanol extract was partitioned into an EtOAc–H2O (1 : 1, v/v) mixture to furnish an EtOAc-soluble fraction (6.84%) and an aqueous phase. The aqueous phase was subjected to Diaion HP-20 column chromatography (H2O→MeOH) to give H2O- and MeOH-eluted fractions (13.50, 4.22%, respectively), as described previously.1) As shown in Table 1, the methanol extract had an inhibitory effect on aromatase (IC50=16.5 µg/mL). A bioassay-guided fractionation revealed that the EtOAc-soluble and MeOH-eluted fractions also showed aromatase inhibitory activities (IC50=2.9, 8.5 µg/mL, respectively), whereas the H2O-eluted fraction showed no noticeable activity.
| Inhibition (%) | IC50 (µg/mL) | ||||
|---|---|---|---|---|---|
| 3 µg/mL | 10 µg/mL | 30 µg/mL | 100 µg/mL | ||
| MeOH Extract | 13.2±3.2 | 41.8±1.6** | 80.0±3.1** | 97.5±0.6** | 16.5 |
| EtOAc-Soluble fraction | 46.3±4.4** | 89.0±1.2** | 100.1±0.6** | 99.9±0.5** | 2.9 |
| MeOH-Eluted fraction | 2.0±3.9 | 70.7±1.2** | 93.0±1.0** | 96.9±0.9** | 8.5 |
| H2O-Eluted fraction | −4.7±4.8 | 1.2±2.2 | −1.7±2.1 | −0.1±4.0 | >100 |
Each value represents the mean±S.E.M. (N=3). Significantly different from the control, ** p<0.01.
In our previous report we described the isolation of 17 coumarins: mammeasins A (3, 0.0293%) and B (4, 0.0115%), surangins B (5, 0.0271%), C (6, 0.0571%), and D (7, 0.0632%), kayeassamins A (8, 0.0578%), E (11, 0.0113%), F (12, 0.0390%), and G (13, 0.0171%), mammeasins A/AC (16, 0.1056%), A/AD (17, 0.0022%), A/AB cyclo D (19, 0.0097%), A/AC cyclo D (20, 0.0109%), B/AB cyclo D (21, 0.0016%), B/AC cyclo D (22, 0.0062%), and E/BB (24, 0.0194%), and deacetylmammea E/BC cyclo D (26, 0.0073%), β-amyrin (0.0072%), and benzoic acid (0.0043%).1) In the present study we additionally isolated two new geranylated coumarins, mammeasins C (1, 0.0008%) and D (2, 0.0047%), from the active EtOAc-soluble fraction together with seven coumarins: 8-hydroxy-5-methyl-7-(3,7-dimethyl-octa-2,6-dienyl)-9-(2-methyl-1-oxobutyl)-4,5-dihydropyrano[4,3,2-de]chromen-2-one4) (9, 0.0015%), 8-hydroxy-5-methyl-7-(3,7-dimethyl-octa-2,6-dienyl)-9-(3-methyl-1-oxobutyl)-4,5-dihydropyrano[4,3,2-de]chromen-2-one4) (10, 0.0012%), mammeas A/AA38) (14, 0.0494%), A/AB38) (15, 0.0048%), A/AA cyclo D38) (18, 0.0035%), E/BA39) (23, 0.0045%), and E/BC cyclo D2) (25, 0.0058%), using normal-phase silica gel and reversed-phase octadecylsilane (ODS) column chromatography, and finally, HPLC (Fig. 1).
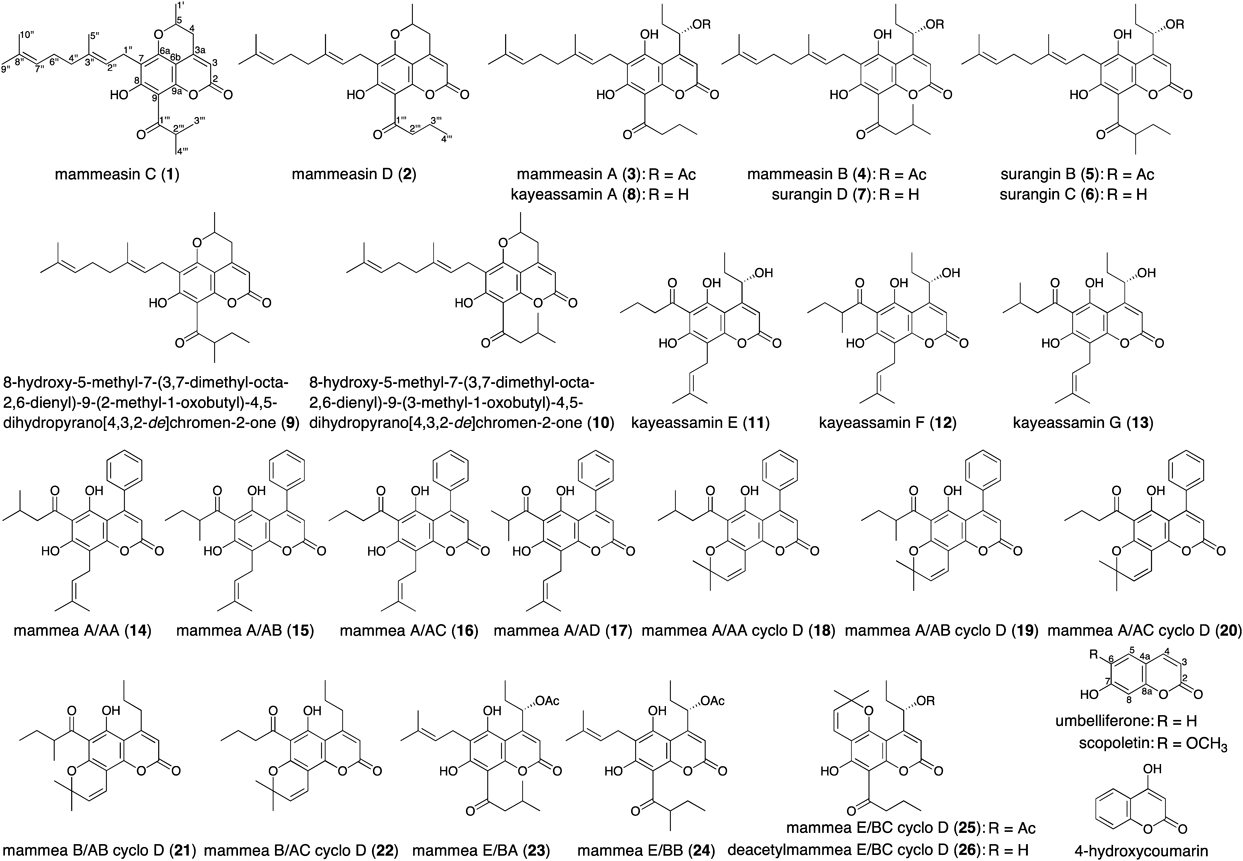
Mammeasin C (1) was obtained as pale yellow oil. Its IR spectrum showed absorption bands at 1748 and 1634 cm−1 assignable to an α,β-unsaturated γ-lactone moiety and a chelated aryl keto group.1,2,4) The UV spectrum exhibited absorption maxima at 221, 292, and 328 nm, similar to those of 5,7-dioxygenated coumarins.1,2,4) The electron ionization (EI)-MS spectrum of 1 showed a molecular ion peak at m/z 424 (M+), and the molecular formula was determined as C26H32O5 by high-resolution (HR)-EI-MS measurement. The 1H- and 13C-NMR spectra of 1 (Tables 2, 3, CDCl3) were assigned with the aid of distortionless enhancement by polarization transfer (DEPT), 1H–1H correlation spectroscopy (COSY), 1H-detected heteronuclear multiple quantum coherence (HMQC), and heteronuclear multiple bond connectivity (HMBC) experiments (Fig. 2). The spectra showed signals assignable to three secondary and three vinyl methyls [δ 1.26, 1.27 (3H each, both d, J=6.6 Hz, 3‴-H3, 4‴-H3), 1.54 (3H, d, J=6.2 Hz, 1′-H3), 1.57 (3H, s, 10″-H3), 1.63 (3H, d, J=0.7 Hz, 9″-H3), 1.78 (3H, s, 5″-H3)]; four methylenes {δ 1.96 (2H, m, 4″-H2), 2.05 (2H, m, 6″-H2), [2.78 (1H, ddd, J=1.4, 11.0, 17.2 Hz), 2.91 (1H, dd, J=2.6, 17.2 Hz), 4-H2], 3.35 (2H, d, J=7.2 Hz, 1″-H2)}; two methines [δ 4.01 (1H, qq, J=6.6, 6.6 Hz, 2‴-H), 4.36 (1H, m, 5-H)]; and two olefinic protons [δ 5.06 (1H, qt, J=0.7, 6.9 Hz, 7″-H), 5.20 (1H, br t, J=ca. 7 Hz, 2″-H)]. The 1H- and 13C-NMR spectroscopic properties of 1 were quite similar to those of 9 and 10, except for the signals due to the 1-oxo-alkyl moiety.4) The 1H–1H COSY experiment on 1 indicated the presence of partial structures, as indicated by the bold lines in Fig. 2. In the HMBC experiment, long-range correlations were observed between the following proton and carbon pairs: 3-H and 2,4,6b-C; 4-H and 3,6b-C; 1″-H2 and 6a,7,8-C; 2″-H and 7,3″,5″-C; 4″-H2 and 3″-C; 5″-H3 and 2″–4″-C; 7″-H2 and 9″,10″-C; 9″-H3 and 7″,8″,10″-C; 10″-H3 and 7″–9″-C; 2‴-H and 1‴-C. On the basis of comprehensive two dimensional (2D)-NMR experiments, we assigned the structure of 1 as 8-hydroxy-5-methyl-7-(3,7-dimethyl-octa-2,6-dienyl)-9-(2-methyl-1-oxopropyl)-4,5-dihydropyrano[4,3,2-de]chromen-2-one.
| Position | 1 | 2 |
|---|---|---|
| δH (J Hz) | δH (J Hz) | |
| 3 | 5.94 (1H, br s) | 5.93 (1H, br s) |
| 4 | 2.78 (1H, ddd, 1.4, 11.0, 17.2) | 2.78 (1H, ddd, 1.5, 10.9, 16.9) |
| 2.91 (1H, dd, 2.6, 17.2) | 2.90 (1H, dd, 2.6, 16.9) | |
| 5 | 4.36 (1H, m) | 4.36 (1H, m) |
| 1′ | 1.54 (3H, d, 6.2) | 1.54 (3H, d, 6.3) |
| 1″ | 3.35 (2H, d, 7.2) | 3.34 (2H, d, 6.9) |
| 2″ | 5.20 (1H, br t, ca. 7) | 5.19 (2H, br t, ca. 7) |
| 4″ | 1.96 (2H, m) | 1.96 (2H, m) |
| 5″ | 1.78 (3H, s) | 1.78 (3H, s) |
| 6″ | 2.05 (2H, m) | 2.05 (2H, m) |
| 7″ | 5.06 (1H, qt, 0.7, 6.9) | 5.05 (1H, qt, 0.9, 6.9) |
| 9″ | 1.63 (3H, d, 0.7) | 1.63 (3H, d, 0.9) |
| 10″ | 1.57 (3H, s) | 1.57 (3H, s) |
| 2‴ | 4.01 (1H, qq, 6.6, 6.6) | 3.26 (2H, br t, ca. 7) |
| 3‴ | 1.26 (3H, d, 6.6)a) | 1.79 (2H, m) |
| 4‴ | 1.27 (3H, d, 6.6)a) | 1.05 (3H, t, 7.5) |
| 8-OH | 14.55 (1H, s) | 14.51 (1H, s) |
a) Assignments may be interchangeable within the same column.
| Position | 1 | 2 | Position | 1 | 2 |
|---|---|---|---|---|---|
| δC | δC | δC | δC | ||
| 2 | 159.8 | 159.7 | 1″ | 21.4 | 21.3 |
| 3 | 105.7 | 105.8 | 2″ | 121.2 | 121.2 |
| 3a | 149.2 | 149.1 | 3″ | 135.8 | 135.8 |
| 4 | 35.1 | 35.0 | 4″ | 39.8 | 39.8 |
| 5 | 72.6 | 72.7 | 5″ | 16.1 | 16.1 |
| 6a | 156.6 | 156.6 | 6″ | 26.7 | 26.7 |
| 6b | 99.6 | 99.6 | 7″ | 124.3 | 124.3 |
| 7 | 113.1 | 113.1 | 8″ | 131.3 | 131.1 |
| 8 | 167.6 | 167.2 | 9″ | 25.6 | 25.7 |
| 9 | 103.2 | 104.0 | 10″ | 17.7 | 17.7 |
| 9a | 154.3 | 154.6 | 1‴ | 210.3 | 205.8 |
| 1′ | 20.7 | 20.7 | 2‴ | 40.1 | 46.3 |
| 3‴ | 19.15a) | 18.0 | |||
| 4‴ | 19.21a) | 13.8 |
a) Assignments may be interchangeable within the same column.

Mammeasin D (2) was also isolated as pale yellow oil. Its molecular formula, C26H32O5, was found to be the same as that of 1 by HR-EI-MS measurement. The 1H- and 13C-NMR spectroscopic properties (Tables 2, 3, CDCl3) of 2 were similar to those of 1, except for the signals due to an 1-oxobutyl moiety in the 9-position [δ 1.05 (3H, t, J=7.5 Hz, 4‴-H3), 1.79 (2H, m, 3‴-H2), 3.26 (2H, br t, J=ca. 7 Hz, 2‴-H2)] instead of the 2-methyl-1-oxopropyl moiety of 1. As shown in Fig. 2, the connectivities of the quaternary carbons in 2 were elucidated on the basis of 1H–1H COSY and HMBC experiments. Thus, the structure of 2 was elucidated to be 8-hydroxy-5-methyl-7-(3,7-dimethyl-octa-2,6-dienyl)-9-(1-oxobutyl)-4,5-dihydropyrano[4,3,2-de]chromen-2-one. Possible biogenetic pathway for the formation of the pyran ring in 9 and 10, having the same moiety with those of 1 and 2, from 6 and 7 have been reported previously.4) These new compounds (1, 2) might be derived through the same pathway. Further studies, e.g. total syntheses of 1 and 2, would be needed to elucidate the absolute stereochemistry and to verify whether these were the artifacts.
Effects of Coumarin Constituents of the Flowers of M. siamensis and Related Compounds on Human Recombinant AromataseTo characterize the active constituents of this plant material, the inhibitory effects of 28 isolates including 26 coumarins (1–26) against aromatase were examined. As shown in Table 4, the coumarin constituents (1–26, IC50=2.7–35.0 µM) possess inhibitory activity, whereas commercially available coumarins such as umbelliferone, scopoletin, and 4-hydroxycoumarin do not. These results suggest that the presence of the side chain at the 4-, 6-, and/or 8-positions of the coumarin skeleton is essential for the inhibitory activity. In particular, mammeasins C (1, 2.7 µM) and D (2, 3.6 µM), and mammea B/AB cyclo D (21, 3.1 µM) show relatively potent activity, comparable to that of aminoglutethimide (2.0 µM).40,41) Therefore, these coumarins may be useful in the treatment of hormone-dependent breast cancer. Detailed structural requirements of coumarins for aromatase inhibitory activity and the mechanism of action, however, need further exploration.
| IC50 (µM) | IC50 (µM) | ||
|---|---|---|---|
| Mammeasin C (1) | 2.7 | Mammea A/AA cyclo D (18) | 7.2 |
| Mammeasin D (2) | 3.6 | Mammea A/AB cyclo D (19) | 24.1 |
| Mammeasin A (3) | 8.7 | Mammea A/AC cyclo D (20) | 35.0 |
| Mammeasin B (4) | 4.1 | Mammea B/AB cyclo D (21) | 3.1 |
| Surangin B (5) | 9.8 | Mammea B/AC cyclo D (22) | 24.6 |
| Surangin C (6) | 8.8 | Mammea E/BA (23) | 16.6 |
| Surangin D (7) | 18.1 | Mammea E/BB (24) | 18.6 |
| Kayeassamin A (8) | 10.0 | Mammea E/BC cyclo D (25) | 11.5 |
| 9 | 7.5 | Deacetylmammea E/BC cyclo D (26) | 16.6 |
| 10 | 8.8 | β-Amyrin | >100 (24.4)a) |
| Kayeassamin E (11) | 14.9 | Benzoic acid | >100 (−3.4)a) |
| Kayeassamin F (12) | 19.7 | ||
| Kayeassamin G (13) | 27.8 | Umbelliferone | >100 (2.6)a) |
| Mammea A/AA (14) | 6.9 | Scopoletin | >100 (−15.9)a) |
| Mammea A/AB (15) | 8.6 | 4-Hydroxycoumarin | >100 (10.9)a) |
| Mammea A/AC (16) | 13.7 | ||
| Mammea A/AD (17) | 11.3 | Aminoglutethimide | 2.0 |
Each value represents the mean±S.E.M. (N=3). a) Values in parentheses present inhibition % at 100 µM.
The following instruments were used to obtain physical data: UV spectra, UV-1600 spectrometer (Shimadzu Co., Kyoto, Japan); IR spectra, FTIR-8100 spectrometer (Shimadzu Co.); EI-MS and HR-EI-MS, JMS-GCMATE mass spectrometer (JEOL Ltd., Tokyo, Japan); 1H-NMR spectra, JNM-ECA500 (500 MHz), and JNM-ECS400 (400 MHz) spectrometers (JEOL); 13C-NMR spectra, JNM-ECA500 (125 MHz), and JNM-ECS400 (100 MHz) spectrometers (JEOL Ltd.) with tetramethylsilane as an internal standard; HPLC detector, SPD-10Avp UV-VIS detector (Shimadzu Co.); HPLC column, Cosmosil 5C18-MS-II (Nacalai Tesque, Inc., Kyoto, Japan), 4.6×250 mm i.d. and 20×250 mm i.d. for analytical and preparative studies, respectively.
The following experimental conditions were used for chromatography (CC): ordinary-phase silica gel column chromatography, silica gel 60N (Kanto Chemical Co., Tokyo, Japan; 63–210 mesh, spherical, neutral); reverse-phase silica gel CC, Diaion HP-20 (Nippon Rensui, Tokyo, Japan) and Chromatorex ODS DM1020T (Fuji Silysia Chemical, Aichi, Japan; 100–200 mesh); normal-phase TLC, pre-coated TLC plates with silica gel 60F254 (Merck, Darmstadt, Germany; 0.25 mm); reversed-phase TLC, pre-coated TLC plates with silica gel RP-18 F254S (Merck, 0.25 mm); reversed-phase HPTLC, pre-coated TLC plates with silica gel RP-18 WF254S (Merck, 0.25 mm), detection was carried out by spraying 1% Ce(SO4)2–10% aqueous H2SO4 on the plates, followed by heating.
Plant MaterialThe flowers of Mammea siamensis were collected from Nakhonsithammarat Province, Thailand, in September 2006, as described previously.1) The plant material was identified by one of the authors (Y.P.). A voucher specimen (2006.09. Raj-04) for this plant has been deposited in our laboratory.
Extraction and IsolationDried flowers of M. siamensis (1.8 kg) were extracted three times with MeOH under reflux for 3 h. Evaporation of the combined extracts under reduced pressure afforded the MeOH extract (463.7 g, 25.66%). An aliquot (413.7 g) of the extract was partitioned into an EtOAc–H2O (1 : 1, v/v) mixture to furnish an EtOAc-soluble fraction (110.34 g, 6.84%) and an aqueous phase. The aqueous phase was subjected to Diaion HP-20 CC (2.4 kg, H2O→MeOH, twice) to give H2O-eluted (217.70 g, 13.50%) and MeOH-eluted (68.10 g, 4.22%) fractions, respectively. An aliquot (89.45 g) of the EtOAc-soluble fraction was subjected to normal-phase silica gel CC [3.0 kg, n-hexane–EtOAc (10 : 1→7 : 1→5 : 1, v/v)→EtOAc→MeOH] to give 11 fractions [Fr. 1 (3.05 g), Fr. 2 (2.86 g), Fr. 3 (11.71 g), Fr. 4 (1.62 g), Fr. 5 (4.15 g), Fr. 6 (6.29 g), Fr. 7 (2.21 g), Fr. 8 (2.94 g), Fr. 9 (10.23 g), Fr. 10 (11.17 g), and Fr. 11 (21.35 g)] as reported previously.1) Fraction 5 (4.15 g) was subjected to reversed-phase silica gel CC [120 g, MeOH–H2O (80 : 20→85 : 15, v/v)→MeOH→acetone] to afford six fractions [fr. 5-1 (115.7 mg), fr. 5-2 (2789.8 mg), fr. 5-3 (515.4 mg), fr. 5-4 (430.0 mg), fr. 5-5 (119.2 mg), and fr. 5-6 (110.0 mg)]. Fraction 5-2 (517.0 mg) was purified by HPLC [Cosmosil 5C18-MS-II, MeOH–1% aqueous AcOH (85 : 15, v/v)] to give mammeas A/AA (14, 101.2 mg, 0.0418%), A/AC (16, 112.9 mg, 0.0466%), A/AA cyclo D (18, 2.7 mg, 0.0011%), and E/BC cyclo D (25, 14.0 mg, 0.0058%). Fraction 5-3 (515.4 mg) was purified by HPLC [Cosmosil 5C18-MS-II, MeOH–1% aqueous AcOH (85 : 15, v/v)] to give 16 (45.6 mg, 0.0035%), 18 (14.9 mg, 0.0011%), mammeas A/AB cyclo D (19, 46.4 mg, 0.0035%), and A/AC cyclo D (20, 30.1 mg, 0.0023%). Fraction 5-4 (430.0 mg) was purified by HPLC [Cosmosil 5C18-MS-II, MeOH–1% aqueous AcOH (90 : 10, v/v)] to give 18 (17.4 mg, 0.0013%), 19 (19.1 mg, 0.0015%), 20 (11.5 mg, 0.0009%), and mammea B/AC cyclo D (22, 9.5 mg, 0.0007%). Fraction 6 (6.29 g) was subjected to reversed-phase silica gel CC [200 g, MeOH–H2O (80 : 20→90 : 10→95 : 5, v/v)→MeOH→acetone] to afford 10 fractions [fr. 6-1 (44.7 mg), fr. 6-2 (157.2 mg), fr. 6-3 (928.8 mg), fr. 6-4 (3117.0 mg), fr. 6-5 (128.8 mg), fr. 6-6 (487.1 mg), fr. 6-7 (230.8 mg), fr. 6-8 (280.5 mg), fr. 6-9 (102.9 mg), and Fr. 6-10 (96.5 mg)]. Fraction 6-3 (514.6 mg) was purified by HPLC [Cosmosil 5C18-MS-II, MeOH–1% aqueous AcOH (80 : 20, v/v)] to give mammea E/BA (23, 32.7 mg, 0.0045%) together with 16 (35.6 mg, 0.0049%), mammeas A/AD (17, 15.8 mg, 0.0022%) and E/BB (24, 140.1 mg, 0.0194%) as reported previously.1) Fraction 6-4 (536.2 mg) was purified by HPLC [Cosmosil 5C18-MS-II, MeOH–1% aqueous AcOH (90 : 10, v/v)] to give mammeas A/AA (14, 17.0 mg, 0.0076%) and A/AB (15, 10.7 mg, 0.0048%) together with mammeasins A (3, 65.8 mg, 0.0293%) and B (4, 21.6 mg, 0.0096%), surangin B (5, 58.2 mg, 0.0259%), and 16 (112.6 mg, 0.0501%) as reported previously.1) Fraction 6-5 (128.8 mg) was purified by HPLC [Cosmosil 5C18-MS-II, MeOH–1% aqueous AcOH (90 : 10, v/v)] to give 4 (24.1 mg, 0.0019%) and 5 (15.1 mg, 0.0012%) as reported previously.1) Fraction 6-6 (487.1 mg) was purified by HPLC [Cosmosil 5C18-MS-II, MeOH–1% aqueous AcOH (90 : 10, v/v)] to give mammeasins C (1, 10.4 mg, 0.0008%) and D (2, 60.9 mg, 0.0047%), 8-hydroxy-5-methyl-7-(3,7-dimethyl-octa-2,6-dienyl)-9-(2-methyl-1-oxobutyl)-4,5-dihydropyrano[4,3,2-de]chromen-2-one (9, 19.8 mg, 0.0015%), and 8-hydroxy-5-methyl-7-(3,7-dimethyl-octa-2,6-dienyl)-9-(3-methyl-1-oxobutyl)-4,5-dihydropyrano[4,3,2-de]chromen-2-one (10, 16.3 mg, 0.0012%) together with 16 (6.0 mg, 0.00050%) as reported previously.1)
Mammeasin C (1)Pale yellow oil. HR-EI-MS: Calcd for C26H32O5 (M+): 424.2250. Found: 424.2243. UV [MeOH, nm (log ε)]: 221 (4.32), 292 (4.22), 328 (4.05). IR (film): 1748, 1717, 1634, 1601, 1456, 1404, 1385, 1237, 1194, 1171, 1132, 1109 cm−1. 1H-NMR (500 MHz, CDCl3) δ: see Table 2. 13C-NMR data (125 MHz, CDCl3) δC: see Table 3. EI-MS m/z: 424 (M+, 37), 301 (100).
Mammeasin D (2)Pale yellow oil. HR-EI-MS: Calcd for C26H32O5 (M+): 424.2250. Found: 424.2243. UV [MeOH, nm (log ε)]: 221 (4.39), 292 (4.29), 324 (4.09). IR (film): 1734, 1717, 1636, 1617, 1456, 1387, 1235, 1192, 1115, 1049 cm−1. 1H-NMR (500 MHz, CDCl3) δ: see Table 3. 13C-NMR data (125 MHz, CDCl3) δC: see Table 3. EI-MS m/z: 424 (M+, 30), 301 (100).
BioassayReagentsDibenzylfluorescein (DBF) and Human CYP19 + P450 Reductase SUPERSOMES (human recombinant aromatase) were purchased from BD Biosciences (Heidelberg, Germany). The other chemicals used in this study were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Inhibitory Effects against Human Recombinant AromataseThe experiments were performed according to the method described previously but with a slight modification.42) A test sample was dissolved in dimethyl sulfoxide (DMSO) and the solution was diluted with potassium phosphate buffer (50 mM, pH 7.4) containing MgCl2 (0.5 mM) to afford the test sample solution (concentration of DMSO: 2%). An enzyme/substrate solution in the buffer (20 µL, 1.6 µM DBF, 8 nM human recombinant aromatase) and the test sample solution (20 µL) were mixed into a 96-well half area black microplate (Greiner Bio-One, Frickenhausen, Germany) at 37°C for 10 min. The enzymatic reaction was initiated by the addition of reduced nicotinamide adenine dinucleotide phosphate (NADPH) solution (40 µL, 500 µM) at 37°C for 30 min. After 30 min incubation, NaOH (30 µL, 2 mM) was added, and the reaction mixture was incubated at 37°C for 2 h to induce the fluorescent signals. Fluorescence was measured using a fluorescence microplate reader (SH-9000, CORONA) at excitation wavelength of 485 nm and emission wavelength of 535 nm. Experiments were performed in triplicate, and IC50 values were determined graphically. The aromatase inhibitor aminoglutethimide was used as a reference compound.
StatisticsValues are expressed as the mean±standard error of the mean (S.E.M.). One-way ANOVA, followed by Dunnett’s test, was used for statistical analysis. Probability (p) values less than 0.05 were considered significant.
This study was supported in part by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018 (T.M.), and a Grant-in-Aid for Scientific Research [KAKENHI, Grant Numbers 15K08008 (T.M.), 15K08009 (K.N.), and 16K0813 (O.M.)]. The authors also acknowledge the financial support extended by Kobayashi International Scholarship Foundation (T.M.).
The authors declare no conflict of interest.