2016 年 64 巻 8 号 p. 1154-1160
2016 年 64 巻 8 号 p. 1154-1160
A safe, facile and low-leaching (up to 0.17 ppm) sulfur-modified glass-supported palladium nanoparticle catalyst has been developed for the Suzuki–Miyaura coupling of aryl halides with aryl boronic acids. Most notably, this catalyst was highly recyclable and could be used up to 10 times without any discernible decrease in its activity.
Palladium catalysts play an important role in organic chemistry where they have been used extensively over the past two decades to affect a wide range of carbon–carbon and carbon–heteroatom (e.g., N, S, O) bond-forming reactions.1,2) Among them, Pd nanoparticle (PdNP) catalysts are particular active, because the surface areas of nanoparticle (NP)-based catalysts are much greater than those of the corresponding bulk catalysts. Furthermore, PdNP catalysts can generate active species under mild and environmentally friendly conditions. For example, it has recently reported that traditional palladium-catalyzed reactions that require a zero valent/divalent palladium catalyst and a suitable ligand (i.e., phosphine ligands and nitrogen-containing heterocyclic carbenes), can progress under ligand-free conditions using PdNPs.3–8) There are therefore numerous advantages associated with the use of PdNP catalysts, including reduced processing costs, shorter reaction times and cleaner reaction profiles, which can have a positive impact on product purification. Based on their many attractive qualities, it is envisaged that PdNPs will become increasingly important for the synthesis of pharmaceutical agents and functional materials. For this reason, considerable research efforts have been directed towards the development of new nanosized catalysts, which have been immobilized on a variety of different solid materials, including metals, as well as several silicon and polymer based supports.9–16)
We previously reported the development of a sulfur-modified Au-supported Pd material (SAPd), which had PdNPs immobilized on its surface. Furthermore, we investigated the application of this material as a catalyst to a variety of different reactions, including the Suzuki–Miyaura, Buchwald–Hartwig amination, carbon (sp3 and sp2)-hydrogen bond activation, double carbonylation, and allyl cleavage reactions. Notably, this catalyst exhibited good recyclability properties in all cases with low palladium leaching.17–27) In 2015, we successfully analyzed the structure of our SAPd catalyst by X-ray absorption fine structure (XAFS) and transmission electron microscopy (TEM) analyses and found that the Au particles in this material simply acted as a solid support for the SAPd membrane.27) SAPd is now commercially available as a safe, facile, recyclable and low-leaching PdNP catalyst. However, one of the big disadvantages of using SAPd as a catalyst is that it contains Au as a key ingredient, making it particularly expensive. In this article, we report the development of a novel sulfur-modified glass-supported palladium (SGlPd) catalyst as a cheaper alternative to the Au-containing SAPd catalyst described above. It is noteworthy that this new SGlPd catalyst holds the PdNPs on its surface in same way as SAPd and exhibits equivalent catalytic activity towards the Suzuki–Miyaura coupling reaction.
To begin with, we screened a wide range of different materials as potential low cost alternatives to Au. A variety of different semiconductor, insulator and metal materials were used to prepare immobilized Pd catalyst in the same way as SAPd.28) Among all of the materials tested, we found glass could be used as a suitable replacement for Au.
Sulfur-modified alkali-free glass was prepared by the treatment of glass with a piranha solution (Chart 1). This material was treated with Pd(OAc)2 in xylene at 100°C for 12 h to allow for the adsorption of palladium onto its surface to give Pd material A. We also investigate the use of several other inexpensive glasses, including quartz glass, white glass and blue glass, which gave materials B–D under the same conditions as those used for the preparation of A. All four of these materials were subsequently evaluated as a catalysts for the Suzuki–Miyaura coupling of iodobenzene (1a) (0.5 mmol) and 4-chlorophenylboronic acid (2a) (117.0 mg, 1.5 eq). Pleasingly, we found that the yields of 3a achieved using catalysts A–D over 10 catalytic cycles were almost identical to those achieved using SAPd (Table 1). This initial result therefore indicated that these new Pd-containing materials (A–D) possessed similar catalytic activities to SAPd. Furthermore, these results indicated that blue glass would be the optimal support material because of its low cost compared with the other glasses tested (Table 1, entry 4). Material D shall therefore be referred to hereafter as a sulfur-modified glass-supported Pd (SGlPd) catalyst29) (Chart 1).

 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Materials | Yields (%) | Cost (¥) | ||||||||||
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | Ave. | |||
| 1a) | Alkali free glass (A) | >99 | 92 | 90 | >99 | 95 | 85 | 80 | 80 | 94 | 98 | 91 | 1500 |
| 2b) | Quartz (B) | 86 | 89 | 86 | 82 | 94 | 87 | 93 | 97 | 94 | 89 | 90 | 1000 |
| 3b) | White glass (C) | 90 | 82 | 91 | 92 | 82 | 89 | 98 | 86 | 98 | 93 | 96 | 800 |
| 4b) | Blue glass (D) | 99 | 94 | 97 | 97 | 96 | 99 | 92 | 98 | 97 | 93 | 96 | 50 |
| 5a) | Au | 97 | 97 | 94 | 90 | >99 | >99 | >99 | 93 | 95 | 98 | 96 | 2000 |
a) Isolated yield. b) HPLC yield.
To obtain information pertaining to the actual active edge structures of the SAPd and SGlPd catalysts, we subjected them to X-ray adsorption near edge structure (XANES) spectroscopy and compared their spectra with those of several standard materials, including Pd foil, PdO, PdSO4, PdS and Pd(PPh3)4. The results revealed that the Pd K-edge XANES spectra of the both SAPd and SGlPd were the same and that they were analogous to that of Pd foil (Fig. 1). These results therefore indicated that the Pd species present in SAPd and SGlPd were the same. Furthermore, the Pd K-edge XANES spectrum of the SGlPd material collected after 10 catalytic cycles of the Suzuki–Miyaura coupling reaction (Table 1) was almost identical to that of fresh SGlPd.
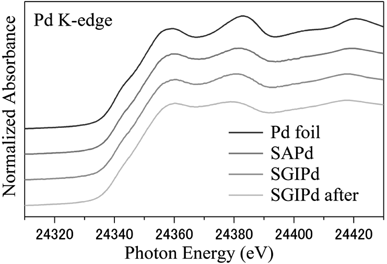
Figure 2 shows the sulfur K-edge XANES spectra of several standard materials, as well as the spectrum of SGlPd. Figures 2(a) and (b) show the spectra of Na2SO4] and PdS, which represent the oxidized and reduced forms of sulfur, respectively. Figures 2(c) and (d) show the spectra of SAPd and SGlPd, respectively. For comparison, we have also provided the spectra of the glass plates both before and after the piranha treatment. The XANES spectrum of SGlPd contained a clear resonance peak at 2481.9 eV. Based on a comparison with the spectra of the standard materials, (a) and (b), the result for SGlPd suggested that the sulfur contained in this material had been strongly oxidized and that it predominantly existed as SO42−. Furthermore, the spectrum obtained for SGlPd (d) was very similar to that of SAPd (c), which indicated that the sulfur contained in these materials existed in the same chemical state. The characteristic peak at 2481.9 eV was not present in the XANES spectrum of the glass before the piranha treatment (f). However, this peak was detected in the spectrum of the glass after the piranha treatment (e). These results therefore demonstrated that the sulfur signal at 2481.9 eV was not derived from an impurity in the glass. One of the characteristic features of SAPd is that it contains approximately 10 layers of multi-layered structure and consists of nanoparticles with a diameter less than 5 nm. Organic materials containing sulfate and xylene as the major ingredients are distributed between the Pd nanoparticles, allowing for the formation of stable high-density PdNPs without condensation.17–27) The results of the XANES analysis suggest that the oxidized sulfur preserves the structure of the PdNPs in SGlPd as well as SAPd.

To identify the species responsible for the catalytic activity of SGlPd, we performed kinetic studies/filtration tests and compared the time conversion plots of a series of reactions involving the resulting materials (reactions A–C). These investigations were performed to determine whether the catalytic activity of SGlPd could be attributed to the leaching of a catalytic Pd-species into the reaction mixture for the Suzuki–Miyaura coupling (Fig. 3). Reaction A was performed by maintaining the conditions shown in Table 1, and the reaction proceeded efficiently to give the coupled product 3a in 99% yield after 3 h. Reactions B and C were also performed under the same conditions, except the SGlPd catalyst was removed from the reaction mixture by filtration after 60 or 5 min, respectively. Reaction B yielded product 3a in 97% yield after 3 h, whereas reaction C gave a yield of only 1% of the product 3a after 3 h. Pd species on SGlPd are strongly bonded on SGlPd, because 3a was not obtained in the following control experiment. A solution of SGlPd in EtOH (3 mL) was heated at 80°C for 3 h and, after SGlPd was removed and 1a (0.5 mmol) and 2a (1.5 eq.) were added to the solution, the mixture was heated at 80°C for 3 h. These results therefore confirmed that the active Pd species was indeed being released from SGlPd and that some time was therefore required for the release of the active Pd-species, representing a similar result to that observed for SAPd.

It is well known that reactions involving the use of polymer-supported Pd suffer from significant contamination problems and the catalyst has to be washed very carefully because of the strong absorption of the starting materials and/or the product to the polymer. As reported in our previous research,17–27) SAPd absorbs very few organic compounds and can therefore be used in combinatorial synthesis because of its small surface area. It was envisaged that SGlPd would perform in a similar manner to SAPd. Thus, we investigated the use of SGlPd as a catalyst for the liquid-phase combinatorial synthesis of different biaryl systems by varying the nature of the iodobenzene derivative used in the Suzuki–Miyaura coupling.
SGlPd was evaluated as a catalyst in the Suzuki–Miyaura coupling of phenylboronic acid 2b with ten different aryl iodides using K2CO3 as a base in EtOH (Table 2). As shown in the table, all 10 reactions proceeded smoothly to give the desired products in near-quantitative yields. Furthermore, all 10 products were obtained in high purity after a standard aqueous work-up. Notably, this catalyst worked well for both aryl (runs 1–8), and heteroaryl (runs 9, 10) iodides. Similar results were also observed when the boronic acid derivative was changed (Table 3). Taken together, these results demonstrate that SGlPd possesses similar catalytic activity to SAPd and that it can therefore be used as an effective Pd reservoir for synthesis without being contaminated by the products or starting materials. Furthermore, SGlPd was repeatedly used in the Suzuki–Miyaura couplings described above without any discernible decrease in its activity. In a typical procedure, a solution of SGlPd (ca. 1 cm2), phenylboronic acid 2b (1.5 equiv.), aryl iodide (0.5 mmol) and K2CO3 (2 equiv.) was heated in EtOH (3 mL) at 80°C for 12 h. The mixture was then cooled and subjected to a standard aqueous work-up to give the corresponding product 3 in high purity.30) The SGlPd was readily removed from the reaction mixture by filtration prior to the work-up and used repeatedly.
 | ||
|---|---|---|
| Run | Ar–I | Yieldb) (%) |
| 1 |  | 3b |
| >99 (>99) | ||
| 2 |  | 3a |
| 98 (>99) | ||
| 3 | 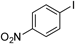 | 3k |
| 99 (99) | ||
| 4 |  | 3d |
| 99 (98) | ||
| 5 |  | 3h |
| 96 (>99) | ||
| 6 |  | 3l |
| >99 (>99) | ||
| 7c) |  | 3e |
| 95 (95) | ||
| 8 | 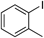 | 3m |
| 95 (92) | ||
| 9 |  | 3n |
| 94 (99) | ||
| 10d) | 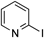 | 3o |
| 91 (92) | ||
a) The same SGlPd was used for all the reaction in this table. b) Yield in parenthesis indicates the isolated yield by using SAPd. c) This reaction was carried out for 5 h. d) This reaction was carried out for 12 h.
 | ||
|---|---|---|
| Entry | Ar B(OH)2 | Yielda) (%) |
| 1 |  | 3a |
| 99 (>99) | ||
| 2 | 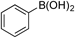 | 3b |
| >99 (>99) | ||
| 3 |  | 3c |
| 91 (>99) | ||
| 4 | 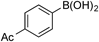 | 3d |
| >99 (99) | ||
| 5 |  | 3e |
| 95 (98) | ||
| 6 |  | 3f |
| 94 (>99) | ||
| 7 | 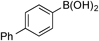 | 3g |
| 95 (97) | ||
| 8 |  | 3h |
| 96 (>99) | ||
| 9 |  | 3i |
| 98 (91) | ||
| 10 | 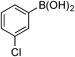 | 3j |
| 96 (98) | ||
a) Yield in parenthesis indicates the isolated yield using SAPd.
Inductively coupled plasma mass spectrometry (ICP-MS) was used to measure the amount of immobilized Pd on the SGlPd catalyst, as well as the amount of Pd leaching into the reaction mixture. The amount of immobilized Pd on the SGlPd catalyst was measured both before and after the application of 10 catalytic cycles, and the results are shown in Table 4. The amount of released Pd after cooling in each cycle was extremely low [0.2–0.7 µg (0.06–0.23 ppm) for a 0.5 mmol scale reaction]. A comparison of the leaching properties of the SAPd and SGlPd catalysts revealed that the latter more released active Pd into the reaction mixture (less than 0.17 ppm) than the SAPd (less than 0.01 ppm). Almost half of immobilized Pd on SGlPd was released in 10 times of Suzuki–Miyaura coupling. Although the amount of Pd leached into the reaction mixture during each cycle was slightly higher for the SGlPd catalyst, this material still allowed for the Suzuki–Miyaura coupling to proceed efficiently from over all 10 cycles under ligand-free conditions.
 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amount of leached-Pd in reaction mixture (µg)a,b,c) | Immobilized Pd on SGlPd (µg) | |||||||||||
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | Total | Before use | After use |
| 0.5±0.2 | 0.4±0.2 | 0.5±0.2 | 0.35±0.15 | 0.35±0.05 | 0.4±0.2 | 0.3±0.1 | 0.35±0.05 | 0.4±0.2 | 0.25±0.05 | 3.5±1.5 | 116.5±18.5 | 59.5±16.5 |
| (0.17) | (0.13) | (0.17) | (0.12) | (0.12) | (0.13) | (0.10) | (0.12) | (0.13) | (0.08) | |||
| (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | |||
a) The entire reaction mixture was acidified and subjected directly to inductively coupled plasma mass spectroscopy measurement. b) The standard deviation was calculated from two sets of samples. c) Numbers in parentheses above and below the dashed line indicate the amount of leached Pd in ppm from SGlPd and SAPd.
We have successfully developed a sulfur-modified glass-supported Pd material (SGlPd) using blue glass as a much cheaper alternative to Au. The catalytic activity of SGlPd was examined in a ligand-free Suzuki–Miyaura coupling reaction, with the reaction only requiring 3 h to achieve high yields, which was much shorter than the same reaction using SAPd (12 h). Although SGlPd leached larger amounts of active Pd species (less than 0.17 ppm for a 0.5 mmol scale reaction) into the reaction mixture compared with SAPd (less than 0.01 ppm for a 0.5 mmol scale reaction), this value is still much lower than the value required by U.S. Food and Drug Administration (i.e., <5 ppm residual metal in product streams). Based on its low leaching, our newly developed SGlPd catalyst could also be recycled up to 10 times without any discernible decrease in its catalytic activity.
Na2S2O8 (4.0 g) was added in small portions to ice-cooled 98% H2SO4 (4.7 g) with continuous stirring, and then crushed ice (13.0 g) and water (4.0 g) were added to the solution while the temperature was maintained below 15°C. When all the salt dissolved to a homogeneous solution at room temperature, the Glass (12×14 mm) was placed in the solution (3.0 mL) for 5 min and washed first with H2O (3.0 mL×10) and then with EtOH (3.0 mL×6). The resulting Glass was placed in a round-bottom flask and dried for 10 min under reduced pressure. The resulting sulfur-modified Glass was placed in a solution of Pd(OAc)2 (5.3 mg, 0.023 mmol) in xylene (3.0 mL) and stirred for 12 h at 100°C under an Ar atmosphere. The Glass was then rinsed with xylene (3.0 mL×50) and, after vacuum drying, placed in xylene (3.0 mL) and heated for 12 h at 135°C. Finally, it was rinsed with xylene (3.0 mL×50) and dried under a vacuum for 10 min to give the SGlPd (immobilized Pd: 116.5±18.5 µg), and this SGlPd was used for the present research.
Filtration Test(Reaction A)A mixture of iodobenzene 1a (102 mg, 0.5 mmol), phenylboronic acid 2a (91.4 mg, 0.75 mmol), and K2CO3 (138 mg, 1.0 mmol) in abs. EtOH (3 mL) was heated at 80°C in the presence of SGlPd (12×14 mm, 116.5±18.5 µg) for 3 h. Time conversion was plotted using HPLC (Condition: Mightysil-RP18 (150 mm, ϕ4.6 mm, 3 µm Kanto Chemical Co., Inc., Tokyo, Japan), MeCN : H2O (70/30), 1 mL/min, 25°C, retention time (tR) 13.7 min).
(Reaction B)A mixture of iodobenzene 1a (102 mg, 0.5 mmol), phenylboronic acid 2a (91.4 mg, 0.75 mmol), and K2CO3 (138 mg, 1.0 mmol) in abs. EtOH (3 mL) was heated at 80°C in the presence of SGlPd (12×14 mm, 116.5±18.5 µg). After 1 h, the reaction was cooled to room temperature, and then the SGlPd was removed from the reaction mixture. In the absence of SGlPd, the reaction mixture was heated at 80°C. Time conversion was plotted using HPLC (Condition: Mightysil-RP18 (150 mm, ϕ4.6 mm, 3 µm Kanto Chemical Co., Inc.), MeCN : H2O (70/30), 1 mL/min, 25°C, tR 13.7 min).
(Reaction C)A mixture of iodobenzene 1a (102 mg, 0.5 mmol), phenylboronic acid 2a (91.4 mg, 0.75 mmol), and K2CO3 (138 mg, 1.0 mmol) in abs. EtOH (3 mL) was heated at 80°C in the presence of SGlPd (12×14 mm, 116.5±18.5 µg). After 5 min, the reaction was cooled to room temperature, and then the SGlPd was removed from the reaction mixture. In the absence of SGlPd, the reaction mixture was heated at 80°C. Time conversion was plotted using HPLC (Condition: Mightysil-RP18 (150 mm, ϕ4.6 mm, 3 µm Kanto Chemical Co., Inc.), MeCN : H2O (70/30), 1 mL/min, 25°C, tR 13.7 min).
Typical Experimental Procedure of Suzuki–Miyaura Coupling (Table 2, entry1)A mixture of 1a (102 mg, 0.5 mmol), phenylboronic acid 2a (91.4 mg, 0.75 mmol), and K2CO3 (138 mg, 1.0 mmol) in EtOH (3 mL) was heated in the presence of SGlPd at 80°C for 3 h under argon an atmosphere without stirring. After the reaction mixture was cooled to room temperature, the SGlPd was removed from the reaction mixture and rinsed several time with EtOH. The raction mixture was poured into 2 M NaOH aq., extracted with AcOEt. The organic layer was washed with sat. aq. NH4Cl, and sat. aq. NaCl, dried over anhydrous NaSO4. Concentration at reduced pressure gave yellowish oil, which was chromatographed on silica gel with hexane as the eluent to give the biphenyl (3a, 77.1 mg, 99%) as a colorless solid. The recovered SGlPd catalyst was again subjected to next reaction for second run. The procedure was repeated a total 10 runs.
Physical Data of Successively Suzuki–Miyaura Coupling Product4-Chlorobiphenyl 3amp 78.0–78.5°C (MeOH) (lit.32) 78.5°C, benzene), colorless plates. 1H-NMR (500 MHz, CDCl3) δ: 7.33–7.47 (m, 5H), 7.50–7.57(m, 4H); 13C-NMR (125 MHz, CDCl3) δ: 127.0, 127.6, 128.4, 128.8, 128.9, 133.3, 139.6, 140.0; low resolution (LR)-MS electron ionization (EI) m/z 188 (100%, M+).
Biphenyl 3bmp 70.0–70.5°C (MeOH) (lit33) 70.0–71.0°C, MeOH), colorless plates. 1H-NMR (500 MHz, CDCl3) δ: 7.33–7.37 (m, 2H), 7.43–7.46 (m, 4H), 7.59–7.61 (m, 4H); 13C-NMR (125 MHz, CDCl3) δ: 127.1, 127.2, 128.7, 141.1; LR-MS electron ionization (EI) m/z 154 (100%, M+).
4-Methylbiphenyl 3cmp 48.0–48.5°C (MeOH) (lit.34) 49–50°C, EtOH), colorless plates. 1H-NMR (500 MHz, CDCl3) δ: 2.40 (s, 3H), 7.24–7.26 (m, 2H), 7.30–7.34 (m, 1H), 7.41–7.44 (m, 2H), 7.49 (d, J=8.0 Hz, 2H), 7.58 (d, J=7.4 Hz, 2H); 13C-NMR (125 MHz, CDCl3) δ: 21.1, 126.9, 127.0, 128.7, 129.5, 137.0, 138.3, 141.1; LR-MS (EI) m/z 168 (100%, M+).
4-Acetylbiphenyl 3dmp 122.5–123.0°C (MeOH) (lit.36)120°C, EtOH), colorless needles. 1H-NMR (500 MHz, CDCl3) δ: 2.65 (s, 3H), 7.39–7.42 (m, 1H), 7.46–7.49 (m, 2H), 7.63 (d, J=8.0 Hz, 2H), 7.69 (d, J=8.3 Hz, 2H), 8.04 (d, J=8.3 Hz, 2H); 13C-NMR (125 MHz, CDCl3) δ: 26.6, 127.1, 127.2, 128.1, 128.8, 128.9, 135.7, 139.7, 145.6, 197.7; LR-MS (EI) m/z 196 (49%, M+).
1-Phenylnaphthalene 3eColorless oil. 1H-NMR (400 MHz, CDCl3) δ: 7.42–7.55 (m, 9H), 7.85–7.92 (m, 3H); 13C-NMR (125 MHz, CDCl3) δ: 125.3, 125.7, 126.0, 126.9, 127.2, 127.6, 128.2 (CH×2), 130.0, 131.6, 133.8, 140.2, 140.7; LR-MS (EI) m/z 204 (100%, M+).
2-Phenylbenzofuran 3fmp 235–236°C (EtOH) (lit.39) 233–235°C, EtOH), colorless plates. 1H-NMR (400 MHz, CDCl3) δ: 7.87 (d, J=8.0 Hz, 2H), 7.58 (d, J=8.0 Hz, 1H), 7.52 (d, J=8.6 Hz, 1H), 7.45 (dd, J=8.0 and 7.4 Hz, 2H), 7.33–7.37 (m, 1H), 7.16–7.30 (m, 3H); 13C-NMR (125 MHz, CDCl3) δ: 155.9, 154.8, 130.4, 129.2, 128.7, 128.5, 124.9, 124.2, 122.9, 120.9, 111.1, 101.3; LR-MS (EI) m/z 194 (100%, M+).
p-Terphenyl 3gmp 214–215°C (EtOH) (lit.40) 212–213°C, EtOH), colorless plates. 1H-NMR (500 MHz, CDCl3) δ: 7.65–7.69 (m, 8H), 7.44–7.49 (m, 4H), 7.35–7.38 (m, 2H); 13C-NMR (125 MHz, CDCl3) δ: 140.7, 140.1, 128.8, 127.5, 127.3, 127.0; LR-MS (EI) m/z 230 (100%, M+).
4-Methoxybiphenyl 3hmp 89.0–89.5°C (MeOH) (lit.32) 90–91°C, MeOH), colorless granules. 1H-NMR (400 MHz, CDCl3) δ: 3.86 (s, 3H), 6.98 (d, J=8.8 Hz, 2H), 7.28–7.32 (m, 1H), 7.40 (m, 2H), 7.52–7.56 (m, 4H); 13C-NMR (125 MHz, CDCl3) δ: 55.3, 114.1, 126.6, 126.7, 128.1, 128.7, 133.7, 140.8, 159.1; LR-MS (EI) m/z 184 (100%, M+).
trans-Stilbene 3imp 125.0–126.0°C (pentane) (lit.42) 122–123°C, petroleum ether), colorless plates. 1H-NMR (400 MHz, CDCl3) δ: 7.11 (s, 2H), 7.24–7.29 (m, 2H), 7.34–7.39 (m, 4H), 7.51–7.54 (m, 4H); 13C-NMR (125 MHz, CDCl3) δ: 126.5, 127.6, 128.6, 137.3; LR-MS (EI) m/z 180 (100%, M+).
3-Chlorobiphenyl 3jColorless oil. 1H-NMR (500 MHz, CDCl3) δ: 7.57–7.58 (m, 1H), 7.56 (d, J=7.4 Hz, 2H), 7.45–7.47 (m, 1H), 7.44 (dd, J=8.0 and 7.4 Hz, 2H), 7.36–7.38 (m, 1H), 7.35 (d, J=8.0 Hz, 1H), 7.32–7.30 (m, 1H); 13C-NMR (125 MHz, CDCl3) δ: 143.0, 139.8, 134.6, 130.0, 128.9, 127.8, 127.3, 127.2, 127.1, 125.3; LR-MS (EI) m/z 188 (100%, M+).
4-Nitrobiphenyl 3kmp 114.5–115.0°C (MeOH) (lit.44) 113–115°C, MeOH), pale yellow columns. 1H-NMR (500 MHz, CDCl3) δ: 7.43–7.52 (m, 3H), 7.62 (d, J=7.4 Hz, 2H), 7.74 (d, J=9.2 Hz, 2H), 8.30 (d, J=9.2 Hz, 2H); 13C-NMR (125 MHz, CDCl3) δ: 124.0, 127.3, 127.7, 128.9, 129.1, 138.6, 147.0, 147.5; LR-MS (EI) m/z 199 (100%, M+).
Biphenyl-4-carboxaldehyde 3lmp 59–60°C (ether) (lit.46) 59–60°C, ether), colorless plates. 1H-NMR (300 MHz, CDCl3) δ: 10.04 (s, 1H), 7.93 (d, J=8.4 Hz, 2H), 7.73 (d, J=8.4 Hz, 2H), 7.60–7.64 (m, 2H), 7.37–7.50 (m, 3H); 13C-NMR (100 MHz, CDCl3) δ: 191.9, 147.2, 139.7, 135.2, 130.3, 129.0, 128.5, 127.7, 127.4; HR-MS (ESI) m/z 183.
2-Methylbiphenyl 3mColorless oil. 1H-NMR (500 MHz, CDCl3) δ: 2.27 (s, 3H), 7.22–7.27 (m, 4H), 7.31–7.36 (m, 3H), 7.38–7.44 (m, 2H); 13C-NMR (125 MHz, CDCl3) δ: 20.5, 125.7, 126.7, 127.2, 128.0, 129.2, 129.8, 130.4, 135.3, 141.9 (C ×2); LR-MS (EI) m/z 168 (100%, M+).
3-Phenylthiophene 3nmp 90.5–91.0°C (EtOH) (lit.31) 89–91°C, EtOH), colorless plates. 1H-NMR (400 MHz, CDCl3) δ: 7.25–7.31 (m, 1H), 7.39–7.45 (m, 5H), 7.59–7.61 (m, 2H); 13C-NMR (125 MHz, CDCl3) δ: 120.2, 126.2, 126.3, 126.4, 127.1, 128.8, 135.8, 142.3; LR-MS (EI) m/z 160 (100%, M+).
2-Phenylpyridine 3oYellow oil.1H-NMR (400 MHz, CDCl3) δ: 8.70 (dt, J=4.8, 1.4 Hz, 1H), 8.03–7.96 (m, 2H), 7.77–7.69 (m, 2H), 7.51–7.45 (m, 2H), 7.45–7.38 (m, 1H), 7.25–7.16 (m, 1H); 13C-NMR (100 MHz, CDCl3) δ: 157.5, 149.7, 139.4, 136.7, 128.9, 128.7, 126.9, 122.1, 120.6. HR-MS m/z (ESI): Calcd for [C11H9N+H]+: 156.0808. Found: 156.0808
XANES Experiments Using Hard X-RayThe X-ray absorption fine structure (XAFS) measurements were performed at hard X-ray beamline BL14B2 of SPring-8 in Japan. The incident hard X-rays were obtained using a silicon double crystal monochromator from synchrotron radiation from the 8GeV storage ring. The net plane is (311) for the Pd K-absorption edge. The higher harmonics of the incident X-rays were reduced using two Rh-coated mirrors. The spectra of standard materials (Pd foil, PdO, PdSO4, PdS and Pd(PPh3)4) were taken in the normal transmission mode. The spectra of SAPd, SGlPd before the 10th cycle of the optimized Suzuki–Miyaura couplings (SAPd before), and after the 10th cycle of the optimized Suzuki–Miyaura (SGlPd after) were measured in the fluorescent X-ray yield method using a 19-elements Ge solid state detector. All the measurements were done at room temperature. The XAFS spectra were analyzed by ATHENA and ARTEMIS XAFS analysis package. For all measurements, data analysis to remove the background and qualitatively analyze the XANES was carried out manually. The data were normalized for variations in the primary X-ray intensity. A linear pre-edge was removed for each spectrum and the data were normalized by the height of the edge-jump. The theoretical scattering amplitude and phase shift function of the single scattering path was calculated by FEFF6.
XANES Experiments Using Soft X-RayThe chemical state of sulfur was analyzed by using b-branch of BL27SU. The radiation from the undulator was mohochromatized by using a double crystal Si(111) monochromator ensures an energy resolution of 0.35 eV. Photon flux on the sample is 1×1011 Ph/s at 2.5 keV (sulfur K-edge). XANES spectra of SAPd and SGlPd amples were recorded by partial-fluorescence yield (PFY) method by using a silicon drift detector (SDD). XANES spectra of standard materials were measured by total electron yield method. SAPd and SGlPd samples were fixed onto a sample holder by screws, and powdered standard materials were fixed on the sample holder by double sided carbon tape.
The XAFS measurements were performed at the BL14B2 and BL27SU of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal Nos. 2011B1761, 2011B1952, 2012A1621, 2012A1770, 2012B1751, 2013A1792, 2014A1786, 2014B1247 and 2015A1917). This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, ACT-C, JST and the Canon Foundation.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.