2016 Volume 64 Issue 9 Pages 1338-1346
2016 Volume 64 Issue 9 Pages 1338-1346
We evaluated the effectiveness of a silicone membrane as an alternative to human skin using the skin permeation parameters of chemical compounds. An in vitro permeation study using 15 model compounds was conducted, and permeation parameters comprising permeability coefficient (P), diffusion parameter (DL−2), and partition parameter (KL) were calculated from each permeation profile. Significant correlations were obtained in log P, log DL−2, and log KL values between the silicone membrane and human skin. DL−2 values of model compounds, except flurbiprofen, in the silicone membrane were independent of the lipophilicity of the model compounds and were 100-fold higher than those in human skin. For antipyrine and caffeine, which are hydrophilic, KL values in the silicone membrane were 100-fold lower than those in human skin, and P values, calculated as the product of a DL−2 and KL, were similar. For lipophilic compounds, such as n-butyl paraben and flurbiprofen, KL values for silicone were similar to or 10-fold higher than those in human skin, and P values for silicone were 100-fold higher than those in human skin. Furthermore, for amphiphilic compounds with log Ko/w values from 0.5 to 3.5, KL values in the silicone membrane were 10-fold lower than those in human skin, and P values for silicone were 10-fold higher than those in human skin. The silicone membrane was useful as a human skin alternative in an in vitro skin permeation study. However, depending on the lipophilicity of the model compounds, some parameters may be over- or underestimated.
Skin forms an interface between the body and environment and functions as a firm barrier against undesirable elements. Many skin permeation studies of therapeutic drugs have been conducted to develop transdermal drug delivery systems (TDDSs) for the purpose of preventing the hepatic first-pass effect, which is observed for some oral dosage forms, and for controlling release over long periods of time.1–3) For the development and evaluation of TDDSs, in vitro skin permeation tests using excised human skin have been performed in the European Union (EU) and United States; however, in Japan, it is difficult to obtain human skin, so permeation studies using human skin are not performed as frequently. Therefore, animal skins, particularly those of hairless rat, mouse, and guinea pig, have been used for the evaluation of percutaneous absorption. Recently, however, sales of cosmetics in the EU,4) for which animal experiments were performed during their development have been prohibited out of concern for animal welfare, and alternative membranes are now required to determine percutaneous absorption. In addition, permeation studies using animal or human skin are associated with several difficulties and limitations for assessing the effects of formulations components, because of the complex nature of this biological tissue and inter- and intra-individuality of skin.
Artificial model membranes offer a simple and reproducible alternative to study the basic physicochemical mechanisms of drug permeation, and provide basic information for understanding more complex drug permeation through animal and human skins. Evaluation of skin permeability using artificial membranes and cultured skin has been performed previously. In vitro permeation studies of various model compounds have been conducted using a three-dimensional cultured human skin model,5–7) a parallel artificial membrane permeability assay (PAMPA),8–10) and Strat-M™,11) and these studies have shown the utility of skin surrogates. A study of silicone membrane permeability has been conducted previously,12) and silicone has been used in various other studies to determine, for example, the effects of vehicles on membrane permeability,13) prediction of skin concentrations of test compounds,14) and skin permeation routes.15) On the other hand, the utility of silicone membrane as an alternative membrane was studied recently by examining the correlation between permeability coefficient (P) values in human skin and in a silicone membrane.16) Subsequently, studies using flux as a permeation parameter have also been conducted, and it was found that a silicone membrane could be used as a substitute for human or animal skin.17,18) However, it also has been reported that silicone membrane permeability is essentially different from human skin permeability,19,20) and it is unclear if silicone membranes were effective as an alternative to human or animal skin.
The physicochemical properties of drugs, such as their molecular weights (MWs) and the lipophilic/hydrophilic balance (i.e., octanol/buffer apparent distribution ratio at a particular pH, log Ko/w), have been shown to markedly affect permeability through the skin.21–23) Most drugs used in TDDSs have MWs <500 Da,24) and the P value through skin was reported previously to markedly decrease with an increase in MW >500 Da. Furthermore, the P values of drugs increased with increasing log Ko/w.21) Depending on the MWs and log Ko/w of the compound used for membrane evaluation, the permeability of silicone membranes and human and animal skin may change. Geinoz et al. reported the utility of a silicone membrane used for seven chemical compounds with MWs between 94.11 (phenol) and 234.40 (lidocaine) and log Ko/w values from 1.23 (nicotine) to 2.59 (4-bromophenol).16) On the other hand, Moss et al. reported that the permeability of silicone membrane and human skin was essentially different for 13 chemical compounds with MWs between 165.19 (benzocaine) and 343.47 (dibucaine) and log Ko/w values from 0.84 (captopril) to 5.36 (captopril hexyl ester).20) The difference in permeability was considered to be caused by the difference between the ranges of MWs and log Ko/w values of the model compounds.
Thus, the P value can be further divided into diffusion (DL−2) and partition (KL) parameters,25) where D, K, and L are the diffusion coefficient, partition coefficient, and thickness of the membrane, respectively. These parameters can be easily obtained from in vitro skin permeation studies, the values of which depend on the characteristics as well as penetration properties of the membrane. Therefore, the determination of these parameters for alternative membranes may prove to be useful in understanding membrane characteristics and their similarities (or differences) to human and animal skin. For instance, it was reported that the log P value in TESTSKIN LSE-high, which is a three-dimensional cultured human skin model, was 10-fold higher than that in humans. Moreover, it was obvious that it did not influence KL but rather DL−2.5) Although P and J are important parameters for evaluating human skin permeability, to understand the skin pharmacokinetics of compounds applied to the skin, it is necessary to evaluate KL and DL−2.14)
In the present study, a permeation experiment using a silicone membrane was performed to obtain parameters comprising P, DL−2, and KL of the test compounds with MWs between 122.12 (benzoic acid) and 270.80 (lidocaine hydrochloride) and log Ko/w values from −1.50 (antipyrine) to 3.86 (flurbiprofen) dissolved in aqueous solutions. The usefulness and characteristics of the silicone membrane were assessed by comparing the permeation parameters of the silicone membrane, human skin, and hairless rat skin.
Aminopyrine, antipyrine, benzoic acid, caffeine, lidocaine hydrochloride, methyl p-aminobenzoate, ethyl p-aminobenzoate, n-propyl p-aminobenzoate, n-butyl p-aminobenzoate, methyl paraben, ethyl paraben, n-propyl paraben, and n-butyl paraben were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Flurbiprofen was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). The MWs and octanol–water coefficients (log Ko/w) of the compounds are shown in Table 1. Silicone membranes were a kind gift from LINTEC Co., Ltd. (Tokyo, Japan). All other reagents were of HPLC grade and were used without further purification.
| No. | Compounds | MW | Log Ko/w |
|---|---|---|---|
| 1 | Antipyrine | 188.2 | −1.51 |
| 2 | Lidocaine hydrochloride | 270.8 | −0.90 |
| 3 | Caffeine | 194.2 | −0.12 |
| 4 | Aminopyrine | 231.3 | 1.10 |
| 5 | Methyl p-aminobenzoate | 151.6 | 1.38 |
| 6 | Lidocaine | 234.3 | 1.40 |
| 7 | Benzoic acid | 122.1 | 1.41 |
| 8 | Ethyl p-aminobenzoate | 165.1 | 1.89 |
| 9 | Methyl paraben | 152.2 | 1.93 |
| 10 | Ethyl paraben | 166.2 | 2.27 |
| 11 | n-Propyl p-aminobenzoate | 179.2 | 2.43 |
| 12 | n-Butyl p-aminobenzoate | 193.2 | 2.70 |
| 13 | Propyl paraben | 180.2 | 2.81 |
| 14 | Butyl paraben | 194.2 | 3.53 |
| 15 | Flurbiprofen | 244.3 | 3.86 |
MW, molecular weight.
An excess of each model compound was added to water and dissolved at 80°C. The suspensions (saturated solutions with excess solids) were stirred using a magnetic bar for 48 h in a water bath maintained at 32°C. The suspensions were then sampled and filtered through a syringe and filter unit (25-mm polyvinylidene fluoride [PVDF] filter media device, 0.45-µm pore size; Millipore). The obtained saturated solutions were adequately diluted with water for determination by ultra-performance liquid chromatography (UPLC).
Permeation Studies Using Silicone MembranesThe silicone membrane was set in a side-by-side two-chamber diffusion cell with an effective permeation area of 0.636 cm2 for determination of the cumulative amounts of model compounds from each suspension that permeated through the membrane. Phosphate-buffered saline pH 7.4 was used as a receiver solution (5.5 mL), except with lidocaine. Citrate-buffered saline (pH 5.0) and carbonated-buffered saline (pH 10.0) were used as lidocaine receiver solutions for symmetric conditions (same solvent in both donor and receptor compartments). Each solution was stirred using a stirrer bar and kept at 32°C by water circulation in a chamber jacket. At 1, 2, 3, 4, 5, 10, 20, 30, 40, and 60 min after application of the model compound, an aliquot (1.0 mL) was withdrawn from the receiver side, and the same volume of fresh buffer was added to maintain constant volume. The concentration of each model compound in the receiver solution was assayed by UPLC to determine the cumulative amount of each compound that permeated through the silicone membrane.
Calculation of Membrane Permeation ParametersP, DL−2, and KL, as permeation parameters, were calculated from the time course of the cumulative amount of compound that permeated through the membrane using the following equations.26)
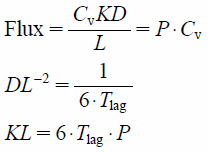 |
The concentration of each model compound was determined by UPLC. The UPLC system consisted of a pump (LC-30ADVP; Shimadzu, Kyoto, Japan), an ultraviolet-visible detector (SPD-20A; Shimadzu), analysis software (LabSolutions, Shimadzu), a system controller (CBM-20A; Shimadzu), an autosampler (SIL-30AC; Shimadzu) and a column oven (CTO-20A; Shimadzu). A Unison UK-C18 column (Imtakt, Kyoto, Japan) was used for lidocaine. A Sunniest C18-HT column (ChromaNik Technologies, Osaka, Japan) was used for methyl paraben, ethyl paraben, propyl paraben, butyl paraben, aminopyrine, antipyrine, caffeine, benzoic acid, and flurbiprofen, and a CAPCELL CORE C18 column (Shiseido, Tokyo, Japan) was used for methyl p-aminobenzoate, ethyl p-aminobenzoate, n-propyl p-aminobenzoate, and n-butyl p-aminobenzoate. All columns were maintained at a temperature of 40°C. Other conditions are listed in Table 2.
| Compounds | Mobile phase | Flow rate (mL/min) | Detection (nm) |
|---|---|---|---|
| Lidocaine | 0.1% phosphoric acid : acetonitrile (80 : 20) | 0.45 | 225 |
| Methyl paraben | 10 mM sodium acetate (pH 4.8 by acetic acid) : acetonitrile (80 : 20) →(20 : 80) | 0.20 | 230 |
| Ethyl paraben | |||
| Propyl paraben | |||
| Butyl paraben | |||
| Caffeine | 10 mM sodium acetate (pH 4.8 by acetic acid) : acetonitrile (80 : 20)→(20 : 80) | 0.45 | 254 |
| Benzoic acid | |||
| Antipyrine | |||
| Aminopyrine | |||
| Flurbiprofen | |||
| Methyl p-aminobenzoate | 20 mM sodium acetate (pH 4.6 by acetic acid) : acetonitrile (60 : 40) | 0.50 | 290 |
| Ethyl p-aminobenzoate | |||
| Propyl p-aminobenzoate | |||
| Butyl p-aminobenzoate |
Pearson’s correlation coefficient was used to characterize the relationship between skin permeation parameters in human and rat skin and the silicone membrane. Significance was to 5% in all evaluations.
P values of the model compounds for the silicone membrane are shown in Table 3. For the silicone membrane, P values of flurbiprofen and butyl paraben were high. In human and hairless rat skin, high P values were obtained for methyl p-aminobenzoate, ethyl p-aminobenzoate, n-propyl p-aminobenzoate, and n-butyl p-aminobenzoate. Low P values were obtained for lidocaine (pH 5.0), antipyrine, and caffeine through the silicone membrane, which corresponded with human and hairless rat skin. The P values of caffeine, which is hydrophilic, in the silicone membrane and in human and hairless rat skin were similar. In contrast, the P values of aminopyrine and n-propyl p-aminobenzoate, which are amphiphilic, in the silicone membrane were 10-fold higher than those in human and hairless rat skin. Furthermore, the P values of butyl paraben and flurbiprofen, which are lipophilic, in the silicone membrane were 100-fold higher than those in human and hairless rat skin.
| No. | Compounds | P (cm/s)×10−6 Silicone membrane | P (cm/s)×10−6 Human skina) | P (cm/s)×10−6 Hairless rat skina) |
|---|---|---|---|---|
| 1 | Antipyrine | 0.13±0.02 | 0.04±0.00b) | 0.04±0.00b) |
| 2 | Lidocaine hydrochloride | 0.40±0.02 | 0.05±0.02 | 0.04±0.01 |
| 3 | Caffeine | 0.22±0.03 | 0.18±0.02 | 0.26±0.02 |
| 4 | Aminopyrine | 3.32±0.17 | 0.33±0.02 | 0.29±0.11 |
| 5 | Methyl p-aminobenzoate | 56.0±8.20 | 18.4±0.83 | 7.22±0.17 |
| 6 | Lidocaine | 18.4±3.28 | 1.97±0.15 | 9.21±3.68 |
| 7 | Benzoic acid | 95.8±14.6 | 1.29±0.10b) | 1.29±0.14b) |
| 8 | Ethyl p-aminobenzoate | 128.0±25.5 | 20.1±2.24 | 10.6±0.25 |
| 9 | Methyl paraben | 18.0±1.64 | 2.49±0.03 | 2.39±0.31 |
| 10 | Ethyl paraben | 51.4±3.33 | 3.86±0.49 | 2.96±0.06 |
| 11 | n-Propyl p-aminobenzoate | 252.0±82.4 | 23.2±3.03 | 19.6±1.61 |
| 12 | n-Butyl p-aminobenzoate | 274.0±29.7 | 20.1±3.02 | 9.49±0.79 |
| 13 | Propyl paraben | 110.0±7.41 | 4.10±0.15 | 3.63±0.15 |
| 14 | Butyl paraben | 289.0±61.9 | 3.54±0.18 | 2.31±0.26 |
| 15 | Flurbiprofen | 333.3±43.2 | 1.39±0.00b) | 0.81±0.01b) |
Figures 1A and B show the relationships between log Ko/w and log P obtained from the experiments using the silicone membrane and human skin and hairless rat skin. The log P values of all membranes increased with the increasing lipophilicity of the compounds. In human and hairless rat skin, the relationships between log P and log Ko/w could be represented by a sigmoidal curve. Furthermore, a significant correlation between log P in the silicone membrane and log Ko/w (log Psilicone=0.721×log Ko/w−5.80; r=0.927) was observed (Fig. 1B).
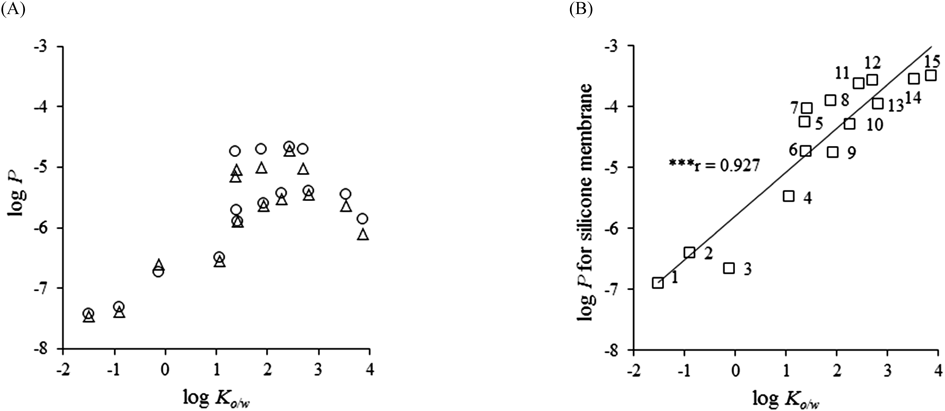
Symbols: silicone membrane (□), excised human skin (○), and excised hairless rat skin (Δ), Pearson’s correlation coefficient showed significance. *** p<0.001. Ko/w, octanol–water coefficient; P, permeability coefficient.
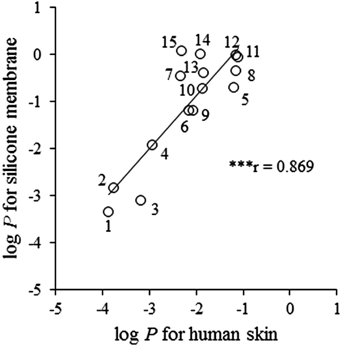
Figures 2A and B show the relationships between the log P values in human skin and the silicone membrane and in hairless rat skin and the silicone membrane, respectively. A significant correlation was observed between the log P values in human skin and silicone (r=0.869) and in hairless rat skin and silicone membrane (r=0.823). Therefore, it was found that the P through human or hairless rat skins could be predicted using log P in the silicone membrane. Linear regression analysis gave the following relationships: log Phuman=0.670×log Psilicone−2.63 and log Phairless rat=0.596×log Psilicone−3.05. Therefore, the ratios of the log P values in human or hairless rat skin versus the log P value in the silicone membrane differed according to the model compound. In the present study, the compounds with low log P values in the silicone membrane (1: antipyrine, 3: caffeine) showed a near order relative to the order in human and hairless rat skin, and the compounds with a high log P value in the silicone membrane (11: n-propyl p-aminobenzoate, 12: n-butyl p-aminobenzoate, 14: butyl paraben, 15: flurbiprofen) were one or two orders higher relative to the order in human and hairless rat skin.

A dashed line shows the relationships with a 1 : 1 correlation. Pearson’s correlation coefficient showed significance. *** p<0.001. P, permeability coefficient.
Figure 3A shows the relationships between log Ko/w and log DL−2 in the silicone membrane, human skin, and hairless rat skin. The log DL−2 values in human and hairless rat skin ranged from −4.90 to −3.69 despite the variation in the log Ko/w of the compounds, whereas those in the silicone membrane ranged from −3.50 to −2.10. Figure 3B shows the relationship between log Ko/w and log KL in the silicone membrane, human skin, and hairless rat skin. The log KL values in each membrane showed the same tendency as shown for P. Briefly, the log KL of all membranes increased with increasing lipophilicity of the chemical compounds, and the relationship between log KL and log Ko/w could be represented by a sigmoidal curve. Furthermore, log KL in the silicone membrane showed a significant correlation with log Ko/w (log KLsilicone=0.941×log Ko/w−3.21; r=0.941).
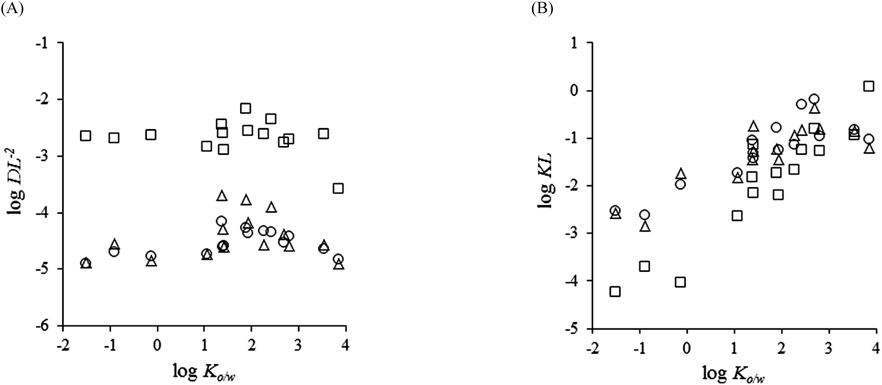
Symbols: silicone membrane (□), excised human skin (○) and excised hairless rat skin (Δ). DL−2, diffusion parameter; KL, partition parameter; Ko/w, octanol–water coefficient.
Figures 4A and B show the relationships between the log DL−2 values in the silicone membrane and human skin or hairless rat skin. Significant correlations were observed between the log DL−2 values in human skin and the silicone membrane (r=0.625) and between those in hairless rat skin and the silicone membrane (r=0.699). DL−2 values of the model compounds, except flurbiprofen, which is lipophilic, in the silicone membrane were approximately 100-fold higher than those in human and hairless rat skin.
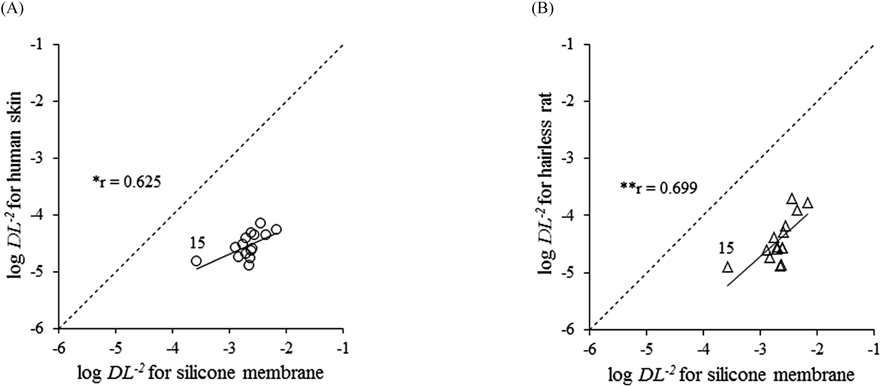
A dashed line shows the relationships with a 1 : 1 correlation. Pearson’s correlation coefficient showed significance. * p<0.05, ** p<0.01. DL−2, diffusion parameter.
Figures 5A and B show the relationships of log KL values between the silicone membrane and human skin or hairless rat skin. A significant correlation was observed between the log KL values in human skin and the silicone membrane (r=0.815) and those in hairless rat skin and the silicone membrane (r=0.791). Linear regression analysis showed the following relationships: log KLhuman=0.424×log KLsilicone−0.396 and log KLhairless rat=0.435×log KLsilicone−0.484. The log KL ratios in human or hairless rat skin versus the silicone membrane differed according to the model compound in a manner similar to that of the log P value. The compounds with a low log KL in the silicone membrane (1: antipyrine, 3: caffeine) had ratios approximately 100-fold higher than those in human and hairless rat skin, and the compounds with a high log KL in the silicone membrane (12: n-butyl p-aminobenzoate, 14: butyl paraben, 15: flurbiprofen) had approximately 10-fold lower, similar, or 10-fold higher ratios than those in human and hairless rat skin.

A dashed line shows the relationships with a 1 : 1 correlation. Pearson’s correlation coefficient showed significance. *** p<0.001. KL, partition parameter.
In vitro permeation studies using excised human and animal skins are very useful for understanding skin permeation profiles and skin concentrations of topically applied chemicals.27,28) However, in vitro experiments using human skin are expensive and sometimes limited because of ethical issues and the low availability of samples. Furthermore, animal testing to evaluate the safety and efficacy of cosmetic ingredients has been banned by Cosmetic Directive 76/768/EEC. For this reason, an alternative membrane for evaluating the permeability of compounds through human and animal skin is required.
P and J values are useful parameters to estimate the blood concentration of topically applied drugs. On the other hand, cosmetic products are expected mainly to have local effects. Thus, measurement of the skin concentration of topically applied chemicals as well as in vitro skin permeation should be evaluated to develop an optimal formulation. Sugibayashi et al. and Hatanaka et al. reported that steady-state chemical concentration in the skin after topical application could be calculated using P and K values obtained from the skin permeation profile.14,29) Thus, understanding of changes in DL−2 and KL against increases in log Ko/w of chemical compounds could be revealed using a silicone membrane to develop an alternative to animal experiments for the evaluation of skin concentrations of compounds. In addition, change in these parameters would provide useful quantitative information regarding development of topical formulation. However, the study with silicone membrane was not really investigated. That is the reason for the observation of the relationship in DL−2 and KL between silicone membrane and human or animal skin in the present study.
Previously, the permeabilities in human skin and hairless rat skin have been shown to correlate with those in alternative membranes, such as a three-dimensional cultured human skin model,5–7) PAMPA,8–10) and Strat-M™.11) On the other hand, silicone membranes have long been available as an alternative membrane in in vitro permeation studies.12) Correlation of P between human skin and a silicone membrane was first reported in 2002 for seven model compounds with MWs between 94.11 (phenol) and 234.40 (lidocaine) and log Ko/w values from 1.23 (nicotine) to 2.59 (4-bromophenol).16) Furthermore, the silicone membrane P, KP(sil) (cm/h), has been calculated using the following equation with human epidermal P, KP(epi), log KP(sil)=1.15 (±0.36)×log KP(epi)+1.29 (±0.58). Figure 6 shows the relationship between the silicone membrane P, Psilicone (cm/h), and human skin P, Phuman (cm/h), obtained in this study. The linear regression equation in the present study was log Psilicone=1.13×log Phuman+1.38; r=0.869. A previous study obtained a linear correlation equation similar to that obtained in the present study, which used 15 model compounds with MWs between 122.12 (benzoic acid) and 270.80 (lidocaine hydrochloride) and log Ko/w values from −1.50 (antipyrine) to 3.86 (flurbiprofen).
Pearson’s correlation coefficient showed significance. *** p<0.001. P, permeability coefficient.
Sloan et al. reported that the J is the only measure of delivery through a membrane that is clinically relevant, because pharmacological activity depends on the amount of exposure and then interacts with a receptor or enzyme. Hence, Sloan et al. assessed the effectiveness of silicone as an alternative membrane using J values.18,30) Furthermore, they proposed the following Roberts–Sloan equation for predicting J from solubility in water (SAQ), solubility in octanol (SOCT) and MW: Log J (Cal.)=−1.893+0.852×log SOCT+0.148×log SAQ−0.00524×MW. Figure 7 shows the relationship between the experimental J and calculated J obtained from the Roberts–Sloan equation using 13 model compounds in the present study. The J values of these 13 model compounds ranged from −1 to 1, and the range was narrow in comparison with that reported by Sloan et al. (from −3 to 3). However, a significant correlation was observed between the experimental flux (JExp.) and calculated flux (JCal.) obtained from the Roberts–Sloan equation in the silicone membrane and similar values were obtained. Consequently, the silicone membrane used in the present study appeared to have the same properties as those of silicone membranes (Silatos™, Silastic®, polydimethylsiloxane: Pillar Surgical) used in previous studies.

Pearson’s correlation coefficient showed significance. *** p<0.001. J, flux.
Alternative membranes that have been used to evaluate skin permeation parameters, such as P, DL−2, and KL, include an artificial membrane, Strat-M™, and a three-dimensional cultured skin model, TESTSKIN LSE-high.5,11) The log Ko/w of the model compounds in the present study ranged from −1.5 to 3.8, which were similar to those of model compounds used in previous reports. The log P and log DL−2 values in Strat-M™ were slightly faster than those in human and hairless rat skins. Furthermore, the log KL values in Strat-M™ were similar to those in human and hairless rat skins. On the other hand, the P and DL−2 values in TESTSKIN LSE-high were 10-fold higher than those in human and hairless rat skins. The log KL values in TESTSKIN LSE-high were similar to those in human and hairless rat skins. KL values in both Strat-M™ and TESTSKIN LSE-high were similar to those in human and hairless rat skins, but the diffusivity values in both Strat-M™ and TESTSKIN LSE-high were different. This result was attributed to differences in skin permeability.
In contrast, P in the silicone membrane correlated with those in human and hairless rat skin, but the log P ratios in human or hairless rat skin versus the silicone membrane differed according to the model compounds (Fig. 2). Flynn proposed a number of algorithms to predict P and showed that log Ko/w and MWs were relevant parameters.31) For example, in the case of compounds in which the MWs exceed 150, if log Ko/w is <0.5, log P (cm/h) will be expected to be −5; and if log Ko/w is >3.5, log P will be expected to be −1.5; and if log Ko/w is between 0.5 and 3.5, log P will be expected to be log Ko/w −5.5. That is, in the case of compounds with MWs >150 and log Ko/w values ranging from 0.5 to 3.5, the values of log P increase linearly as the values of log Ko/w increase. The relationships between log P, log KL, and log DL−2 in a silicone membrane and in human skin using the model compounds within these limits are shown in Fig. 8. A significant correlation was observed between log P (r=0.911), log KL (r=0.915), and log DL−2 (r=0.723) values in human skin and silicone membrane. The correlation coefficients became higher than those calculated for all of the examined model compounds. Linear regression analysis showed the following relationships: log Phuman=0.915×log Psilicone−0.396, log KLhuman=0.813×log KLsilicone−0.421, log DL−2human=0.619×log DL−2silicone−2.83. When compounds that have P values in humans that increase linearly with log Ko/w are used, the P values with a silicone membrane were 10-fold higher than those in humans, the KL values in the silicone membrane were 10-fold lower than those in humans, and DL−2 values for silicone were 100-fold higher than those in humans.
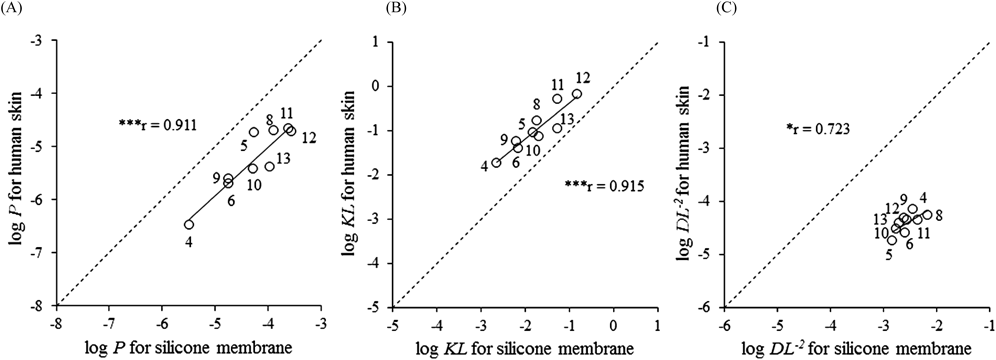
Model compounds with MWs>150, 0.5≤log Ko/w≤3.5. A dashed line shows the relationships with a 1 : 1 correlation. Pearson’s correlation coefficient showed significance. * p<0.05, *** p<0.001. DL–2, diffusion parameter; KL, partition parameter; Ko/w, octanol–water coefficient; MW, molecular weight; P, permeability coefficient.
On the other hand, the P values of antipyrine and caffeine, which are hydrophilic (log Ko/w<0.5), in the silicone membrane and human skin were similar. The KL values in the silicone membrane were 100-fold lower than those in human skin, and the DL−2 values in the silicone membrane were 100-fold higher than those in human skin. Because the silicone membrane has no hydrophilic pathway, such as hair follicles and sweat ducts as in human and animal skin, the KL values of the hydrophilic compounds in the silicone membrane were lower than those in humans. However, because the DL−2 values for silicone were higher than those in humans, it was thought that the P values calculated as the product of KL and DL−2 were similar. Furthermore, the P values of butyl paraben and flurbiprofen, which are lipophilic (log Ko/w>3.5), in the silicone membrane were 100-fold higher than those in human skin. The KL value of butyl paraben in the silicone membrane was similar to that in human skin, and the DL−2 value in the silicone membrane was 100-fold higher than that in human skin. On the other hand, the KL and the DL−2 values of flurbiprofen (log Ko/w: 3.86) with the silicone membrane was 10-fold higher than those in human skin. As a result, the P values of flurbiprofen through the silicon membrane were 100-fold higher than other model compounds. Although the KL values of butyl paraben and flurbiprofen in human skin showed almost similar values, those with the silicone membrane increased with an increase of log Ko/w values. These findings can be explained by the fact that the lipophilicity of the silicone membrane was higher than that in human skin.
In this study, we investigated the relationships between the permeabilities of silicone membranes and human and hairless rat skin using P, DL−2, and KL. Similar to the findings in previous studies, we also showed a good correlation between log P values in the silicone membrane and human skin, and that the calculated flux obtained from the Roberts–Sloan equation was similar to the experimental flux. We also found that the relationship between the permeabilities of the silicone membrane and human or hairless rat skins differed depending on the lipophilicity of the model compounds. Moss et al. reported that silicone membrane permeability is essentially different from that of human skin.19,20) The log Ko/w of the model compounds showed wide lipophilicity range from 0.84 to 5.36. On the other hand, in the present study, significance correlations of permeation parameters between silicone membrane and human or animal skin in the lipophilicity range of 0.5≤log Ko/w≤3.5 of model compounds. This result suggested that difference in the lipophilicity range for the applied model compound might affect the usefulness of silicone membrane.
The skin consists of the stratum corneum, which is lipophilic, and viable epidermis and dermis, which are hydrophilic, so it is a heterogeneous membrane that also has sweat glands and pores. Because the silicone membrane was a homogeneous membrane, the permeabilities of the membrane and of human and hairless rat skin were different. The characteristics of the silicone membrane are homogeneous; therefore, it is not necessary to consider its effect on skin permeation due to individual differences, such as needed for human skin or animal skin. Therefore, it is effective to consider the effect of the formulations and vehicles that act on the stratum corneum.32,33) Because DL−2 of the silicone membrane in this study was 100-fold higher, regardless of the polarity of the compound, it has been found that permeability can be evaluated in a shorter time. Therefore, it was suggested that a silicone membrane is useful for high-throughput screening prior to in vitro permeation studies. In addition, KL of the silicone membrane showed higher and lower values compared with human skin for hydrophilic and lipophilic compounds, respectively. These results suggested that underestimation for lipophilic chemicals or overestimation for hydrophilic chemicals in skin concentration might occur. Thus, evaluation of DL−2 and KL as well as P of the silicone membrane could be useful to analyze the obtained permeation data in detail. The present results would be helpful to understand the permeation mechanism of applied compounds and to develop a topical formulation with permeation study with silicone membrane. Further experiments should be done with various types of hydrophilic and lipophilic bases to understand the possibilities for the use of silicone membrane as an alternative to animal experiment in the evaluation of skin permeation.
The permeation parameters of chemical compounds through a silicone membrane were calculated from the obtained permeation profiles, in particular for chemical compounds with MWs between 122 and 271 and log Ko/w values from −1.5 to 3.9. For the hydrophilic compounds in this study, including antipyrine and caffeine, KL values for the silicone membrane were 100-fold lower than those in human and hairless rat skin, and P values for the silicone membrane were similar to those in human and hairless rat skin. On the other hand, in the hydrophobic model compounds, including n-butyl paraben and flurbiprofen, KL values for the silicone membrane were similar to or 10-fold higher than those in human and hairless rat skin, and P values for the silicone membrane were 100-fold higher than those in human and hairless rat skin. The permeation parameters of model compounds with MWs between 151.16 (methyl p-aminobenzoate) and 234.34 (lidocaine) and log Ko/w values from 1.10 (aminopyrine) to 2.81 (propyl paraben) for the silicone membrane and human or hairless rat skin showed a significant correlation. Therefore, silicone membranes can be used to evaluate the permeability of model compounds. However, depending on the lipophilicity of the model compounds, under- or overestimation of permeability relative to those in human skin or hairless rat skin may occur.
The authors declare no conflict of interest.