Abstract
During the search for new antitrypanosomal drug leads, four antitrypanosomal compounds, of three depsipeptides and one nortriterpenoid, were isolated from cultures of the mutant strain IU-3 of the insect pathogenic fungus Ophiocordyceps coccidiicola NBRC 100683. Their structures were identified by the analysis of high resolution-electron ionization (HR-EI)-MS and HR-FAB-MS, and 1H- and 13C-NMR spectra, including extensive two dimensional (2D)-heteronuclear NMR experiments, and comparison with literature data for destruxin A (1), destruxin B (2), destruxin E chlorohydrin (3) and helvolic acid (4). Compounds 1–4 showed in vitro antitrypanosomal activity against Trypanosoma brucei brucei GUTat3.1 with IC50 values of 0.33, 0.16, 0.061 and 5.08 µg/mL, respectively.
Human African trypanosomiasis, also known as sleeping sickness, is a potentially fatal parasitic disease transmitted by the bite of the tsetse fly that plagues many regions of Africa. Although the number of people being infected with the disease has declined due to better diagnosis and treatment, an estimated 30000 people are currently infected with 7000 new infections in 20121) without treatment, sleeping sickness is fatal. Moreover current therapies often have unpleasant side effects.
During the course of our screening program for compounds that treat the disease, which is caused by the parasite Trypanosoma brucei, we had reported on various natural products, such as microbial and fungi metabolites and plant products that exhibit potent antitrypanosomal properties.2–5) This time we focused on metabolites from cultures of cordyceps that showed antitrypanosomal activity. The “dong chóng xìa căo” in Chinese pronunciation, means “summer herb-winter worm” is traditionally used for herbal medicines and health foods in East to Southeast Asia. That is an entomopathogenic fungi, Ophiocordyceps sinensis (BERK.) G.H. SUNG et al. parasitizing to a lepidopteran larvae, Thitarodes spp. Members of the Ophiocordycepitaceae, and taxonomically allied Cordycipitaceae and Clavicipitaceae are known as the biggest entomopathogenic group among fungi, and possible to parasite to over ten orders of insects, spider, mites and other ascomycetes fungi, Elaphomyces spp.6–8) They are distributed in worldwide, especially in East to Southeast Asia. At least about 500 species were named in the world.9) This high and unique biodiversity is expected as screening sources for new drugs.
Ophiocordyceps coccidiicola (KOBAYASI) G.H. SUNG et al. was described in 1978 from Japan, while no collection report from other countries. It occurs on a cadaver of scale insects that inhabiting on and sucking the deciduous shrubs in humidity area like valley. The morphology is similar to Hirsutella coccidiicola KOBAYASI & SHIMIZU, and probably the both names are teleomorph–anamorph relationship while Kobayasi did not mention (personal opinion, Ban).
To the best of our knowledge, there have been no reports for the study of metabolites of O. coccidiicola up to date. Here, we report the isolation, structural identification of four known compounds (1–4), and antitrypanosomal activity.10,11)
Results and Discussion
The wild-type strain NBRC 100683 was isolated from single ascospore, which specimen was collected from Umegase Valley, Ichihara, Chiba Pref., Japan, occurring on a scale insect on a branch of Hydrangea sp. The mutant strain IU-3 was obtained from subculture NBRC 100683 in potato sucrose broth (PSB, l-1: extract of 200 g potato and 20 g sucrose; pH 5.6). The mutant is lacking pigments and faster vegetative growth.
Colonies of strain IU-3 grows 24–25.5 mm diam. on potato sucrose agar (PSA, l-1: add 20 g agar to PSB) at 25°C in 18 d. Colony mycelium is white, reverse white, become tan with age, the texture is floccose by arising aerial hypha. Conidial structures are observed at a part of colony which stimulating, for example, by cutting mycelium. According to the original description, phialides of H. coccidiicola are 13–18×2 µm, conidia are 2.5–3.5×1.5–2 µm. The strain IU-3 sometimes produces conspicuously longer conidia on agar medium than in nature. To clarify it was not contamination, we conducted sequence analysis of ribosomal RNA (rRNA) internal transcribed spacer (ITS) region. The result showed the mutant strain was 100% much with the parent strain NBRC 100683.
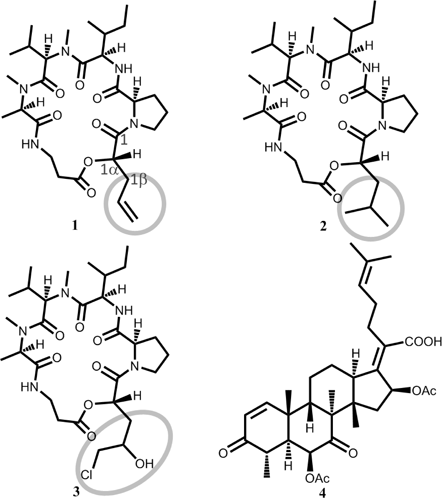
The IU-3 was cultured for 2 weeks at 25°C in PSB. The culture solution (15.5 L) of IU-3 was separated from the mycelia by filtration and subsequently absorbed on Diaion HP-20 resin. And then, the absorbed fraction was eluted with MeOH. The MeOH eluted fraction was evaporated to an aqueous concentrate and successively extracted with EtOAc. The EtOAc extracted material (1.1 g) was chromatographed on silica gel by n-hexane and an increasing ratio of CHCl3 and by repeated preparative HPLC on an ODS C-18 column, using 55–80% MeOH in H2O to afford four known compounds, destruxin A (1) (51.4 mg), destruxin B (2) (37.2 mg), destruxin E chlorohydrin (3) (46.8 mg) and helvolic acid (4) (9.1 mg). Their structures were identified by the analysis of high resolution-electron ionization (HR-EI)-MS and HR-FAB-MS, and 1H- and 13C-NMR spectra, (see supporting information) including extensive two dimensional (2D)-heteronuclear NMR experiments, and comparison with literature data for destruxin A (1), destruxin B (2), destruxin E chlorohydrin (3) and helvolic acid (4).10,11) Cyclic depsipeptides (1–3) were already isolated from Metarhizium anisopliae, formerly known as Entomophthora anisopliae (basionym), is a fungus that grows naturally in soils throughout the world and causes disease in various insects by acting as a parasitoid.10) Meanwhile, a nortriterpenoid derivative, helvolic acid (4), was also isolated from M. anisopliae grown in medium with insect component.11)
Compounds 1–4 showed in vitro antitrypanosomal activity against Trypanosoma brucei brucei GUTat3.1 with IC50 values of 0.33, 0.16, 0.061 and 5.08 µg/mL, respectively, which were comparable to suramin (commonly used therapeutic drug for sleeping sickness). They displayed weak cytotoxicity against normal human diploid fibroblasts (MRC-5 cells) with IC50 values of 4.63, 3.75, 0.36 and >100 µg/mL, with selectivity indices of 14.0, 24.3, 5.9 and 19.7, respectively. Destruxin B (2) with an isopropyl group at C-1α position showed two times stronger activity than destruxin A (1) with a 2-propenyl group. Furthermore, destruxin E chlorohydrin (3) with a hydroxyl group at C-1γ position and a chlorine atom at C-1δ position showed the strongest activity among these three compounds. This activity of 3 is around 26 times of suramin, which are used for the remedy of African trypanosomiasis (Fig. 1, Table 1).
Table 1.
In Vitro Anti-trypanosomal Activity against
Trypanosoma brucei brucei GUTat3.1 and Cytotoxicity in MRC-5 Cells of Compounds
1–
4| Compound | IC50 (µg/ mL) |
|---|
| Anti-tryp activity (T. b. b. GUTat 3.1) | Cytotoxicity (MRC-5) | Selectivity index (MRC-5/Try) |
|---|
| Destruxin A (1) | 0.33 | 4.63 | 14.0 |
| Destruxin B (2) | 0.16 | 3.75 | 24.3 |
| Destruxin E chlorohydrin (3) | 0.061 | 0.36 | 5.9 |
| Helvolic acid (4) | 5.08 | >100 | 19.7 |
| Suramin (positive control) | 1.58 | >100 | >63 |
In conclusion, this paper describes the identification of entomopathogenic fungal metabolites produced by O. coccidiicola, possessing in vitro anti-trypanosomal activity. To the best of our knowledge, this is first report for the study of metabolites of O. coccidiicola. Among them, destruxins 1–3 are belonging to cyclodepsipeptides. We had reported other anti-trypanosomal cyclodepsipeptides, cardinalisamides A–C, from the insect pathogenic fungus Cordyceps cardinalis NBRC 103832.2) Helvolic acid (4) is an antibacterial nortriterpenoid that is an inhibitor of the protein synthesis through elongation factor G (EF-G) on the bacterial ribosome. Although fusidic acid (analog of 4) was reported to show anti-protozan activities against malaria, babesia and so on,12) this is the first report for anti-trypanosomal activity of 4.
Experimental
General Experimental ProceduresOptical rotations were recorded on a JASCO P-1030 polarimeter. IR spectra were measured on a Shimazu FTIR-8400S instrument. NMR spectra were obtained on a Varian UNITY 600 NMR spectrometer. The chemical shifts were given in δ (ppm), and coupling constants were reported in Hz. HR-MS spectra were obtained on a JEOL JMS-700 instrument. Kieselgel 60 (230–400 mesh, Merk) was used for column chromatography, and silica gel 60 F-254 (Merck) for TLC. HPLC was performed on a JASCO-PU 1580 instrument with a COSMOSIL C18 P-MS II (250×20 mm).
Material, Extraction and IsolationThe fungus O. coccidiicola NBRC 100683 was identified by Dr. Ban of the Nite Biological Resource Center (NBRC). A voucher specimen (O. coccidiicola NBRC 100683 and IU-3) is deposited at the NBRC Culture Collection. IU-3 was cultured for 2 weeks at 25°C in potato sucrose medium (15 L culture). The culture solution (15 L) of IU-3 was separated from the mycelia by filtration and subsequently absorbed on Diaion HP-20 resin. And then, the absorbed fraction was eluted with MeOH. The MeOH eluted fraction was evaporated to an aqueous concentrate and successively extracted with EtOAc (500 mL×3). The EtOAc-soluble portion (1.1 g) was repeatedly chromatographed on silica gel (Kanto Kagaku silicagel 60N, 40–50 µm) by n-hexane and an increasing ratio of CHCl3. Further purification was carried out by repeated preparative HPLC on an ODS C-18 column (COSMOSIL MS-II 20 20×250 mm for 1–3, COSMOSIL AR-II 20 20×250 mm for 4), using 55–80% MeOH in H2O (flow rate; 3 mL/min, room temperature; 23°C and isocratic) to afford four compounds, destruxin A (1) (60% MeOH, 90 min) (51.4 mg), destruxin B (2) (60% MeOH, 39.6 min) (37.2 mg), destruxin E chlorohydrin (3) (55% MeOH, 54.8 min) (46.8 mg) and helvolic acid (4) (80% MeOH, 22 min) (9.1 mg).
TrypanosomeThe bloodstream forms of T. b. brucei strain GUTat 3.1 parasites were used for experimentation, as described previously.13)
In Vitro AssayThe in vitro antitrypanosomal assays using T. b. brucei strain GUTat 3.1 have been described previously.13) In brief, 95 µL of parasite suspension was incubated with 5 µL of drug solution for 72 h and Alamar Blue was used for parasite survival determination to calculate IC50 values.
Cytotoxicity assay against human diploid embryonic cell line MRC-5 was carried out as previously described.12)
Acknowledgments
We are indebted to Dr. M. Tanaka for measurements of the 600 MHz NMR spectra, Faculty of Pharmaceutical Sciences of Tokushima Bunri University. This work was supported by a Grant from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan-Senryaku project (No. S0891078).
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Materials
The online version of this article contains supplementary materials.
References
- 1) Home page of World Health Organization: WHO.: ‹http://www.who.int/mediacentre/factsheets/fs259/en/›, 2015.
- 2) Umeyama A., Takahashi K., Grudniewska A., Shimizu M., Hayashi S., Kato M., Okamoto Y., Suenaga M., Ban S., Kumada T., Ishiyama A., Iwatsuki M., Otoguro K., Ōmura S., Hashimoto T., J. Antibiot., 67, 163–166 (2014).
- 3) Umeyama A., Ohta C., Shino Y., Okada M., Nakamura Y., Hamagaki T., Imagawa H., Tanaka M., Ishiyama A., Iwatsuki M., Otoguro K., Ōmura S., Hashimoto T., Tetrahedron, 70, 8312–8315 (2014).
- 4) Inahashi Y., Iwatsuki M., Ishiyama A., Namatame M., Nishihara-Tsukashima A., Matsumoto A., Hirose T., Sunazuka T., Yamada H., Otoguro K., Takahashi Y., Ōmura S., Shiomi K., J. Antibiot., 64, 303–307 (2011).
- 5) Iwatsuki M., Kinoshita Y., Niitsuma M., Hashida J., Mori M., Ishiyama A., Namatame M., Nishihara-Tsukashima A., Nonaka K., Masuma R., Otoguro K., Yamada H., Shiomi K., Ōmura S., J. Antibiot., 63, 331–333 (2010).
- 6) Kobayasi Y., Shimizu D., Bull. Natl. Sci. Mus., Tokyo, 5, 69–85 (1960).
- 7) Imai S., Transactions of the Sapporo Natural History Society, 14, 101–106 (1936).
- 8) Sung G. H., Hywel-Jones N. L., Sung J. M., Luangsa-Ard J. J., Shrestha B., Spatafora J. W., Stud. Mycol., 57, 5–59 (2007).
- 9) Home page of Index Fungorum.: ‹http://www.indexfungorum.org/›
- 10) Gupta S., Roberts D. W., Renwick J. A. A., J. Chem. Soc., Perkin Trans. 1, 1989, 2347–2357 (1989).
- 11) Lee S.-Y., Kinoshita H., Ihara F., Igarashi Y., Nihira T., J. Biosci. Bioeng., 105, 476–480 (2008).
- 12) Otoguro K., Kohana A., Manabe C., Ishiyama A., Ui H., Shiomi K., Yamada H., Ōmura S., J. Antibiot., 54, 658–663 (2001).
- 13) Otoguro K., Ishiyama A., Namatame M., Nishihara A., Furusawa T., Masuma R., Shiomi K., Takahashi Y., Yamada H., Ōmura S., J. Antibiot., 61, 372–378 (2008).