2016 Volume 64 Issue 9 Pages 1256-1261
2016 Volume 64 Issue 9 Pages 1256-1261
This study investigated how the inclusion of magnesium oxide (MgO) maintained tablet hardness during storage in an unpackaged state. Tablets were prepared with a range of MgO levels and stored at 40°C with 75% relative humidity for up to 14 d. The hardness of tablets prepared without MgO decreased over time. The amount of added MgO was positively associated with tablet hardness and mass from an early stage during storage. Investigation of the water sorption properties of the tablet components showed that carmellose water sorption correlated positively with the relative humidity, while MgO absorbed and retained moisture, even when the relative humidity was reduced. In tablets prepared using only MgO, a petal- or plate-like material was observed during storage. Fourier transform infrared spectrophotometry showed that this material was hydromagnesite, produced when MgO reacts with water and CO2. The estimated level of hydromagnesite at each time-point showed a significant negative correlation with tablet porosity. These results suggested that MgO suppressed storage-associated softening by absorbing moisture from the environment. The conversion of MgO to hydromagnesite results in solid bridge formation between the powder particles comprising the tablets, suppressing the storage-related increase in volume and increasing tablet hardness.
Tablets are frequently used as a drug dosage form that is convenient for patients. However, uncoated tablets are affected by the temperature, humidity, and light-exposure of their storage environment; humidity in particular often affects the physical properties of tablets by reducing hardness and changing (accelerating or delaying) their disintegration time.1–4) For this reason, uncoated tablets are usually packaged using materials with a high moisture resistance, such as pillow-type packaging with aluminum foil. In recent years, however, uncoated tablets have also often been handled in an unpackaged state, such as in automatic tablet packing machines, to provide unit-dose packages that aim to improve the reliability of, and compliance to, drug therapies. The packaging materials used for unit-dose packaging are not generally completely moisture-resistant, and the tablets in these packages reportedly show decreased hardness.4,5)
Magnesium oxide (MgO) has frequently been used as a laxative and antacid and has been proven to be effective and safe. MgO tablets have also become widespread in recent years because they are easy to take.6–8) When stored for three months in an unpackaged state at 30°C with 75% relative humidity, these MgO tablets (Magmitt Tab®, and Maglax Tab®) showed a 1.7–3.8-fold increase in hardness.9) MgO accounts for 86–88% of the mass of these tablets, strongly implying that MgO is involved in this increase in hardness. We have previously shown that MgO is useful as an excipient that suppresses the decline in tablet hardness caused by humidity.10)
The present study therefore investigated the mechanism by which the addition of MgO suppressed tablet softening during storage.
The present study used Japanese Pharmacopoeia acetaminophen (Iwaki Seiyaku Co., Ltd., Tokyo, Japan) as a model drug; anhydrous dibasic calcium phosphate (GS grade, Kyowa Chemical Industry Co., Ltd., Kagawa, Japan) and lactose granules (Tablettose® 80, MEGGLE JAPAN Co., Ltd., Tokyo, Japan) as excipients; carmellose (NS-300®, NICHIRIN CHEMICAL INDUSTRIES, Ltd., Hyogo, Japan) as a disintegrant; and magnesium stearate or calcium stearate (both vegetable-derived; TAIHEI CHEMICAL INDUSTRIAL Co., Ltd., Osaka, Japan) as lubricants. We also used MgO (Kyowa Chemical Industry Co., Ltd.) that had been passed through a 150-µm sieve. Other reagents complied with the Japanese Pharmacopoeia, sixteenth edition (JP16), or were of analytical grade.
Preparation of Powder FormulationsEach stock powder (total amount: 990 g) was mixed using the constituents shown in Table 1 for five minutes with rotation at 30 rpm and revolution at 38 rpm using a mixer (PowMixer, Cross Rotary Model-Laboratorie Type [CM-3], TSUKASA INDUSTRY Co., Ltd., Aichi, Japan). Magnesium stearate (10 g) was then added to the mixed powders to achieve a final concentration of 1%, followed by two more minutes of mixing under the same conditions to obtain the formulation powders.
| Formulation | ||||
|---|---|---|---|---|
| MgO 0% | MgO 10% | MgO 30% | MgO 50% | |
| Acetaminophen | ← | 5% (12.5 mg) | → | |
| Lactose granules | 59% (147.5 mg) | 49% (122.5 mg) | 29% (72.5 mg) | 9% (22.5 mg) |
| Anhydrous dibasic calcium phosphate | ← | 30% (75.0 mg) | → | |
| Carmellose | ← | 5% (12.5 mg) | → | |
| Magnesium oxide (MgO) | 0% | 10% (25.0 mg) | 30% (75.0 mg) | 50% (125.0 mg) |
| Magnesium stearate | ← | 1% (2.5 mg) | → | |
| Total | 100% (250.0 mg) | |||
The formulation powders (250 mg) shown in Table 1 or 200 mg MgO powder were tableted at a compression pressure of 7 kN by a tableting machine (HANDTAB-200, Ichihashi Seiki Co., Ltd., Kyoto, Japan), using a flat punch with a 9-mm diameter. When MgO was being tableted as a single ingredient, the punch and die were coated with calcium stearate dissolved in acetone.
Tablet StorageThe prepared tablets were kept for one day in a desiccator with a bed of silica gel on the bottom. The tablets were then stored for a maximum of 14 d in a storage stability testing chamber (LH20-14M, Nagano Science Co., Ltd., Osaka, Japan) at 40°C with 75% relative humidity. After the experiment, the tablets were again placed in a desiccator lined with silica gel for at least one day prior to examination.
Measuring Tablet Tensile Strength (TS)A Schleuniger tablet hardness tester (8M, Freund Corporation, Tokyo, Japan) was used to measure the load required to break each tablet in the radial direction. This load was then converted to TS (MPa), a measure of tablet hardness, using formula (1):
 | (1) |
Dynamic water vapor sorption by the sample powder (5–20 mg) was measured using DVS-Intrinsic (East Core Ltd., Tokyo, Japan), in which the relative humidity of the sample chamber was increased or decreased by 10% increments at 25°C.
Observing the Tablet Surfaces and Cross-SectionsGold was deposited onto the tablets for ten seconds using an Auto Fine Coater (JFC-1600, JEOL Ltd., Tokyo, Japan) under reduced pressure (≤8 Pa). Using a field emission scanning electron microscope (SEM; JSM-7600F, JEOL Ltd., Tokyo, Japan), we observed the middle part of the tablet surface and the center of the tablet cross-section using an acceleration voltage of 15 kV.
Fourier Transform Infrared Spectrophotometry (FT-IR) MeasurementsThe tablets were shaved with sandpaper in 0.4-mm increments from the surface in the horizontal plane, and samples were collected at each depth. The resulting sample (0.2 mg) was mixed with 20 mg KBr and ground using an agate mortar and pestle; it was then compressed into a disc using a hydraulic press and analyzed using an FT/IR-4100 (JASCO Corporation, Tokyo, Japan). Scanning was conducted 15 times over the 400–4000 cm−1 range at a speed of 2 mm/s and a resolution of 4 cm−1.
Calculation of Tablet PorosityThe porosity of tablets prepared using MgO was calculated using the following procedure:
[1] The mean mass (mg) of the tablet before storage (0 d) was subtracted from the mean mass (mg) of the tablet after storage; this difference represented the total mass (mg) of H2O and CO2 absorbed by the tablet.
[2] Subject to the reaction formula (A) shown below, MgO, CO2, and H2O reacted at a molar ratio of 5 : 4 : 5. The molecular weights of MgO, CO2, and H2O were 40.3, 44.0, and 18.0, respectively, and the mass ratio was MgO : (CO2+H2O)=201.5 : 266.0. Accordingly, 0.76 mg of MgO was consumed per 1-mg increase in mass during storage. Therefore, the mass found in [1] was multiplied by 0.76 to calculate the mass of MgO (mg) involved in the reaction.
 | (A) |
[3] The sum of [1] and [2] was the mass (mg) of hydromagnesite produced. Volume was calculated by dividing this mass by the true density of hydromagnesite (2.2 g/mL11,12)).
[4] The mass of the residual unreacted MgO after storage was calculated by subtracting [3] from the mean mass of the tablet (mg) after storage. The true density of MgO was measured using a dry automatic pycnometer (AccuPyc 1330, Shimadzu Corporation, Kyoto, Japan) and the volume was calculated by dividing this mass by the determined density (3.17 g/mL).
[5] The tablet void volume (mL) was calculated by subtracting the sum of the volumes determined in [3] and [4] from the tablet volume (mL), which was calculated using the mean size and thickness after storage. The porosity was this void volume, expressed as a percentage of the tablet volume.
The tablets were prepared using 0–50% MgO (Table 1). The changes in tablet mass, volume, and TS during storage for up to 14 d at 40°C with 75% relative humidity are shown in Fig. 1.

●, 0% MgO tablets; ■, 10% MgO tablets; ◆, 30% MgO tablets; ×, 50% MgO tablets. Data represent the mean±standard deviation (S.D.) (n=10).
Tablets lacking MgO (0% MgO tablet) showed reduced TS on and after storage day 1, and this decrease was maintained until day 14. In contrast, all tablets containing MgO showed an increase in TS on and after storage day 1. In particular, the TS of tablets containing ≥30% MgO had increased markedly by day 1, and this trend was maintained until day 14 (Fig. 1A). Tablet MgO content tended to associate with an increase in TS during storage (10% MgO tablets<30% MgO tablets<50% MgO tablets) (Fig. 1A) and with increased tablet mass (Fig. 1B) over time. However, all tablets showed an increase in volume over time, with a particularly prominent increase on storage day 1 (Fig. 1C). This volume increase was inversely related to the tablet MgO content.
The data presented in Fig. 1 showed that tablet mass increased during storage and we therefore investigated the dynamic vapor sorption properties of the four major tablet components (Fig. 2). The mass of carmellose, which was used as a disintegrant, correlated positively with changes in relative humidity. In contrast, MgO showed a rapid increase in mass when the relative humidity was increased above around 80%, but its mass only decreased slightly when the relative humidity was lowered. The masses of anhydrous dibasic calcium phosphate and lactose did not change substantially when the relative humidity was altered.

Thus the results presented in Figs. 1 and 2 suggest the following possibilities: (1) MgO and carmellose absorbed water during storage, and this affected tablet hardness; (2) moisture absorption by carmellose reduced tablet TS, whereas moisture absorption by MgO increased tablet TS and mass; or (3) moisture absorption by MgO suppressed the change in tablet volume.
Changes in Single-Component MgO Tablet Properties during StorageTo investigate how MgO affected tablet TS, we prepared tablets with MgO as the only component and investigated the changes that took place during storage (Fig. 3).

Data represent the mean±S.D. (n=10).
Similar to the tablets prepared by adding MgO into the formulation, the tablets prepared with 100% MgO also showed increased tablet hardness and mass (Fig. 3A) during storage at 40°C with 75% relative humidity. In particular, the hardness of the 100% MgO tablets increased greatly within the first hour of storage, after which it continued to increase gradually, reaching about 2.5 times the initial hardness after one day (Fig. 3A). The tablet hardness on storage day 2 and later actually exceeded the measurement limits of the equipment employed. Compared to these changes in hardness, the changes in mass were more gradual, increasing over the first two days of storage and subsequently increasingly more gradually (Fig. 3B). In contrast, the volume of the 100% MgO tablets did not change significantly during the storage period (Fig. 3C). These results imply that the increase in tablet volume observed in the mixed formulation tablets during storage was due to the moisture absorption and swelling of carmellose. This swelling expanded the distance between the formulation powder particles in the tablets, reducing the van der Waals forces between them and decreasing the hardness of 0% MgO tablets (Fig. 1A). In tablets containing 10–50% MgO, its moisture absorption increased tablet hardness.
In order to clarify the mechanism by which MgO increased tablet hardness, we used SEM to observe changes in tablet morphology during storage (Fig. 4). A change was observed in the state of the 100% MgO tablet surface after three and seven days of storage; a petal-like or plate-like material that was different from the original MgO was observed in void portions (Fig. 4). In the tablet cross-sections, plate-like crystals were observed on storage day 7 (Fig. 4).

Surface images were observed at the upper tablet surface and cross-sections were observed at the center of a vertical section.
We therefore explored whether these structural changes progressed from the tablet surface toward the tablet center. The 100% MgO tablets that had been stored for one, six, or 24 h were shaved to yield samples collected at a depth of 0.4 or 0.8 mm from the surface for assessment by FT-IR (Fig. 5). All samples showed a characteristic absorption near 1400–1500 cm−1 and near 3000–3600 cm−1; the absorption intensities increased during storage. When compared within the same storage time, however, samples taken from closer to the surface showed greater absorption. In their study of the effect of pH on the generation of magnesium carbonate hydrates (magnesite [MgCO3], nesquehonite [MgCO3·3H2O], and hydromagnesite [Mg5(CO3)4(OH)2·4H2O]), Zhang et al. reported that the main product was hydromagnesite at higher pH conditions; this has a planar structure and its examination by FT-IR showed that the CO32− absorption was around 1400–1500 cm−1, while the OH and crystal water absorption was around 3400–3600 cm−1.13) These results suggested that the tablet mass changes during storage involved water absorption by MgO and also absorption of CO2 gas, and that hydromagnesite was produced more predominantly at the tablet surface, from the early stages of storage. This indicated that hydromagnesite produced near the surface forms solid bridges between particles and also fills the pores that pass through the center; this reduces the access of moisture and CO2 to the center, slowing hydromagnesite production. This can be explained by the results of our previous investigation,10) which indicated that the pore surface area and the open pore ratio of tablets composed of 100% MgO decreased after storage. This was also supported by the appearance of the petal- or plate-like material in the 100% MgO tablets from day 3 (tablet surface) and day 7 (cross-section).

When 100% MgO tablets were stored at 40°C with 75% relative humidity, the results indicated that hydromagnesite was the predominant product, which increased tablet hardness. However, the storage duration associated with SEM crystal changes (Fig. 4) was not coincident with the increase in 100% MgO tablet hardness (Fig. 3A). This may be because the hydromagnesite produced at the particle contact points contributed to increased tablet hardness during the early stages of storage. We previously reported that 100% MgO tablets prepared at 7 kN had a mean pore size of 30–40 nm, which is in the mesoporous range.10) There were therefore mesopores immediately adjacent to the contact point(s) of each MgO particle within the tablet, indicating that moisture from the air could undergo capillary condensation between and/or around these particles. When these tablets were exposed to moisture and CO2 gas, the MgO eluted into the capillary-condensed water where it was converted to hydromagnesite, crystallized, and formed solid bridges between the MgO particles.
Based on this reasoning and assuming that all of the tablet mass increase during storage was due to the H2O and CO2 incorporated for hydromagnesite production according to the reaction formula (A), we estimated the amount of hydromagnesite produced and the porosity of the 100% MgO tablets, and investigated the correlations between these parameters (Fig. 6A). The mean tablet mass and volume values presented in Fig. 3 were used and the true densities of MgO and hydromagnesite were understood to be 3.17 and 2.2 g/mL,11,12) respectively.
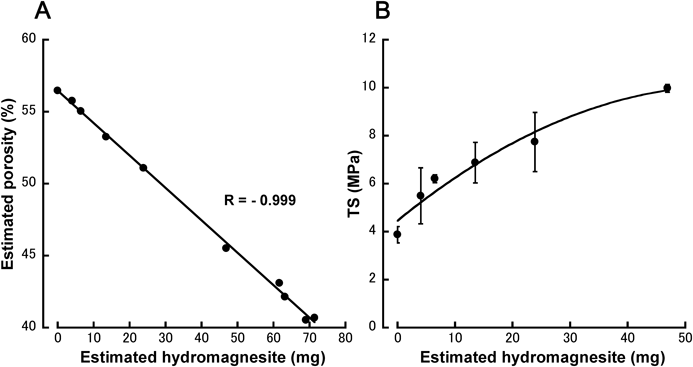
Estimated hydromagnesite (mg) and estimated porosity (%) were calculated on the basis of the mean values of tablet mass and volume at each storage period. (B) TS values represent the mean±S.D. (n=10).
The resulting plot showed a significant negative correlation (R=−0.999). This indicated that as the amount of hydromagnesite increased, tablet porosity decreased. It has been shown in a previous investigation that the porosity of 100% MgO tablets decreases after storage.10) Since hydromagnesite has a lower true density than MgO, its generation should be accompanied by an increase in volume; however, the volume of the 100% MgO tablets remained almost constant throughout the storage period (Fig. 3C). This indicated that the generated hydromagnesite increased tablet hardness by filling the gaps between particles within the tablets.
The relationship between tablet TS and the estimated amount of hydromagnesite produced was not a straight line, because the rate of increase in TS declined as the level of hydromagnesite increased (Fig. 6B). This was consistent with the observation that TS showed a greater rate of increase than did tablet mass (Figs. 3A, B), particularly at the early stages of storage (by day 1). This reflected the early exposure of MgO powder particles located near the tablet surface to H2O and CO2, causing them to form solid bridges of hydromagnesite at the contact points. This implies that in tablets formulated using a disintegrant (Fig. 1) as well as MgO, the swelling-associated volume increase was suppressed, and TS increased, rather than decreasing. Additionally, during longer storage periods, hydromagnesite levels increased, even in the tablet interior; the resultant formation of new solid bridges, or the growth of existing solid bridges, reduced the voids within the tablet, which further increased the TS.
The findings of this study indicated that addition of MgO suppressed the reduction in hardness during tablet storage under accelerated test conditions by absorbing moisture and CO2 from the environment, causing hydromagnesite production. The resulting hydromagnesite forms solid bridges between the powder particles present in the tablets, suppressing the storage-associated increase in tablet volume and increasing tablet hardness.
The authors declare no conflict of interest.