2016 Volume 64 Issue 9 Pages 1304-1309
2016 Volume 64 Issue 9 Pages 1304-1309
The objective of this study was to establish the key factor of the lauryl sulfate (LS) salt/complex for sustained release of a hydrophilic drug at various physiological pH levels. Mirabegron is a hydrophilic drug that exhibits pH-dependent solubility. Sodium lauryl sulfate (SLS) bound to mirabegron in a stoichiometric manner. The formation of the LS salt/complex significantly reduced mirabegron solubility and helped achieve sustained release of mirabegron over a wide range of pH levels. In addition to SLS, other additives containing a sulfate group formed salts/complexes with mirabegron and reduced its solubility at different pH levels. Furthermore, octyl sulfate (OS), myristyl sulfate (MS), and cetyl sulfate (CS) salts/complexes, which contain alkyl chains of different lengths, showed a lower solubility than mirabegron and promoted sustained release of mirabegron. The rank order of solubility and dissolution rate were as follows: OS salt/complex>LS salt/complex>MS salt/complex>CS salt/complex, which corresponded to the rank of alkyl chain lengths. We conclude that the presence of a sulfate group and the length of the alkyl chain are key factors of the LS salt/complex for sustained release of a hydrophilic drug at various physiological pH levels.
Oral sustained release dosage forms have been developed in an attempt to improve patients’ quality of life by reducing the inconvenience caused by frequent administrations of conventional dosage form. Among these, multiple-unit dosage forms are preferred over single-unit systems because of their better predictability, reproducible therapeutic effects, and less frequent side effects.1) Multiple-unit dosage forms distribute more uniformly in the gastrointestinal tract resulting in more reproducible drug release profiles, more predictable gastric emptying, homogeneous drug absorption, and reduced local irritation. Multiple-unit dosage forms also minimize the risk of dose dumping and avoid unwanted intestinal retention of the polymeric material.
Development of oral sustained release dosage forms with a constant release rate for hydrophilic drugs has always been a challenge for pharmaceutical technology. The majority of hydrophilic drugs, if not formulated properly, may readily release the drug at a fast rate and are likely to produce toxic concentrations when administered orally. In addition, with sustained release dosage forms, drug release in vitro should preferably be independent of the pH of the release medium in order to minimize biopharmaceutical variability.2)
The use of ionic complexes between the oppositely charged drug and additive has attracted much attention as a sustained release technology for hydrophilic drugs, because ionic complexes can be prepared using very simple and chemically mild processes. In recent years, a number of studies addressed the issue of ionic complex formation in relation to the sustained release formulations. For example, ion-exchange resins, water-insoluble polymers that contain acidic or basic functional groups in a repeating pattern, have been widely used to form reversible weak ionic bonds with oppositely charged drug molecules, resulting in sustained release of the drug from the drug–resin complex.3–6) Tannic acid, a polyphenol with the chemical formula C76H52O46, which corresponds to decagalloyl glucose, acts as a cross-linking agent in the formulation of nano- and microsystems due to its ability to interact with amino groups to form salts/complexes through ionic, hydrophobic interactions and/or hydrogen bonding.7) Tannic acid is also used as a salt/complex to sustain the drug release.8–10) Chitosan is a cationic polymer and has already been widely exploited in many drug delivery applications in ionic complex with the protonated amine group of chitosan for sustained release action.11–13) Carrageenan can also serve as an appropriate carrier for sustained release dosage forms due to ionic complex formation.14,15) However, these complexes could not singly achieve adequate sustained release of tested drugs at various pH levels.
To overcome these drawbacks, we have developed a novel ionic complex with a drug and sodium lauryl sulfate (SLS). SLS is an anionic surfactant with excellent wetting properties across a wide pH range that is commonly used in many pharmaceutical and cosmetic applications.16–18) SLS is listed as a Generally Regarded as Safe (GRAS) chemical and is included in the Food and Drug Administration (FDA) Inactive Ingredients Guide (dental preparations; oral capsules, suspensions, and tablets; topical and vaginal preparations), in the list of nonparenteral medicines licensed in the U.K., and in the Canadian List of Acceptable Non-medicinal Ingredients.19) SLS was used as a solubilizer to improve the solubility and dissolution rate of cyclosporine A.20) Recently, SLS was reported to form salts/complexes between the lauryl sulfate (LS) anion and protonated drug forms.21,22) The LS salt/complex has been utilized to increase the lipophilicity of insulin23,24) and to enhance bioavailability of the poorly absorbed RWJ-445167 compound within an in situ gelling formulation.25) However, to the best of our knowledge, there have been no published accounts of the use of the LS salt/complex for oral sustained release of drugs.
In this study, we intended to establish the key factor of the LS salt/complex for sustained release of a hydrophilic drug at various pH levels within the simulative physiological range. For this purpose, quantitative characteristics of drug solubility and drug release from salts/complexes containing alkyl chains of variable lengths were determined. Furthermore, drug insolubilization induced by some additives containing a sulfate group was evaluated at various pH levels.
Mirabegron (Fig. 1) was used as a model hydrophilic drug that exhibits pH-dependent solubility. Mirabegron, [2-(2-amino-1,3-thiazol-4-yl)-N-[4-(2-ethyl)phenyl]acetamide] is a β3-adrenergic receptor agonist used for the treatment of overactive bladder.26–28) Mirabegron was provided by Astellas Pharma Inc. (Tokyo, Japan). SLS was purchased from Cognis GmbH (Monheim, Germany). Sodium myristyl sulfate (SMS) and sodium cetyl sulfate (SCS) were kindly supplied by Nikko Chemicals Co., Ltd. (Tokyo, Japan). Sodium octyl sulfate (SOS) and sodium benzoate were purchased from Kanto Chemical (Tokyo, Japan). Dextran sulfate sodium was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Aminoalkyl methacrylate copolymer (Eudragit® E) and methacrylic acid copolymer (Eudragit® L100) were provided by Evonik Industries AG (Essen, Germany). Hypromellose (TC-5E) was purchased from Shin-etsu Chemical (Tokyo, Japan). Carboxyvinyl Polymer (Carbopol® 971P) was purchased from CBC (Tokyo, Japan). All other chemicals were of reagent grade.
Preparation of LS Salt/ComplexTo prepare the LS salt/complex, 1 g (2.52 mmol) of mirabegron was dissolved in 50 mL of 0.1 N HCl. SLS solutions of different molar concentrations were also prepared by dissolving appropriate amounts of SLS (0.25, 1.26, 2.52, 5.04, 7.57, 1244.61, 25.22 mmol) in 50 mL of purified water. The SLS solution was added slowly into the mirabegron solution under magnetic stirring at room temperature. The solution became cloudy and a white precipitate was formed. The white precipitates were separated by filtration (0.45 µm polytetrafluoroethylene (PTFE) membrane filter, Millipore Omnipore™, Japan) and dried at 40°C for 12h. To determine the composition of the LS salt/complex, the white precipitate was dissolved in methanol. The solution was further diluted with mobile phase before injection into an HPLC. Mirabegron concentration was measured. The amount of LS in the salt/complex was indirectly calculated based on the measured amount of mirabegron in the salt/complex under an assumption that the salt/complex consisted of the compound and LS only. Also, the cloudy solution was centrifuged at 3000 rpm for 15 min at room temperature. The supernatants were filtered (0.45 µm polyvinylidene difluoride (PVDF) syringe filter, Millipore Millex-HV, Japan) and suitably diluted before injection into a HPLC. Mirabegron concentration in supernatants was separated using a column (Develosil ODS-HG-3, 4.6×150 mm; Nomura Chemical Co., Ltd., Japan) and the mirabegron soluble fraction was calculated based on the initial amount of mirabegron added. The mobile phase consisted of perchloric acid solution–acetonitorile (7 : 3). The column temperature was set at 40°C, and the flow rate was maintained at 0.9 mL/min. The detection wavelength was 250 nm.
Preparation of Octyl Sulfate (OS), Myristyl Sulfate (MS), and Cetyl Sulfate (CS) Salts/ComplexesFor each preparation, 1 g of mirabegron was dissolved in 100 mL of 0.1 N HCl. A corresponding amount of SMS (2 : 1, mirabegron/SMS molar ratio) was dissolved in 100 mL of distilled water and added to the mirabegron solution. Dissolution was aided by magnetic stirring for 2 h in a water bath set to 50°C. The solution became cloudy and a white precipitate was formed. The white precipitates were separated by filtration and dried at 40°C for 12 h. Similar method was applied to prepare OS and CS salts/complexes with the following exceptions: OS salt/complex was prepared without a water bath, whereas during CS salt/complex formation the water bath was set to 70°C.
Differential Scanning Calorimetry (DSC) AnalysisDSC curves were obtained with a DSC 6220 (Rigaku, Tokyo). SLS, mirabegron, physical mixture (PM) of SLS and mirabegron, and the LS salt/complex were loaded into aluminium pans separately and heated from 30 to 230°C under a nitrogen atmosphere at a heating rate of 10°C /min.
Scanning Electron Microscopy (SEM)Morphological characteristics of SLS, mirabegron, and the LS salt/complex were studied using a scanning electron microscope (VHX-D510, KEYENCE, Osaka, Japan).
Solubility StudiesMirabegron or salts/complexes were added in excess to 100 mL of the following solvents: 0.1 N HCl (pH 1.0), 0.001 N HCl (pH 3.0), or purified water (pH 6.0). The samples were shaken at a speed of 200 rpm at room temperature for 24 h and then centrifuged at 3000 rpm for 15 min. The supernatant was filtered through a 0.45 µm PVDF syringe filter and suitably diluted and a 10 µL aliquot was analyzed by HPLC at 250 nm.
Dissolution StudiesDissolution tests were conducted using formulations containing 25 mg of mirabegron. The tests were performed in accordance with Dissolution Test Method 2 (paddle method) (Japanese Pharmacopoeia XIV, 2001b) using an automatic 6-series dissolution testing device (Toyama Sangyo Co., Ltd., Japan) with a UV-Vis spectrophotometer (Shimazu Co., Japan). A phosphate buffer with a pH 6.8 (second fluid, identified in the Disintegration Test cited in Japanese Pharmacopoeia XIV, 2001a), 0.1 N HCl and water were used as the test fluid. The volume of test fluid was 900 mL. The wavelength used to detect mirabegron were 248 and 450 nm. Due to a low solubility of MS and CS salts/complexes, sink conditions could not be obtained. Therefore, amounts of MS and CS salts/complexes were set to 12.5 and 3.125 mg of mirabegron, respectively, so that they could completely dissolve in this test medium. In order to evaluate sensitively the difference of each salt/complex, the paddle rotation speed was set on 50 rpm.
Evaluation of Drug Insolubilization with Some AdditivesTo evaluate mirabegron insolubilization, 200 mg of mirabegron was dissolved in 10 mL of 0.1 N HCl. In addition, 400 mg of some additives was dissolved/dispersed in 20 mL of water and added into mirabegron solution. The mixed solution became cloudy and a precipitate was formed. Then the pH of the solution was adjusted to different levels (2.0, 4.0, or 6.0). The concentration of soluble mirabegron in each supernatant was measured by HPLC as described in the above section. Furthermore, dissolution tests were carried out using prepared precipitates that contained 25 mg of mirabegron. The tests were performed in accordance with the dissolution test method as described in the above section.
SLS has a hydrophilic sulfate head group and longer alkyl chains with a molecular weight of 288.4 g/mol, while mirabegron has a thiazol group (pKa 4.5) and an amine group (pKa 8.0) with a molecular weight of 396.5 g/mol. Under acidic conditions, an amine group and a thiazol group of mirabegron become protonated. On the other hand, the sulfate group of SLS was ionized in water.

Upon a slow addition of the SLS solution (pH 9.5) to the mirabegron solution (pH 2.3), positively charged amine group and thiazol group of mirabegron bound to the negatively charged sulfate group of SLS. Thus, the mixed solution became cloudy and the formed ionic complex precipitated out of the solution due to its low aqueous solubility. As shown in Fig. 2, the addition of a stoichiometric amount of SLS to the mirabegron solution (2 : 1, SLS/mirabegron molar ratio) resulted in the lowest mirabegorn soluble fraction in the solution (pH 2.7) and a maximum yield of the precipitate. This precipitate was the LS salt/complex. The result indicates that positive charged mirabegron can interact stoichiometrically with negatively charged SLS.

After reaching the stoichiometric molar ratio (SLS/mirabegron; 2 : 1), the addition of an excessive amount of SLS into the mirabegron solution decreased the amount of the precipitate. This can be attributed to the resolubilization of the LS salt/complex by the micelles formed by an excessive amount of SLS. At a stoichiometric ratio of SLS to mirabegron, all compounds in the solution were bound with SLS. An additional amount of SLS then formed micelles where a salt/complex precipitate would be resolubilized. This hypothesis was further confirmed by conducting another experiment in which the mirabegron solution (50.4 mM) was slowly added dropwise into a 100.9 mM concentration of SLS solution instead. In this experiment, the SLS concentration was above its critical micelle concentration (CMC) value (8.32 mM)22) and thus micelles were formed before the compound was added. However, neither clouding nor precipitation was observed at the beginning, indicating that LS salt/complex formed initially was resolubilized in SLS micelles. Precipitation occurred suddenly once the amount of mirabegron exceeded its stoichiometric ratio to SLS. This phenomenon was also observed previously with other compound.25)
In addition, we directly analyzed the precipitates to determine the composition of the LS salt/complex by HPLC. These results showed an approximately stoichiometric ratio of SLS to mirabegron in the LS salt/complex (Table 1).
| Feeding molar ratio (SLS/mirabegron) | Calculated molar ratio (LS/mirabegron) |
|---|---|
| 1/1 | 1.85/1 |
| 2/1 | 1.96/1 |
| 3/1 | 1.89/1 |
| 5/1 | 1.97/1 |
The LS salt/complex was characterized by DSC (Fig. 3). The DSC thermogram of SLS showed two melting endotherms: between 90–110°C and between 190–198°C, respectively. The DSC thermogram of mirabegron showed a melting endotherm at around 140°C indicating crystallinity of mirabegron used in this study. Thermogram of the PM of SLS and mirabegron was similar to separate DSC thermograms of SLS and mirabegron. On the other hand, LS salt/complex was found to be not amorphous, but crystalline powder. The finding that the complex was amorphous observed in previous study,25) however LS salt/complex in this study indicated no amorphicity. Thermogram of LS salt/complex showed three melting endotherms: between 55–60°C, between 70–100°C and between 140–150°C, respectively. The weak endotherms between 55–60°C and between 70–100°C were not observed in the thermogram of SLS and mirabegron. This endotherm might indicate the formation of new solid phase due to the LS salt/complex. Also, the endotherms between 140–150°C might show mirabegron not having interaction with SLS.

SEM picture of SLS showed agglomerated particles with a diameter of 300–500 µm, while mirabegron formed rod-like crystals. In contrast to SLS and mirabegron, the LS salt/complex formed distinctly shaped particles with a diameter of 10–70 µm (Fig. 4).

Solubility data for various formulations is shown in Table 2. Mirabegron is a basic molecule and has a pH-dependent solubility. Generally, it exhibit low solubility at high pH and high solubility a low pH. It is accepted that basic molecule ionize at low pH and cause increased interaction between the drug and solvent molecules and hence increase in the solubility.29) Mirabegron was found to have a higher solubility at lower pH (12933 µg/mL at pH 1), while reversed trend was observed at higher pH (116 µg/mL at pH 6). On the other hand, the LS salt/complex significantly reduced solubility and showed pH-independent solubility, which comprised 87, 87, and 95 µg/mL at pH 1, 3, and 6, respectively. Less ionization occurs at high pH due to weekly basic nature of mirabegron and hence stronger association between mirabegron and SLS. Similarly, at low pH converse phenomenon occurs.
| pH 1 (µg/mL) | pH 3 (µg/mL) | pH 6 (µg/mL) | |
|---|---|---|---|
| Mirabegron | 12933.0±272.8 | 501.4±5.5 | 116.1±1.8 |
| OS salt/Complex (C8) | 1137.9±10.1 | 786.1±27.5 | 832.8±26.0 |
| LS salt/Complex (C12) | 87.1±3.4 | 87.2±9.0 | 95.3±3.1 |
| MS salt/Complex (C14) | 54.4±8.8 | 32.7±5.6 | 9.8±0.1 |
| CS salt/Complex (C16) | 9.9±0.2 | 5.0±0.1 | 4.4±1.5 |
Each data represents the mean±S.D. (n=3).
In addition, we examined the effect of alkyl chain length on the solubility of salts/complexes formed by LS, OS, MS, and CS, which have 12, 8, 14, and 16 carbon atoms in their alkyl chains, respectively. OS salt/complex did not reduce the solubility compared with LS salt/complex. However, MS and CS salts/complexes exhibited a lower solubility than the LS salt/complex, suggesting that the solubility of a salt/complex decreases significantly with the increase in the alkyl chain length of an alkyl sulfate salt. The solubility of alkyl sulfate salts decreased in the following order: SOS (C8)>SLS (C12)>SMS (C14)>SCS (C16). The solubility of salts/complexes showed the same pattern as the solubility of alkyl sulfate salts.
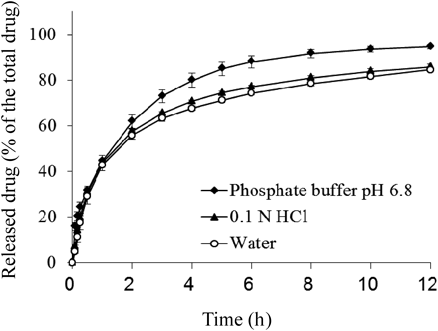
Dissolution of Salts/ComplexesDissolution profiles of salts/complexes and PM, as a reference, in the phosphate buffer (pH 6.8) are shown in Fig. 5. Over 80% of the drug was released from the OS salt/complex in 0.5 h. The dissolution rate was a little slower than that of PM of SLS and mirabegron, however the release was not sustained. The release from the LS salt/complex amounted to 45% in 1.0 h and reached an equilibrium at about 10.0 h, which was more sustained compared with the dissolution of PM and OS salt/complex. In addition, the LS salt/complex exhibited a sustained release pattern even in 0.1 N HCl and water (Fig. 6), demonstrating that the release of drug from the LS salt/complex was insensitive to pH variations within the simulative physiological range due to pH-independent solubility. Moreover, MS and CS salts/complexes exhibited a more prolonged release profile compared with the kinetics of release from the LS salt/complex. The rank order of the dissolution rate from the complexes was as follows: PM>OS salt/complex>LS salt/complex>MS salt/complex>CS salt/complex. This ranking was in agreement with the alkyl chain lengths of salts/complexes. The alkyl chain length may affect solubility and wettability. Therefore, salts/complexes with longer alkyl chain length showed a more prolonged released release profile.

Error bars represent standard deviations of three measurements.
Error bars represent standard deviations of three measurements.
Mirabegron soluble fraction remaining in solution with various additives is presented in Table 3. The salts/complexes precipitated as soon as additives containing a carboxyl group was added to the mirabegron solution. After adjusting the pH of the solution to 6.0, mirabegron soluble fraction in solution showed low tendency except in the case of sodium benzoate. However, the salts/complexes with a carboxyl group dissolved after adjusting the pH of the solution to 2.0 and the solution showed high mirabegron soluble fraction. On the other hand, additives containing a sulfate group readily formed salts/complexes and reduced the mirabegron soluble fraction in solution regardless of the pH value. This suggests that despite mirabegron has a pH-dependent solubility, pH-independent lower solubility of salts/complexes may be achieved by making use of additives containing a sulfate group. In addition, we found that the cationic additive, aminoalkyl methacrylate copolymer, and the nonionic additive, hypromellose, did not affect the mirabegron soluble fraction. Such results suggest that these additives failed to promote salt/complex formation. These observations indicate that the presence of the sulfate group is a key factor in mediating pH-independent lower solubility of salt/complex. Such salts/complexes with a sulfate group are ideally suited for preparing sustained release formulations due to pH-independent lower solubility.
| Mirabegron soluble fraction (%) | |||
|---|---|---|---|
| pH 2 | pH 4 | pH 6 | |
| Mirabegron | 103.2 | 103.8 | 103.0 |
| Anionic additives | |||
| Carboxyl group (COO−) | |||
| Methacrylic acid copolymer | 96.1 | 41.8 | 2.9 |
| Carboxyvinyl polymer | 92.3 | 65.8 | 0.0 |
| Sodium benzoate | 90.6 | 77.1 | 91.4 |
| Sulfate group (OSO3−) | |||
| SLS | 5.4 | 6.1 | 10.9 |
| Dextran sulfate sodium | 4.6 | 4.8 | 7.9 |
| Carrageenan | 46.2 | 42.6 | 39.4 |
| Cationic additive | |||
| Aminoalkyl methacrylate copolymer | 103.6 | 102.5 | 100.9 |
| Nonionic additive | |||
| Hypromellose | 101.7 | 100.0 | 100.6 |
Each data represents the mean value (n=1–3). Mirabegron (200 mg) was dissolved in 10 mL of 0.1 N HCl, and 20 mL of water dissolved/dispersed some additives (400 mg) was added. Then, mirabegron soluble fraction represents as present of the maximum concentration (200 mg/30 mL).
Dissolution profiles of salts/complexes formed by additives containing a sulfate group in the phosphate buffer medium (pH 6.8) are shown in Fig. 7. Salts/complexes were prepared using 1 : 2 weight ratio of mirabegron to additive. However, actual mirabegron-additive ratios of salts/complexes were different for each complex, therefore over 100% of mirabegron was released from some salts/complexes including excessive mirabegron. In order to normalize the mirabegron-additive ratio, the dissolution rate was recalculated, as the reached equilibrium point was 100%. SLS, dextran sulfate sodium and carrageenan all have sulfate groups. However, the salt/complex formed by SLS, LS salt/complex, exhibited the slowest drug release rate. This finding suggests that in addition to the sulfate group, another key factor promoting sustained release of mirabegron is the presence of an alkyl chain.

Error bars represent standard deviations of three measurements.
In this study, we used the LS salt/complex to achieve adequate sustained release of a hydrophilic drug with pH-dependent solubility. We demonstrated that the LS salt/complex significantly decreased both solubility and dissolution rate of a hydrophilic drug in the simulative physiological pH range. The MS and CS salts/complexes exhibited a lower solubility and a slower sustained release rate compared with the LS salt/complex. SLS is commonly used in a wide range of pharmaceutical applications, while SMS and SCS are predominantly used in cosmetics. Therefore, LS salt/complex is the most suitable for the development of novel drug delivery systems.
The rank order of solubility and dissolution rate were as follows: OS salt/complex>LS salt/complex>MS salt/complex >CS salt/complex, which corresponded to the rank of alkyl chain lengths. Furthermore, not only SLS but also other additives containing a sulfate group formed salts/complexes with hydrophilic drug and reduced its solubility regardless of the pH value. Thus, our results indicate that key factors for the sustained release from LS salt/complex at various pH within the simulative physiological range are the presence of a sulfate group and the length of the alkyl chain.
Y. K., K. Y., T. Y. and K. S. are employees of Astellas Pharma Inc. (Tokyo, Japan). S. U. and N. N. have no conflict of interest.