2016 年 64 巻 8 号 p. 1136-1141
2016 年 64 巻 8 号 p. 1136-1141
A series of 1-(2-aminophenyl)-3-arylurea novel derivatives were synthesized and evaluated against Ephrin type-A receptor 2 (EphA2) and histone deacetylases (HDACs) kinase. Most of the compounds exhibited inhibitory activity against EphA2 and HDAC. The antiproliferative activities were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (thiazolyl blue, tetrazolium blue) against the human cancer cell lines HCT116, K562 and MCF7. Compounds 5a and b showed the most potent inhibitory activity against EphA2 and HDAC. However, compound 5b exhibited higher potency against HCT116 (IC50=5.29 µM) and MCF7 (IC50=7.42 µM). 1-(2-Aminophenyl)-3-arylurea analogues may serve as new EphA2–HDAC dual inhibitors.
Mechanism-based targeted therapies as a treatment for tumors has been proclaimed as one of the major breakthroughs in human cancer research in the past several decades. However, the clinical effectiveness of such treatment is generally unsustainable in the long run and relapses are almost inevitable after the treatment.1) One explanation is that, the core hallmark capabilities of cancer are regulated by partially redundant signaling molecules. As such, a targeted therapeutic agent inhibiting only one key molecule in a tumor may not completely shut off the core hallmark capability, allowing some cancer cells to survive with residual function.1) To address this problem, the medicine will have to incorporate, within a single molecule, elements that simultaneously tackle multiple targets for cancer therapy.2)
The erythropoietin-producing hepatocellular (Eph) receptor tyrosine kinases (RTKs) has been recognized increasingly as key regulators of both normal development and disease including organ development, tissue remodeling, neuronal signaling, vascular development and tumor progression.3–7) To date, Eph receptor tyrosine kinases are classified in the EphA (EphA1–10) and EphB (EphB1–6) subclasses based on sequence homology and binding affinity for ephrins.8) One family member in particular, the EphA2 was identified from human epithelial cDNA library and widespread expression in epithelial cells.9) An oncogenic role for EphA2 has been suggested due to its overexpression in breast, prostate, lung cancer as well as malignant melanoma.10) The level of EphA2 expression on tumor cells correlates with the degree of malignant progression and poor prognosis.11) The possibility of targeting EphA2/ephrin therapeutically may be the most straightforward in the context of inhibiting Eph/ephrins signaling in the vasculature as a means of preventing tumor angiogenesis.7) Currently only a few small molecule EphA2 inhibitors have been reported in the literature.12,13)
The reversible acetylation of lysine residues in histone tails plays a critical role in transcriptional activation and repression.14) Histone acetyltransferases (HATs) catalyze the acetylation of lysine residues on histone and non-histone proteins. The reverse reaction is catalyzed by histone deacetylases (HDACs), thus promoting a more closed chromatin structure where transcription is repressed.15,16) HDAC inhibitors have been applied to treatment of human cancers.17,18) Zolinza (vorinostat, SAHA) was approved by the Food and Drug Administration (FDA) for the treatment of advanced cutaneous T-cell lymphoma (CTCL).19) A benzamide HDAC inhibitor, MS275, is reported to be a selective HDAC inhibitor targeting class I HDACs, which is a moderately potent HDAC inhibitor in phase II clinical trial.20) Several small molecule HDAC inhibitors have entered clinical trials for the treatment of a variety of haematological and solid tumors.21) Preclinical data with numerous cancer cell lines has shown synergistic and additive effects when combining HDAC inhibitors with various antitumor therapies, suggesting HDAC might be an ideal target for combination.22)
HDAC inhibitors have been shown to synergize with other agents, including RTK inhibitors, to suppress proliferation and induce apoptosis in tumor cells.23,24) This evidence suggests that simultaneous EphA2 activation and HDAC inhibition could be a promising approach in cancer therapy. Here, compound N-(4-(3-(2-aminophenyl)ureido)phenyl)-4-methylbenzamide (5e) was disclosed to inhibit EphA2 and HDAC based on the virtual screening. Then we designed a series of 1-(2-aminophenyl)-3-arylurea derivatives and a simple and convenient route was applied for synthesis of compounds 5a–h (Chart 1). In addition, biological assay showed some compounds exhibited potential antiproliferative activity across a range of human cancer cell lines.

Reagents and conditions: (a) SOCl2, DMF, reflux, 4 h; (b) 4-nitroaniline, TEA, 0°C; (c) Fe, NH4Cl, C2H5OH, H2O, reflux, 6 h; (d) 1-isocyanato-2-nitrobenzene, toluene, reflux, 3 h; (e) Fe, HOAc, 95% C2H5OH, reflux, 6 h.
The substituted aromatic acid was treated with thionyl chloride, affording the corresponding acyl chloride 1. The reaction was conducted using 1–2 drops of N,N-dimethylformamide (DMF) as the catalyst under anhydrous conditions. After the thionyl chloride was evaporated, the crude product was used for the next step without purification. Then the acyl chloride 1 was reacted with 4-nitroaniline in dry dichloromethane in the presence of anhydrous triethylamine at 0°C to afford the corresponding amide 2. Reduction with iron powder in water/ethanol gave desired amine 3. Compound 3 reacted with 1-isocyanato-2-nitrobenzene to afford the urea 4. The target compounds 5a–h were synthesized by reduction of the corresponding urea 4 with iron powder in 95% ethanol.
PharmacologyTo study biological activity, compounds 5a–h were evaluated against EphA2 kinase activity assays with Dasatinib as positive control and HDACs enzyme assay with MS-275 as positive control. EphA2 was expressed from CHO-CK cell-line and HDACs were prepared from HeLa cell extracts. Compounds 5a–h were initially screened at final concentrations of 10.0, 1.0 and 0.1 µM and an enzymatic assay measuring total HDACs activity. IC50s of compounds 5a–h and the reference compounds Dasatinib and MS-275 were calculated according to inhibitory rates, the in vitro data for enzymatic inhibition are listed in Table 1.
 | |||
|---|---|---|---|
| Compound | R | IC50 (µM) | |
| EphA2 | HDACs | ||
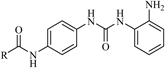
| 5a | 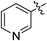 | 0.68 | 0.94 |
| 5b |  | 0.57 | 1.52 |
| 5c |  | 1.89 | 29.1 |
| 5d |  | 0.51 | 12.3 |
| 5e |  | 5.72 | 2.54 |
| 5f | 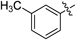 | 8.20 | 15.5 |
| 5g |  | 9.33 | 6.43 |
| 5h |  | 0.79 | 18.5 |
| Dasatinib |  | 0.43 | Nt |
| MS-275 | 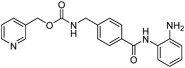 | Nt | 0.83 |
Nt: Not tested.
The data in Table 1 demonstrated that compounds 5a–h exhibited the inhibitory activity against EphA2 and HDACs, especially compound 5a (IC50=0.68 µM against EphA2, IC50=0.94 µM against HDACs) and 5b (IC50=0.57 µM against EphA2, IC50=1.52 µM against HDACs). Compounds 5b–d, h relatively exhibited higher inhibitory activities than that of substituted aryl compounds against EphA2. These results suggest that methoxy groups at benzene ring are favorable for EphA2 inhibition. The activity of compound 5c against EphA2 was found to be less potent than 5b, d and h. This means that the methoxy group at 2-position is unfavorable for EphA2 inhibition. The inhibitory activity against EphA2 of compound 5a was close to that of compounds 5b and d, so it means that the pyridine is an important group for EphA2 inhibition.
As shown in Table 1, compounds 5a–h exhibited inhibitory activity against HDACs. Compared with reference compound MS-275, all the synthesized compounds inhibit HDACs activity with reduced potency. Only the activity of compound 5a (IC50=0.94 µM) against HDACs was close to that of MS-275 (IC50=0.83 µM). It seems that the effect of pyridine substituting benzene ring can obviously affect EphA2 and HDACs inhibitory activity. Compound 5b (IC50=1.52 µM) also had higher activity against HDACs than other substituted phenyl compound.
To test the anticancer activities of the synthesized compounds we evaluated antiproliferative activities of compounds 5a–h against human colon carcinoma cell line (HCT116), erythroleukemic cell line (K562) and breast cancer cell line (MCF7) by applying the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. The results were summarized in Table 2.
| Compound | IC50 (µM) | ||
|---|---|---|---|
| HCT116 | K562 | MCF7 | |
| 5a | 32.17 | 8.36 | 15.38 |
| 5b | 5.29 | 9.17 | 7.42 |
| 5c | >100 | >100 | >100 |
| 5d | 9.31 | >100 | 7.55 |
| 5e | 85.21 | 65.42 | >100 |
| 5f | >100 | >100 | >100 |
| 5g | 19.87 | 26.54 | 10.81 |
| 5h | 6.36 | 68.13 | 9.17 |
| Dasatinib | 5.50 | 0.022 | 3.90 |
| MS-275 | 0.76 | 0.989 | 16.69 |
Compounds 5a, b, g and h exhibited potential effects against HCT116, K562 and MCF7 in vitro (Table 2). In the case of against K562, all synthesized compounds showed less inhibitory activities than reference compounds. When tested against HCT116 and MCF7, compound 5b displayed enhanced inhibition (IC50=5.29 µM against HCT116) as compared with Dasatinib (IC50=5.50 µM against HCT116) and compounds 5a (IC50=15.38 µM against MCF7), 5b (IC50=7.42 µM against MCF7), 5d (IC50=7.55 µM against MCF7), 5g (IC50=10.81 µM against MCF7) and 5h (IC50=9.17 µM against MCF7) showed a higher potency than MS-275 (IC50=16.69 µM against MCF7).
Docking Studies of Compound 5b on EphA2 as Well as on HDACThe binding mode of the representative compound 5b to the EphA2 and HDAC are shown in Fig. 1. It was possible to dock compound 5b in an energetically favorable conformation into the model (Fig. 1A). The hydrogen bonds of the benzoylamido NH with the backbone of M695 (hinge), of the ureido NH with the side chain T692 (hinge) are reproduced. The 2-aminophenyl moiety is aligned with a hydrophobic pocket composed of I690, G663, M667 and L646. 4-Methoxybenzene moiety is at the entrance of the active site. As shown in Fig. 1B, the carbonyl and 2-aminophenyl NH are coordinated with the catalytic zinc ion. The hydrogen bonds of 2-amino substituent with the backbone of A168, of the ureido NH with the carbonyl group of G140 may contribute to the tight fit. The phenyl ring of the ureidophenyl moiety is stacked between P141, P198 and H170.

Compound 5b was shown in light gray. EphA2 and HDAC is represented by ribbons with the interacting residues represented as sticks. Hydrogen-bonding interactions and coordination with zinc ion are drawn as dashed lines.
In summary, a series of 1-(2-aminophenyl)-3-arylurea derivatives were synthesized efficiently as novel HDAC–EphA2 dual inhibitors. As expected, these compounds exhibited distinct inhibitory activity against HDAC and EphA2 and antiproliferative activities in vitro. Compounds 5a, b, g and h exhibited potential effects against HCT116, K562 and MCF7 in vitro. Especially compound 5b exhibited higher potency against HCT116 than Dasatinib and against MCF7 than MS275. According to the docking study, the binding mode was discussed. The results suggested that 1-(2-aminophenyl)-3-phenylurea motif offered us some useful information to lead future anticancer design efforts targeting EphA2 and HDAC proteins.
All chemicals were reagent grade and purchased from commercial suppliers. Melting points were determined in open capillaries on a WRS-1A digital melting point apparatus (Shenguang). The NMR spectra were recorded on a Bruker Avance 500 (Bruker), using tetramethylsilane, or trimethylsilyl (TMS) as the internal standard. The chemical shifts are reported in ppm (δ) and coupling constants (J) values are given in Hertz (Hz). The IR spectra were performed on a FTIR-8400S (Shimadzu) in KBr pellets; the frequencies are expressed in cm−1. Mass spectra were obtained from Agilent 1100 LC/MSD (Agilent) or Q-tof micro MS (Micromass) and the high-resolution (HR) electrospray ionization-time of flight (ESI-TOF)-MS was recorded on Agilent 6224 A (TOF) LC/MS.
General Synthesis of N-(4-Nitrophenyl)arylamide (2)The substituted aromatic acid (1.0 mmol), DMF (0.05 mL) and SOCl2 (6.0 mL) were mixed and stirred at reflux for 4 h, then cooled and evaporated to give reactive acyl chloride (1), respectively. A solution of acyl chloride 1 (1.0 mmol) in CH2Cl2 (5.0 mL) was added dropwise to the solution of 4-nitroaniline (1.0 mmol) and triethylamine (0.5 mL) in 20 mL CH2Cl2 at 0°C. After stirring overnight at room temperature, the reaction mixture was then poured in excess of diluted NaOH and extracted with CH2Cl2. The extraction liquid was purified by a flash chromatography with petroleum ether–EtOAc (10 : 1, v/v) to give N-(4-nitrophenyl)arylamide as a yellow powder.
General Synthesis of N-(4-Aminophenyl)arylamide (3)To a solution of 2 (1.0 mmol) in H2O (5 mL) were added NH4Cl (0.1 mmol), iron powder (3.0 mmol) and EtOH (0.5 mL). The mixture was stirred at reflux for 6 h, and filtered. The solution was extracted with EtOAc, and the organic layer was washed with saturated brine and dried over MgSO4. The solvent was removed under reduced pressure. The residue was purified by a flash chromatography with petroleum ether–EtOAc (2 : 1, v/v) to give N-(4-aminophenyl)arylamide as a white powder.
General Synthesis of N-(4-(3-(2-Nitrophenyl)ureido)phenyl)arylamide (4)1-Isocyanato-2-nitrobenzene (1.0 mmol) was added to a solution of 3 (1.0 mmol) in dry toluene (10 mL). The mixture was refluxed for 3 h. The solvent was removed under reduced pressure, and the mixture was washed with CH2Cl2. The product was recrystallized from tetrahydrofuran (THF) as a yellow solid.
N-(4-(3-(2-Nitrophenyl)ureido)phenyl)nicotinamide (4a)Yellow powder, yield: 57%; mp: 263–266°C; 1H-NMR (dimethyl sulfoxide (DMSO)-d6, 500 MHz): δ, ppm: 10.42 (s, 1H), 9.92 (s, 1H), 9.83 (s, 1H), 8.78 (s, 1H), 8.32–8.29 (m, 2H), 8.13–8.10 (m, 1H), 7.70–7.62 (m, 2H), 7.54–7.40 (m, 3H), 7.36 (d, J=8.0 HZ, 1H), 7.30–2.18 (m, 2H); HR-ESI-MS m/z: 400.1023 (Calcd for C19H17N5O2Na [M+Na]+: 400.1016).
4-Methoxy-N-(4-(3-(2-nitrophenyl)ureido)phenyl)benzamide (4b)Yellow powder, yield: 58%; mp: 276–278°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.14 (s, 1H), 9.93 (s, 1H), 9.82 (s, 1H), 8.36–8.32 (m, 1H), 8.15–8.13 (m, 1H), 7.87 (d, J=8.0 Hz, 2H), 7.72–7.68 (m, 1H), 7.56 (d, J=8.0 Hz, 2H), 7.42–7.39 (m, 1H), 7.21–7.10 (m, 4H), 3.94 (s, 3H); HR-ESI-MS m/z: 429.1185 (Calcd for C19H17N5O2Na [M+Na]+: 429.1169).
2-Methoxy-N-(4-(3-(2-nitrophenyl)ureido)phenyl)benzamide (4c)Yellow powder, yield: 55%; mp: 276–278°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.09 (s, 1H), 9.90 (s, 1H), 9.78 (s, 1H), 8.33–8.30 (m, 1H), 8.10–8.07 (m, 1H), 7.72–7.68 (m, 1H), 7.60–7.45 (m, 4H), 7.43–7.40 (m, 1H), 7.21–7.10 (m, 4H), 3.95 (s, 3H); HR-ESI-MS m/z: 429.1177 (Calcd for C19H17N5O2Na [M+Na]+: 429.1169).
3-Methoxy-N-(4-(3-(2-nitrophenyl)ureido)phenyl)benzamide (4d)Yellow powder, yield: 49%; mp: 225–228°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.22 (s, 1H), 9.91 (s, 1H), 9.80 (s, 1H), 8.35–8.32 (m, 1H), 8.14–8.10 (m, 1H), 7.74–7.70 (m, 3H), 7.57–7.53 (m, 2H), 7.42–7.40 (m, 1H), 7.19–7.05 (m, 3H), 6.75–6.72 (m, 1H), 3.95 (s, 3H); HR-ESI-MS m/z: 429.1182 (Calcd for C19H17N5O2Na [M+Na]+: 429.1169).
4-Methyl-N-(4-(3-(2-nitrophenyl)ureido)phenyl)benzamide (4e)Yellow powder, yield: 60%; mp: 249–251°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.15 (s, 1H), 9.93 (s, 1H), 9.82 (s, 1H), 8.34–8.31 (m, 1H), 8.12–8.09 (m, 1H), 7.75–7.67 (m, 3H), 7.55 (d, J=7.9 Hz, 2H), 7.43–7.40 (m, 1H), 7.24–7.16 (m, 4H), 2.53 (s, 3H); HR-ESI-MS m/z: 413.1232 (Calcd for C19H17N5O2Na [M+Na]+: 413.1220).
3-Methyl-N-(4-(3-(2-nitrophenyl)ureido)phenyl)benzamide (4f)Yellow powder, yield: 58%; mp: 235–238°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.17 (s, 1H), 9.94 (s, 1H), 9.84 (s, 1H), 8.33–8.29 (m, 1H), 8.10–8.07 (m, 1H), 7.81–7.70 (m, 3H), 7.56 (d, J=8.0 Hz, 2H), 7.41–7.38 (m, 1H), 7.26–7.18 (m, 4H), 2.53 (s, 3H); HR-ESI-MS m/z: 413.1239 (Calcd for C19H17N5O2Na [M+Na]+: 413.1220).
4-Chloro-N-(4-(3-(2-nitrophenyl)ureido)phenyl)benzamide (4g)Yellow powder, yield: 51%; mp: 285–287°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.14 (s, 1H), 9.95 (s, 1H), 9.84 (s, 1H), 8.32–8.29 (m, 1H), 8.10–8.07 (m, 1H), 7.86–7.83 (m, 2H), 7.71–7.68 (m, 1H), 7.56–7.43 (m, 4H), 7.41–7.38 (m, 1H), 7.17 (d, J=8.0 Hz, 2H); HR-ESI-MS m/z: 433.0692 (Calcd for C19H17N5O2Na [M+Na]+: 433.0674).
3,4,5-Trimethoxy-N-(4-(3-(2-nitrophenyl)ureido)phenyl)benzamide (4h)Yellow powder, yield: 52%; mp: 232–234°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.32 (s, 1H), 9.93 (s, 1H), 9.83 (s, 1H), 8.33–8.29 (m, 1H), 8.12–8.09 (m, 1H), 7.71–7.68 (m, 1H), 7.54 (d, J=8.0 Hz, 2H), 7.42–7.38 (m, 1H), 7.25–7.18 (m, 4H), 3.88 (s, 6H), 3.79 (s, 3H); HR-ESI-MS m/z: 489.1393 (Calcd for C19H17N5O2Na [M+Na]+: 489.1381).
General Synthesis of N-(4-(3-(2-Aminophenyl)ureido)phenyl)arylamide (5)To a solution of 4 (1.0 mmol) in 95% EtOH (5 mL) was added iron powder (3.0 mmol) and HOAc (0.1 mL). The mixture was stirred at reflux for 6 h, and filtered. The solvent was removed under reduced pressure, and the residue was purified by a flash chromatography with CH2Cl2–MeOH (10 : 1, v/v) to give N-(4-(3-(2-aminophenyl)ureido)phenyl)arylamide.
N-(4-(3-(2-Aminophenyl)ureido)phenyl)nicotinamide (5a)White powder, yield: 84%; mp: 239–243°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.34 (s, 1H), 9.10 (d, J=2.0 Hz, 1H), 8.76–8.74 (m, 2H), 8.28 (d, J=8.1 Hz, 1H), 7.70–7.65 (m, 3H), 7.58–7.54 (m, 1H), 7.44 (d, J=8.9 Hz, 2H), 7.34 (dd, J=7.9 Hz and 1.4 Hz, 1H), 6.84 (dt, J=8.5 Hz and 1.5 Hz, 1H), 6.74 (dd, J=7.8 Hz and 1.4 Hz, 1H), 6.58 (dt, J=7.8 Hz and 1.5 Hz, 1H), 4.78 (s, 2H); 13C-NMR (DMSO-d6, 125 MHz): δ, ppm: 168.1, 157.9, 151.5, 149.1, 148.2, 138.3, 137.8, 135.3, 134.6, 129.7, 129.1, 128.9, 127.8, 126.5, 121.2, 119.4, 117.6; HR-ESI-MS m/z: 370.1297 (Calcd for C19H17N5O2Na [M+Na]+: 370.1274); IR (KBr, cm−1): 3298, 1643, 1572, 1510, 1312, 1232, 835, 746, 710, 660.
N-(4-(3-(2-Aminophenyl)ureido)phenyl)-4-methoxybenzamide (5b)White powder, yield: 85%; mp: 230–232°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.02 (s, 1H), 8.83 (s, 1H), 7.97–7.67 (m, 4H), 7.85 (s, 1H), 7.43–6.81 (m, 7H), 6.65 (s, 1H), 4.03 (s, 3H); 13C-NMR (DMSO-d6, 125 MHz): δ, ppm: 170.4, 169.1, 157.7, 155.5, 139.7, 138.5, 134.4, 129.5, 129.0, 128.1, 125.8, 125.0, 123.9, 122.8, 118.4, 60.5; HR-ESI-MS m/z: 399.1461 (Calcd for C21H20N4O3Na [M+Na]+: 399.1428); IR (KBr, cm−1): 3348, 1639, 1547, 1510, 1398, 1250, 837, 756, 675, 572.
N-(4-(3-(2-Aminophenyl)ureido)phenyl)-2-methoxybenzamide (5c)White powder, yield: 80%; mp: 202–206°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 9.97 (s, 1H), 8.74 (s, 1H), 7.71 (s, 1H), 7.66 (dd, J=7.5 Hz and 1.5 Hz, 1H), 7.62 (d, J=9.0 Hz, 2H), 7.49 (dt, J=8.5 Hz and 1.5 Hz, 1H), 7.40 (d, J=9.0 Hz, 2H), 7.34 (dd, J=8.0 Hz and 1.0 Hz, 1H), 7.17 (d, J=8.5 Hz, 1H), 7.06 (t, J=7.5 Hz, 1H), 6.84 (t, J=7.5 Hz, 1H), 6.73 (dd, J=7.5 Hz and 1.0 Hz, 1H), 6.57 (dt, J=7.5 Hz, 1H), 4.76 (s, 2H), 3.91 (s, 3H); 13C-NMR (DMSO-d6, 125 MHz): δ, ppm: 169.8, 162.5, 158.1, 155.5, 139.1, 138.5, 138.0, 133.6, 130.7, 130.2, 129.2, 126.0, 125.6, 125.0, 123.3, 122.4, 119.2, 115.6, 58.8; HR-ESI-MS m/z: 399.1455 (Calcd for C21H20N4O3Na [M+Na]+: 399.1428); IR (KBr, cm−1): 3352, 1647, 1553, 1514, 1402, 1244, 1022, 837, 752, 667.
N-(4-(3-(2-Aminophenyl)ureido)phenyl)-3-methoxybenzamide (5d)White powder, yield: 80%; mp: 213–216°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.12 (s, 1H), 8.74 (s, 1H), 7.70–7.64 (m, 3H), 7.53 (d, J=7.7 Hz, 1H), 7.48–7.41 (m, 4H), 7.34 (d, J=6.9 Hz, 1H), 7.14 (dd, J=8.2 Hz and 2.4 Hz, 1H), 6.84 (dt, J=8.0 Hz and 1.4 Hz, 1H), 6.74 (d, J=6.5 Hz, 1H), 6.56 (t, J=8.0 Hz, 1H), 4.79 (s, 2H), 3.84 (s, 3H); 13C-NMR (DMSO-d6, 125 MHz): δ, ppm: 171.1, 165.7, 158.0, 154.9, 139.8, 139.0, 137.4, 135.1, 130.5, 129.8, 127.4, 126.5, 124.8, 124.1, 123.0, 120.8, 116.5, 115.6, 59.4; HR-ESI-MS m/z: 399.1449 (Calcd for C21H20N4O3Na [M+Na]+: 399.1428); IR (KBr, cm−1): 3348, 1651, 1553, 1514, 1310, 1240, 831, 752, 667, 523.
N-(4-(3-(2-Aminophenyl)ureido)phenyl)-4-methylbenzamide (5e)White powder, yield: 86%; mp: 202–204°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.02 (s, 1H), 8.68 (s, 1H), 7.87–7.86 (m, 2H), 7.67 (s, 1H), 7.65 (d, J=9.5 Hz, 2H), 7.40 (d, J=9.5 Hz, 2H), 7.33 (m, 3H), 6.84 (dt, J=9.0 Hz and 1.5 Hz, 1H), 6.74 (dd, J=8.0 Hz and 1.5 Hz, 1H), 6.58 (dt, J=7.5 Hz and 1.5 Hz, 1H), 4.75 (s, 2H), 2.50 (s, 3H); 13C-NMR (DMSO-d6, 125 MHz): δ, ppm: 168.5, 157.8, 154.5, 146.5, 140.6, 137.9, 136.0, 133.7, 132.0, 130.4, 129.6, 127.3, 126.3, 123.5, 123.0, 118.7, 27.7; HR-ESI-MS m/z: 383.1519 (Calcd for C21H20N4O2Na [M+Na]+: 383.1478); IR (KBr, cm−1): 3325, 1649, 1553, 1516, 1310, 1242, 752, 669, 627, 521.
N-(4-(3-(2-Aminophenyl)ureido)phenyl)-3-methylbenzamide (5f)White powder, yield: 72%; mp: 222–226°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.10 (s, 1H), 8.73 (s, 1H), 7.76–7.71 (m, 3H), 7.66–7.65 (m, 2H), 7.43–7.38 (m, 4H), 7.34 (dd, J=7.5 Hz and 1.0 Hz, 1H), 6.84 (dt, J=8.0 Hz and 1.5 Hz), 6.74 (dd, J=7.5 Hz and 1.0 Hz, 1H), 6.58 (dt, J=7.5 Hz and 1.0 Hz, 1H), 4.77 (s, 2H), 2.40 (s, 3H); 13C-NMR (DMSO-d6, 125 MHz): δ, ppm: 168.0, 157.2, 154.2, 144.6, 140.2, 139.4, 138.7, 137.1, 136.5, 134.7, 129.9, 129.5, 128.5, 127.4, 126.1, 123.8, 123.3, 118.9, 26.9; HR-ESI-MS m/z: 383.1506 (Calcd for C21H20N4O2Na [M+Na]+: 383.1478); IR (KBr, cm−1): 3281, 1645, 1556, 1510, 1402, 1231, 744, 694, 625, 523.
N-(4-(3-(2-Aminophenyl)ureido)phenyl)-4-chlorobenzamide (5g)White powder, yield: 79%; mp: 289–291°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.19 (s, 1H), 8.96 (s, 1H), 7.98 (d, J=8.5 Hz, 2H), 7.94 (s, 1H), 7.64 (d, J=8.5 Hz, 2H), 7.59 (d, J=8.5 Hz, 2H), 7.43 (d, J=8.5 Hz, 2H), 7.36 (d, J=9.0 Hz, 1H), 6.83 (t, J=8.0 Hz, 1H), 6.73 (d, J=6.5 Hz, 1H), 6.57 (t, J=7.5 Hz, 1H), 4.80 (s, 2H); 13C-NMR (DMSO-d6, 125 MHz): δ, ppm: 170.9, 157.9, 155.0, 144.4, 139.9, 138.7, 135.4, 135.1, 133.5, 131.2, 130.1, 127.2, 126.5, 124.1, 123.9, 118.6; HR-ESI-MS m/z: 403.0968 (Calcd for C20H17ClN4O2Na [M+Na]+: 403.0932); IR (KBr, cm−1): 3425, 2920, 1645, 1533, 1462, 1313, 1018, 837, 750, 648.
N-(4-(3-(2-Aminophenyl)ureido)phenyl)-3,4,5-trimethoxybenzamide (5h)White powder, yield: 78%; mp: 197–200°C; 1H-NMR (DMSO-d6, 500 MHz): δ, ppm: 10.00 (s, 1H), 8.77 (s, 1H), 7.77 (s, 1H), 7.61 (d, J=9.0 Hz, 2H), 7.43 (d, J=9.0 Hz, 2H), 7.36–7.35 (m, 1H), 7.3 (s, 2H), 6.84 (dt, J=8.5 Hz and 1.5 Hz, 1H), 6.74 (dd, J=8.0 Hz and 1.5 Hz, 1H), 6.56 (dt, J=7.5 Hz and 1.0 Hz, 1H), 4.77 (s, 2H), 3.87 (s, 6H), 3.73 (s, 3H); 13C-NMR (DMSO-d6, 125 MHz): δ, ppm: 171.4, 159.2, 158.1, 155.1, 148.8, 140.6, 138.7, 130.6, 130.1, 129.5, 127.8, 126.8, 123.6, 123.0, 119.4, 111.3, 65.2, 60.6; HR-ESI-MS m/z: 459.1647 (Calcd for C23H24N4O5Na [M+Na]+: 459.1639); IR (KBr, cm−1): 3354, 1738, 1645, 1551, 1514, 1483, 1307, 742, 667, 521.
EphA2 Inhibition AssayThe inhibitory activities of the compounds to EphA2 were determined by using a time resolved fluorescence resonance energy transfer (TR-FRET) assay. To evaluate inhibitory activity of compounds, the ATP concentration (3 µM) was adjusted to equal Km and the concentration of EphA2 (0.02 µg/mL) was used at an EC50 value. TK-substrate-biotin (0.5 µM) and SEB (20 nM) were used for EphA2 kinase reaction. The total reaction volume was 10 µL and test compounds were preincubated with enzyme for 10 min before adding peptide substrate and ATP. Kinase reactions were conducted for 30 min at room temperature in standard 384 well plates and then 10 µL of detection mixture including 10 mM ethylenediaminetetraacetic acid (EDTA) and 1 µM europium-tagged antibody was added to the reaction plates 1 h before reading the plates. Following the addition of reagents for detection, the TR-FRET signal was measured using an EnVision multi-label reader. The instrument settings used were 340 nm for excitation and 615 nm and 665 nm for emission with a 100 µs delay time. The TR-FRET counts are expressed as ratio F665/F615nm×104, where F665 and F615nm are fluorescence counts at 665 and 615 nm for fluorescein and europium, respectively. IC50 was calculated by a non-linear regression using Prism version 5.01.
HDAC Inhibition AssayIn vitro HDAC inhibition assays were conducted as described in reference.25) Briefly, 10 µL of HeLa nuclear extract was mixed with tested compound (50 µL). Five minutes later, fluorogenic substrate tert-butoxycarbonyl (Boc)-Lys (acetyl)-7-amino-4-methyl-coumarin (AMC) (40 µL) was added, and the mixture was incubated at 37°C for 30 min and then stopped by addition of 100 µL of developer containing trypsin and p-toluenesulfonic acid (TSA). After incubation at 37°C for 20 min, fluorescence intensity was measured using a microplate reader at excitation and emission wavelengths of 390 and 460 nm, respectively. The inhibition ratios were calculated from the fluorescence intensity readings of tested wells relative to those of control wells. Experiment with triplicate data were performed. The IC50 values were calculated using a regression analysis of the concentration/inhibition data.
In vitro Antiproliferative AssayThe anti-proliferative activities were measured using MTT method assay in vitro, using Dasatinib and MS275 as the reference standard. In brief, HCT116, K562 and MCF7 cells in 100 µL culture medium were seeded into 96-well microplates, respectively, and incubated at 37°C for 24 h prior to drug exposure. Cell numbers were titrated to keep control cells growing in the exponential phase throughout the 48 h incubation period. Cells were treated with final concentrations of 100.0, 10.0, 1.0, 0.1 and 0.01 µM of tested compounds simultaneously and incubated for 48 h and then 50 µL of MTT solution (5 mg/mL in medium) was added to each well and incubated for 2.5 h. The formed blue formazan crystals were pelleted to the bottom of the well by centrifugation, separated from the supernatant, and dissolved in 150 µL of DMSO. The optical density at 570 nm was determined by an enzyme-linked immunosorbent assay (ELISA) reader. Two separate experiments with triplicate data were performed to obtain mean cell viability. The IC50 values were calculated according to the inhibition ratios.
Molecular ModelingThe molecular docking studies were performed with the molecular modeling program (GOLD v3.0) for searching binding space and ligand conformational space.26) The following crystal structures from protein data bank were used as raw models for the docking of compound 5b with HDAC (PDB ID: 1C3R) and EphA2 (PDB ID: 1MQB), respectively. In the protein preparation, all the water molecules were included, hydrogen atoms were added, and bond orders for crystal protein were adjusted. The active site was defined within a 10 Å radius around the ligand present in the crystal structure. The compound 5b was docked into ligand binding site. The GOLD fitness score is calculated from the contributions of hydrogen bond and van der Waals interactions between the protein and ligand. The interacting ability of a compound depends on the fitness score, greater the GOLD fitness score and better the binding affinity.
This study was supported by the National Natural Science Foundation of China (Grant No. 81202410), Ph.D. Programs Foundation of Ministry of Education of China (Grant No. 20120096120010) and Fundamental Research Funds for the Central Universities (Grant No. PY2014LX0010).
The authors declare no conflict of interest.