2016 Volume 64 Issue 9 Pages 1356-1363
2016 Volume 64 Issue 9 Pages 1356-1363
In the present study, a novel series of 2-(2-(3-aryl-5-substituted-1,3,4-thiadiazol-2(3H)-ylidene)hydrazinyl)-4,4-diphenyl-1H-imidazol-5(4H)-one derivatives were designed and prepared via the reaction of the most versatile, hitherto unreported 2-(5-oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)-N-phenylhydrazinecarbothioamide with the appropriate hydrazonoyl halides. In addition, some thiazole derivatives were prepared. The structures of the newly synthesized compounds were established based on spectroscopic evidences and their alternative syntheses. Some of the newly synthesized compounds have been evaluated for their anticancer activity against a liver carcinoma cell line HEPG2-1. Moreover, their structure–activity relationship (SAR) was explored for further development in this area. The results indicated that many of the tested compounds showed moderate to high anticancer activity with respective to doxorubicin as a reference drug. Consequently, the new synthesized series of thiadiazole–imidazole derivatives are considered as powerful anticancer agents.
There are plenty of imidazole containing drugs, such as angiotensin II antagonistic,1–4) antimalarial,5) antibacterial,6,7) anticancer,8) anti-inflammatory9) and anticytokine agents.10) Moreover, many literature reviews11–13) showed that the 1,3,4-thiadiazole nuclei and annelated 1,3,4-thiadiazoles have antimicrobial, anti-inflammatory, anticancer, anticonvulsant, antidepressant, antioxidant, radio protective, and anti-leishmanial activities. These important biological activities encouraged several research groups to find out different methods for synthesis of new thiadiazoles using different synthones, such as thiosemicarbazides, thiocarbazides, dithiocarbazates, thioacylhydrazines, acylhydrazines, and bithioureas.14)
In continuation of our studies dealing with the utility of hydrazonoyl halides for synthesis of various bridgehead nitrogen polyheterocycles,15–24) we wish to report herein a new facile synthesis of various functionalized derivatives of 1,3,4-thiadiazoles bearing imidazole moiety that have not been reported hitherto as potential anticancer agents.
2-(5-Oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)-N-phenylhydrazinecarbothioamide (2) was prepared via reaction of 2-hydrazinyl-4,4-diphenyl-1H-imidazol-5(4H)-one (1)6) with phenyl isothiocyanate in EtOH as depicted in Chart 1. The structure of the product was confirmed based on its spectral data. For example, the IR spectrum showed absorption bands at ν=3428–3270, 3217 (4NH) and 1657 (C=O) cm−1; 1H-NMR revealed the presence of five signals (ArH, 4NH); in addition to the mass spectrum which exhibited a peak at m/z 401 (M+).

The behavior of the N-phenylhydrazinecarbothioamide derivative 2 towards different hydrazonoyl chlorides was investigated. Thus, when compound 2 was treated with N′-phenylbenzohydrazonoyl chloride (3) in EtOH, the presence of a catalytic amount of triethylamine (TEA), afforded a single product identified as the corresponding 2-(2-(3,5-diphenyl-1,3,4-thiadiazol-2(3H)-ylidene)hydrazinyl)-4,4-diphenyl-1H-imidazol-5(4H)-one (5) (Chart 1). The structure 5 was elucidated on the basis of spectral analysis and elemental analysis (see Experimental).
The structure assigned for product 5 was further evidenced via its alternative method. Thus, reaction of 2-mercapto-4,4-diphenyl-1H-imidazol-5(4H)-one (6) with 2-hydrazono-3,5-diphenyl-2,3-dihydro-1,3,4-thiadiazole (7) in ethanol under reflux, afforded a product which is typical in all respects with that obtained from the reaction of 2 with 3 (Chart 1).
The reaction proceeded through the alkylation of thiol group in thioamide moiety to give the non-isolable intermediates 4. Intramolecluar cyclization of intermediate 4 followed by elimination of aniline molecule afforded the 1,3,4-thiadiazole 5.
Similarly, treatment of compound 2 with 2-oxo-N′-arylpropanehydrazonoyl chlorides 8a–h afforded the respective 1,3,4-thiadiazoles 10a–h rather than thiadiazine 11a–h or 1,3-thiazole 12a–h (Chart 2). 1,3,4-Thiadiazole 10a–h was formed through intramolecluar cyclization of NH group in hydrazone moiety with an activated imino group in the non-isolable intermediates 9a–h, followed by elimination of aniline molecule gave the respective 1,3,4-thiadiazole 10a–h (Chart 2). The structures of products 10a–h were established based on analytical and spectral data (see Experimental). The 1H-NMR spectrum of compound 10a, taken as a typical example, showed 4 signals at δ: 2.45 (s, 3H, CH3), 7.08–7.45 (m, 15H, Ar-H), 10.38, 11.20 (2s, br, 2H, 2NH, D2O-exchangeable). Also 13C-NMR spectrum revealed two signals at δ: 25.17 and 194.1 assignable for CH3CO group. The mass spectra revealed, in each case, a peak corresponds to the molecular ion which is consistent with the expected molecular formula.

The structure of 10 was proved chemically via an alternative method (Chart 2). Thus, the reaction of methyl 2-(5-oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)hydrazinecarbodithioate (13) with 8a in ethanol in the presence of TEA at reflux led to formation of product which is identical in all respects (mp, mixed mp and IR) with compound 10a.
In a similar manner, ethyl 2-chloro-2-(2-arylhydrazono)acetate 14a–c reacted with the compound 2 to furnish the corresponding 1,3,4-thiadiazole derivatives 16a–c (Chart 3).
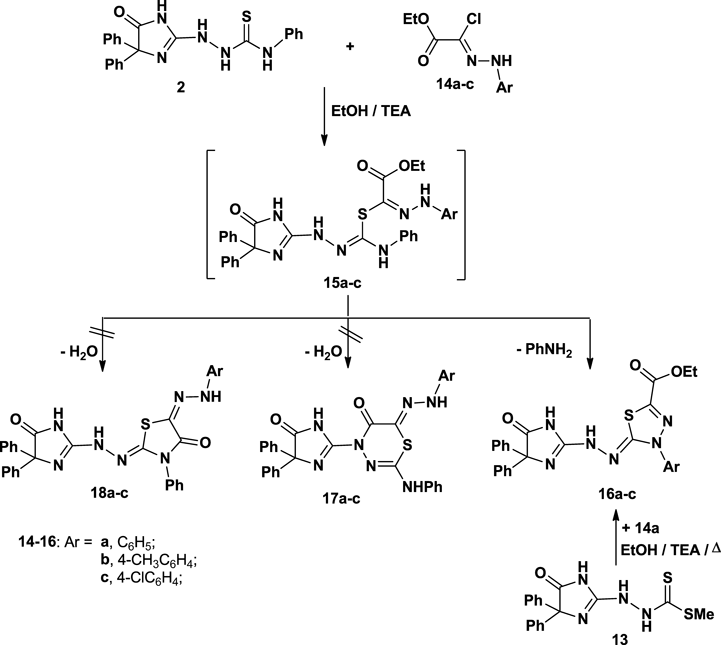
As can be seen in Experimental, the IR, 1H- and 13C-NMR spectra of 16a taken as an example of the prepared series, showed the presence of the ester CO2CH2CH3 group and disappearance of the hydrazone-NH function ruling out the other structure 15a. The mass spectrum of the reaction products 16a–c showed, in each case, a peak corresponding to their molecular ions.
Also, the structure of 16 was proved chemically via an alternative method (Chart 3). Thus, the reaction of compound 13 with 14a, in ethanol in the presence of TEA at reflux, led to formation of product, which is identical in all respects (mp, mixed mp and IR) with compound 16a.
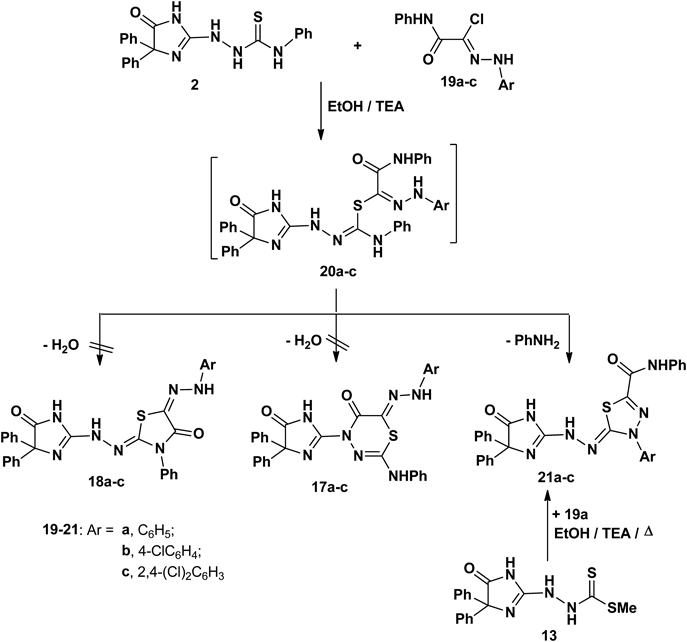
Also, 2-oxo-N′-aryl-2-(phenylamino)acetohydrazonoyl chloride 19a–c reacted with the compound 2 to furnish the corresponding 1,3,4-thiadiazole derivatives 21a–c (Chart 4). The 1H-NMR spectrum of compound 21a, revealed a signal corresponding to the carboxamide proton at 11.73 ppm, in addition to an aromatic multiplet in the region 7.02–7.78 ppm. The mass spectrum of compound 21a revealed a molecular ion peak at m/z=545 which is in agreement with the proposed structure (see Experimental). In addition, compound 21 was proved chemically via an alternative method from the reaction of compound 13 with 19a led to formation of product, which is identical in all respects (mp, mixed mp and IR) with compound 21a (Chart 4).
Furthermore, thioamide derivative 2 reacted with ethyl chloroacetate (22) under thermal conditions in EtOH in the presence of TEA to afford a single product 23 that was identified as 2-(2-(5-oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)hydrazono)-3-phenylthiazolidin-4-one as outlined in Chart 5. The structure of the isolated product 23 was inferred from its elemental analysis and spectral data. Its IR spectrum showed absorption bands at ν=3383, 3180 (2NH), and 1737, 1650 (2C=O) cm−1, its 1H-NMR spectrum revealed singlet signal at δ: 3.96 ppm assignable to thiazolidinone (CH2) group (see Experimental).
Alternatively, compound 2 reacted with 2-chloro-3-oxo-N-phenylbutanamide (24) in refluxing EtOH in the presence of TEA to afford 4-methyl-2-(2-(5-oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)hydrazono)-N,3-diphenyl-2,3-dihydrothiazole-5-carboxamide (25) as outlined in Chart 5. The structure of compound 25 was elucidated based on their elemental analysis and spectral data (see Experimental).

Finally, TEA catalyzed reaction of the phenacyl bromide 26 with compound 2 in refluxing ethanol afforded one isolable product 27 named as 2-(2-(3,4-diphenylthiazol-2(3H)-ylidene)hydrazinyl)-4,4-diphenyl-1H-imidazol-5(4H)-one (Chart 5). The structure of the product 27 was established based on its elemental analysis and spectral data (see Experimental).
Anticancer Activity EvaluationThe antitumor activity of the newly synthesized compounds was determined against a liver carcinoma cell line HEPG2-1. Doxorubicin was used as a reference standard and showed IC50=0.72 µM against a liver carcinoma cell line. Data generated were used to plot a dose–response curve of which the concentration (µM) of test compounds required to kill 50% of cell population (IC50) was determined. Cytotoxic activity was expressed as the mean IC50 of three independent experiments.
The results revealed that most of the tested compounds showed a great variable activity compared to reference drug as shown in Table 1. The descending order of activity of the newly synthesized compounds was as follow: 16c>21c>10g>21b>10h>10d>16a>21a>10f>10e>16b>10a>10b>10c>5.
 | |||
|---|---|---|---|
| Compd. No. | R | X | IC50 (µM) |
| 5 | Ph | H | 74.20 |
| 10a | Ac | H | 11.26 |
| 10b | Ac | 4-Me | 19.06 |
| 10c | Ac | 4-OMe | 51.15 |
| 10d | Ac | 4-Cl | 4.20 |
| 10e | Ac | 3-Cl | 7.43 |
| 10f | Ac | 4-Br | 5.25 |
| 10g | Ac | 4-NO2 | 1.08 |
| 10h | Ac | 2,4-(Cl)2 | 1.44 |
| 16a | CO2Et | H | 4.25 |
| 16b | CO2Et | 4-Me | 7.81 |
| 16c | CO2Et | 4-Cl | 0.86 |
| 21a | CONHPh | H | 5.82 |
| 21b | CONHPh | 4-Cl | 1.17 |
| 21c | CONHPh | 2,4-(Cl)2 | 1.02 |
| Doxorubicin | — | — | 0.72 |
Examination of the structure–activity relationship (SAR) leads to the following conclusions (Chart 6).
•The 1,3,4-thiadiazole derivatives 16c, 21c, 10g, 21b and 10h (IC50=0.86, 1.02, 1.08, 1.17, 1.44 µM, respectively) have promising antitumor activity against liver carcinoma cell line (HEPG2-1) while 1,3,4-thiadiazole derivatives 10d, 16a, 21a, 10f, 10e, 16b, 10a and 10b have moderate activity (IC50=4.20–19.06 µM). On the other hand, 1,3,4-thiadiazole derivative 10c and 5 has poor antitumor activity against liver carcinoma cell line (IC50>50 µM).
•For substituent at position 2 of 1,3,4-thiadiazole: the ester group (CO2Et) gives higher activity than the amide group (CONHPh) than the acetyl group (Ac) than the phenyl moiety (Ph).

Melting points were measured with an Electrothermal IA 9000 series digital melting point apparatus. IR spectra were recorded in potassium bromide discs on Pye Unicam SP 3300 and Shimadzu FTIR 8101 PC infrared spectrophotometers. NMR spectra were recorded on a Varian Mercury VX-300 NMR spectrometer operating at 300 MHz (1H-NMR) and run in deuterated dimethyl sulfoxide (DMSO-d6). Chemical shifts were related to that of the solvent. Mass spectra were recorded on a Shimadzu GCeMS-QP1000 EX mass spectrometer at 70 eV. Elemental analyzes were measured by using a German made Elementar Vario LIII CHNS analyzer. Antitumor activity was evaluated by the Regional Center for Mycology and Biotechnology, Al-Azhar University, Cairo, Egypt. Hydrazonoyl halides25) were prepared as previously reported in the respective literature.
Synthesis of 2-(5-Oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)-N-phenylhydrazine Carbothioamide (2)A mixture of 2-hydrazinyl-4,4-diphenyl-1H-imidazol-5(4H)-one (1) (2.66 g, 10 mmol) and phenyl isothiocyanate (1.35 g, 10 mmol) in absolute ethanol (50 mL) was refluxed for 4 h. The formed solid after cooling was filtered off, washed with ethanol, dried and finally crystallized from ethanol to give pure product of compound 2, as yellow crystals (74%); mp=175–177°C; IR (KBr) ν: 3428–3270, 3217 (4NH), 3085, 3059, 2916 (CH), 1657 (C=O), 1596 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 7.11–7.64 (m, 15H, Ar-H), 9.02, 9.22, 10.03, 11.32 (4s, br, 4H, 4NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 71.0, 123.7, 124.4, 126.7, 127.9, 128.1, 128.4, 139.0, 140.2, 158.3, 166.9, 180.3; MS m/z (%): 401 (M+, 35), 308 (100), 247 (42), 165 (98), 77 (64). Anal. Calcd for C22H19N5OS (401.13): C, 65.81; H, 4.77; N, 17.44. Found: C, 65.73; H, 4.62; N, 17.28%.
Synthesis of 1,3,4-Thiadiazole Derivatives 5, 10a–i, 16a–c and 21a–cA mixture of compound 2 (0.401 g, 1 mmol) and the appropriate hydrazonoyl halides 3, 8a–i, 14a–c, and 19a–c (1 mmol) in ethanol (20 mL) containing TEA (0.1 g, 1 mmol) was refluxed for 4–8 h. (monitored by TLC). The formed precipitate was isolated by filtration, washed with methanol, dried and recrystallized from N,N-dimethylformamide (DMF) to give products 5, 10a–i, 16a–c and 21a–c, respectively. The physical properties and spectral data of the obtained products are listed below.
2-(2-(3,5-Diphenyl-1,3,4-thiadiazol-2(3H)-ylidene)hydrazinyl)-4,4-diphenyl-1H-imidazol-5(4H)-one (5)Yellow solid (70%); mp 230–232°C; IR (KBr) ν: 3414, 3248 (2NH), 3027, 2937 (CH), 1650 (C=O), 1599 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 7.16–7.92 (m, 20H, Ar-H), 9.06, 10.47 (2s, br, 2H, 2NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 75.3, 112.3, 115.5, 118.6, 123.7, 126.8, 127.6, 128.1, 128.6, 131.4, 131.7, 132.0, 132.7, 136.1, 138.2, 149.5, 166.3; MS m/z (%): 502 (M+, 17), 354 (65), 311 (50), 247 (68), 117 (51), 77 (100), 59 (90). Anal. Calcd for C29H22N6OS (502.16): C, 69.30; H, 4.41; N, 16.72. Found C, 69.19; H, 4.33; N, 16.59%.
2-(2-(5-Acetyl-3-phenyl-1,3,4-thiadiazol-2(3H)-ylidene)hydrazinyl)-4,4-diphenyl-1H-imidazol-5(4H)-one (10a)Yellow solid (75%); mp 188–190°C; IR (KBr) ν: 3434, 3152 (2NH), 3022, 2957 (CH), 1712, 1649 (2C=O), 1597 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 2.45 (s, 3H, CH3), 7.08–7.45 (m, 15H, Ar-H), 10.38, 11.20 (2s, br, 2H, 2NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 25.1, 72.4, 114.4, 114.9, 123.5, 125.3, 128.9, 129.6, 133.4, 134.7, 141.9, 145.3, 151.7, 167.9, 194.1; MS m/z (%): 468 (M+, 14), 354 (69), 247 (73), 149 (43), 92 (100), 65 (61). Anal. Calcd for C25H20N6O2S (468.14): C, 64.09; H, 4.30; N, 17.94. Found C, 64.01; H, 4.19; N, 17.78%.
2-(2-(5-Acetyl-3-(p-tolyl)-1,3,4-thiadiazol-2(3H)-ylidene)hydrazinyl)-4,4-diphenyl-1H-imidazol-5(4H)-one (10b)Yellow solid (77%); mp 165–167°C; IR (KBr) ν: 3442, 3157 (2NH), 3021, 2920 (CH), 1719, 1646 (2C=O), 1597 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 2.38 (s, 3H, CH3), 2.44 (s, 3H, CH3), 7.18–7.64 (m, 14H, Ar-H), 10.31, 11.20 (2s, br, 2H, 2NH, D2O-exchangeable); MS m/z (%): 482 (M+, 11), 382 (63), 261 (70), 106 (100), 80 (59), 64 (68). Anal. Calcd for C26H22N6O2S (482.15): C, 64.71; H, 4.60; N, 17.42. Found C, 64.59; H, 4.47; N, 17.30%.
2-(2-(5-Acetyl-3-(4-methoxyphenyl)-1,3,4-thiadiazol-2(3H)-ylidene)hydrazinyl)-4,4-diphenyl-1H-imidazol-5(4H)-one (10c)Yellow solid (70%); mp 159–161°C; IR (KBr) ν: 3431, 3152 (2NH), 3029, 2957 (CH), 1712, 1649 (2C=O), 1597 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 2.45 (s, 3H, CH3), 3.76 (s, 3H, OCH3), 7.00–7.59 (m, 14H, Ar-H), 10.37, 11.30 (2s, br, 2H, 2NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 24.9, 55.3, 72.9, 114.9, 116.2, 122.5, 127.5, 129.6, 133.4, 134.7, 135.3, 141.9, 144.6, 156.0, 167.6, 194.3; MS m/z (%): 498 (M+, 36), 318 (53), 352 (61), 247 (66), 92 (100), 64 (73). Anal. Calcd for C26H22N6O3S (498.15): C, 62.64; H, 4.45; N, 16.86. Found C, 62.61; H, 4.33; N, 16.65%.
2-(2-(5-Acetyl-3-(4-chlorophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)hydrazinyl)-4,4-diphenyl-1H-imidazol-5(4H)-one (10d)Yellow solid (76%); mp 215–217°C; IR (KBr) ν: 3416, 3166 (2NH), 3028, 2950 (CH), 1716, 1648 (2C=O), 1598 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 2.42 (s, 3H, CH3), 7.18–7.73 (m, 14H, Ar-H), 10.21, 11.74 (2s, br, 2H, 2NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 25.2, 72.7, 116.4, 124.1, 125.6, 126.9, 127.4, 128.3, 128.8, 129.2, 129.3, 130.1, 140.3, 167.9, 194.5; MS m/z (%): 504 (M++2, 5), 502 (M+, 16), 328 (19), 208 (75), 190 (96), 80 (57), 64 (100). Anal. Calcd for C25H19ClN6O2S (502.10): C, 59.70; H, 3.81; N, 16.71. Found C, 59.57; H, 3.68; N, 16.55%.
2-(2-(5-Acetyl-3-(3-chlorophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)hydrazinyl)-4,4-diphenyl-1H-imidazol-5(4H)-one (10e)Yellow solid (71%); mp 183–185°C; IR (KBr) ν: 3436, 3152 (2NH), 3018, 2979, 2929 (CH), 1710, 1651 (2C=O), 1596 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 2.43 (s, 3H, CH3), 6.98–7.49 (m, 14H, Ar-H), 10.06, 11.04 (2s, br, 2H, 2NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 25.3, 72.6, 113.4, 118.1, 122.4, 126.7, 127.6, 128.1, 128.6, 130.3, 131.1, 133.9, 135.7, 143.9, 155.6, 167.5, 192.8; MS m/z (%): 504 (M++2, 3), 502 (M+, 12), 404 (52), 238 (72), 190 (84), 80 (52), 64 (100). Anal. Calcd for C25H19ClN6O2S (502.10): C, 59.70; H, 3.81; N, 16.71. Found C, 59.57; H, 3.69; N, 16.58%.
2-(2-(5-Acetyl-3-(4-bromophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)hydrazinyl)-4,4-diphenyl-1H-imidazol-5(4H)-one (10f)Yellow solid (71%); mp 183–185°C; IR (KBr) ν: 3422, 3156 (2NH), 3063, 2924 (CH), 1707, 1658 (2C=O), 1597 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 2.42 (s, 3H, CH3), 6.96–7.53 (m, 14H, Ar-H), 10.13, 11.19 (2s, br, 2H, 2NH, D2O-exchangeable); MS m/z (%): 548 (M++2, 10), 546 (M+, 11), 512 (95), 466 (90), 327 (100), 172 (50), 91 (85), 64 (55). Anal. Calcd for C25H19BrN6O2S (546.05): C, 54.85; H, 3.50; N, 15.35. Found C, 54.69; H, 3.37; N, 15.19%.
2-(2-(5-Acetyl-3-(4-nitrophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)hydrazinyl)-4,4-diphenyl-1H-imidazol-5(4H)-one (10g)Yellow solid (71%); mp 246–248°C; IR (KBr) ν: 3428, 3153 (2NH), 3052, 2926 (CH), 1717, 1655 (2C=O), 1599 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 2.46 (s, 3H, CH3), 7.07–7.66 (m, 14H, Ar-H), 10.01, 11.37 (2s, br, 2H, 2NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 25.0, 72.7, 114.6, 121.3, 124.9, 125.7, 125.8, 133.3, 138.5, 141.6, 143.7, 144.5, 151.6, 167.2, 192.3; MS m/z (%): 513 (M+, 22), 448 (53), 327 (69), 238 (63), 153 (100), 92 (83), 64 (70). Anal. Calcd for C25H19N7O4S (513.12): C, 58.47; H, 3.73; N, 19.09. Found C, 58.35; H, 3.54; N, 19.00%.
2-(2-(5-Acetyl-3-(2,4-dichlorophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)hydrazinyl)-4,4-diphenyl-1H-imidazol-5(4H)-one (10h)Yellow solid (75%); mp 213–215°C; IR (KBr) ν: 3426, 3166 (2NH), 3052, 2938 (CH), 1711, 1652 (2C=O), 1598 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 2.40 (s, 3H, CH3), 7.18–7.71 (m, 13H, Ar-H), 10.25, 10.89 (2s, br, 2H, 2NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 25.8, 72.2, 114.6, 116.4, 119.4, 129.1, 130.6, 130.9, 131.4, 131.8, 132.0,, 132.9, 133.2, 145.6, 161.2, 172.4, 193.3; MS m/z (%): 536 (M+, 16), 424 (36), 238 (61), 153 (75), 92 (100), 64 (82). Anal. Calcd for C25H18Cl2N6O2S (536.06): C, 55.87; H, 3.38; N, 15.64. Found C, 55.79; H, 3.28; N, 15.49%.
Ethyl 5-(2-(5-Oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)hydrazono)-4-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate (16a)Yellow solid (73%); mp 171–173°C; IR (KBr) ν: 3373, 3186 (2NH), 3061, 2926 (CH), 1754, 1654 (2C=O), 1598 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 1.18 (s, 3H, J=5.4 Hz, CH2CH3), 4.14 (s, 2H, J=5.4 Hz, CH2CH3), 7.02–7.58 (m, 15H, Ar-H), 10.73, 11.07 (2s, br, 2H, 2NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 14.1, 61.5, 74.4, 114.9, 121.6, 123.8, 125.5, 129.0, 129.8, 133.4, 134.2, 139.2, 144.5, 150.2, 162.8, 169.7; MS m/z (%): 498 (M+, 12), 354 (47), 247 (70), 149 (43), 92 (100), 64 (61). Anal. Calcd for C26H22N6O3S (498.15): C, 62.64; H, 4.45; N, 16.86. Found C, 62.51; H, 4.36; N, 16.73%.
Ethyl 5-(2-(5-Oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)hydrazono)-4-(p-tolyl)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate (16b)Yellow solid (74%); mp 166–168°C; IR (KBr) ν: 3444, 3175 (2NH), 3028, 2989, 2913 (CH), 1751, 1677 (2C=O), 1605 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 1.18 (s, 3H, J=5.4 Hz, CH2CH3), 2.27 (s, 3H, CH3), 4.19 (s, 2H, J=5.4 Hz, CH2CH3), 7.16–7.52 (m, 14H, Ar-H), 10.79, 11.04 (2s, br, 2H, 2NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 14.0, 20.3, 61.5, 74.5, 114.5, 118.4, 123.8, 125.5, 129.7, 131.7, 133.4, 134.2, 139.2, 140.2, 150.4, 162.8, 169.9; MS m/z (%): 512 (M+, 17), 442 (96), 321 (100), 164 (33), 106 (61), 79 (69). Anal. Calcd for C27H24N6O3S (512.16): C, 63.27; H, 4.72; N, 16.40. Found C, 63.14; H, 4.58; N, 16.26%.
Ethyl 4-(4-Chlorophenyl)-5-(2-(5-oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)hydrazono)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate (16c)Yellow solid (77%); mp 184–186°C; IR (KBr) ν: 3425, 3171 (2NH), 3062, 2982, 2929 (CH), 1748, 1677 (2C=O), 1600 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 1.120 (s, 3H, J=5.4 Hz, CH2CH3), 4.19 (s, 2H, J=5.4 Hz, CH2CH3), 6.97–7.63 (m, 14H, Ar-H), 10.71, 11.07 (2s, br, 2H, 2NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 14.0, 20.3, 61.1, 74.9, 116.6, 117.4, 121.3, 124.1, 126.7, 128.2, 129.0, 135.1, 138.6, 141.7, 145.6, 162.2, 169.8; MS m/z (%): 534 (M++2, 8), 532 (M+, 23), 413 (59), 367 (64), 165 (100), 118 (62), 77 (86). Anal. Calcd for C26H21ClN6O3S (532.11): C, 58.59; H, 3.97; N, 15.77. Found C, 58.59; H, 3.97; N, 15.77%.
5-(2-(5-Oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)hydrazono)-N,4-diphenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxamide (21a)Yellow solid (76%); mp 231–233°C; IR (KBr) ν: 3440, 3382, 3157 (2NH), 3055, 3019, 2951 (CH), 1655, 1645 (2C=O), 1599 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 7.02–7.78 (m, 20H, Ar-H), 10.21, 11.09, 11.73 (3s, br, 3H, 3NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 74.1, 114.6, 118.4, 120.9, 122.7, 123.0, 124.1, 128.6, 129.3, 133.4, 134.7, 135.6, 138.3, 142.2, 145.3, 150.7, 162.6, 170.0; MS m/z (%): 545 (M+, 11), 401 (100), 282 (55), 104 (37), 92 (86), 77 (98). Anal. Calcd for C30H23N7O2S (545.16): C, 66.04; H, 4.25; N, 17.97. Found C, 66.13; H, 4.22; N, 17.86%.
4-(4-Chlorophenyl)-5-(2-(5-oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)hydrazono)-N-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxamide (21b)Yellow solid (74%); mp 256–258°C; IR (KBr) ν: 3377, 3350, 3157 (3NH), 3060, 3019, 2932 (CH), 1663, 1648 (2C=O), 1597 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 7.10–7.75 (m, 19H, Ar-H), 10.15, 11.09, 11.70 (3s, br, 3H, 3NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 74.9, 113.7, 117.3, 119.9, 120.5, 121.0, 123.8, 124.5, 126.7, 127.6, 128.4, 129.1, 132.4, 137.5, 140.2, 143.5, 162.8, 170.4; MS m/z (%): 581 (M++2, 4), 579 (M+, 14), 542 (53), 367 (48), 165 (84), 80 (100), 64 (98). Anal. Calcd for C30H22ClN7O2S (579.12): C, 62.12; H, 3.82; N, 16.90. Found C, 62.03; H, 3.81; N, 16.75%.
4-(2,4-Dichlorophenyl)-5-(2-(5-oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)hydrazono)-N-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxamide (21c)Yellow solid (72%); mp 244–246°C; IR (KBr) ν: 3421, 3352, 3159 (3NH), 3061, 2953 (CH), 1667, 1649 (2C=O), 1597 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 7.113–7.74 (m, 18H, Ar-H), 10.21, 11.05, 11.75 (3s, br, 3H, 3NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 74.9, 114.1, 116.7, 118.6, 120.8, 121.5, 124.9, 126.9, 127.2, 127.8, 128.9, 129.7, 130.2, 132.2, 135.7, 137.6, 139.8, 145.5, 162.9, 171.2; MS m/z (%): 613 (M+, 20), 512 (44), 542 (53), 305 (61), 165 (76), 80 (100), 64 (79). Anal. Calcd for C30H21Cl2N7O2S (613.09): C, 58.64; H, 3.44; N, 15.96. Found C, 58.61; H, 3.28; N, 15.78%.
Alternate Synthesis of Thiadiazole 5A mixture of equimolar amounts of the 2-mercapto-4,4-diphenyl-1H-imidazol-5(4H)-one (6) (0.268 g, 1 mmol) and 2-hydrazono-3,5-diphenyl-2,3-dihydro-1,3,4-thiadiazole (7) (0.268 g, 1 mmol) in 20 mL of ethanol was refluxed till all of the starting materials have been disappeared and H2S ceased to evolve (12 h, monitored by TLC). The solvent was evaporated and the residue was triturated with methanol. The solid that formed was filtered and recrystallized from DMF to give compound 5 that was identical in all respects (mp, mixed mp and IR spectra) with that obtained from reaction of 2 with 3.
Alternate Synthesis of Thiadiazole Derivatives 10a, 16a and 21aTo a mixture of methyl 2-(5-oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)hydrazinecarbodithioate (13) (0.356 g, 1 mmol) and hydrazonoyl chloride 8a or 14a or 19a (1 mmol) in absolute EtOH (20 mL), was added TEA (0.07 mL, 1 mmol). The reaction mixture was stirred at room temperature till methyl mercaptan ceased to evolve (3h). The solvent was evaporated and the residue was treated with ice/HCl mixture. The solid product was collected, washed with EtOH, dried, and finally recrystallized from DMF to give the respective compounds 10a, 16a, or 21a, that was identical in all respects (mp, mixed mp and IR spectra) with that obtained from reaction of 8a or 14a or 19a with 3.
Synthesis of Thiazole Derivatives 23, 25 and 27A mixture of compound 2 (0.401 g, 1 mmol) and ethyl chloroacetate (22) (0.122 g, 1 mmol) or 2-chloro-3-oxo-N-phenylbutanamide (24) (0.211 g, 1 mmol) or phenacyl bromide (26) (0.197 g, 1 mmol) in ethanol (20 mL) containing TEA (0.1 g, 1 mmol) was refluxed for 4–6 h (monitored by TLC). The product started to separate out during the course of reaction. The solid product was filtered, washed with water, dried and recrystallized from EtOH to give the corresponding compounds 23 or 25 or 27, respectively.
2-(2-(5-Oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)hydrazono)-3-phenylthiazolidin-4-one (23)Yellow solid (70%); mp 163–165°C; IR (KBr) ν: 3383, 3180 (2NH), 3055, 2936 (CH), 1737, 1650 (2C=O), 1597 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 3.96 (s, 2H, CH2), 7.14–7.94 (m, 15H, Ar-H), 9.78, 10.49 (2s, br, 2H, 2NH, D2O-exchangeable); Anal. Calcd for C24H19N5O2S (441.13): C, 65.29; H, 4.34; N, 15.86. Found C, 65.17; H, 4.30; N, 15.77%.
4-Methyl-2-(2-(5-oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)hydrazono)-N,3-diphenyl-2,3-dihydrothiazole-5-carboxamide (25)Yellow solid (72%); mp 193–195°C; IR (KBr) ν: 3421, 3283, 3164 (3NH), 3047, 2926 (CH), 1668, 1648 (2C=O), 1598 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 2.12 (s, 3H, CH3), 7.13–7.65 (m, 20H, Ar-H), 9.88, 10.71, 12.03 (3s, br, 3H, 3NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 13.0, 71.0, 120.5, 121.0, 123.6, 124.4, 126.5, 126.7, 127.8, 128.7, 128.9, 129.4, 130.3, 138.0, 138.8, 142.8, 151.1, 160.4, 174.2; MS m/z (%): 558 (M+, 21), 372 (100), 243 (57), 183 (53), 91 (68), 77 (63). Anal. Calcd for C32H26N6O2S (558.18): C, 68.80; H, 4.69; N, 14.92. Found C, 68.67; H, 4.69; N, 15.01%.
2-(2-(3,4-Diphenylthiazol-2(3H)-ylidene)hydrazinyl)-4,4-diphenyl-1H-imidazol-5(4H)-one (27)Yellow solid (70%); mp 225–227°C; IR (KBr) ν: 3414, 3183 (2NH), 3053, 2932 (CH), 1648 (C=O), 1599 (C=N) cm−1; 1H-NMR (DMSO-d6) δ: 5.88 (s, 3H, thiazole-CH), 7.28–7.54 (m, 20H, Ar-H), 10.70, 11.09 (2s, br, 2H, 2NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ: 70.6, 126.5, 126.7, 127.0, 127.1, 127.8, 128.0, 128.8, 129.2, 129.7, 135.0, 139.6, 140.2, 140.7, 149.5, 152.4, 154.9, 173.6; MS m/z (%): 501 (M+, 21), 372 (100), 243 (57), 183 (53), 91 (68), 77 (63). Anal. Calcd for C30H23N5OS (501.16): C, 71.83; H, 4.62; N, 13.96. Found C, 71.68; H, 4.60; N, 13.79%.
Biological EvaluationEvaluation of the Antitumor Activity Using Viability AssayHuman hepatocellular carcinoma (HEPG2) cell line was obtained from the American Type Culture Collection (ATC C, Rockville, MD, U.S.A.). The cells were grown on RPMI-1640 medium supplemented with 10% inactivated fetal calf serum and 50 µg/mL gentamycin. The cells were maintained at 37°C in a humidified atmosphere with 5% CO2 and were subcultured two to three times a week. Potential cytotoxicity of the compounds was evaluated on tumor cells using the method of Gangadevi and Muthumary.26) The cells were grown as monolayers in growth RPMI-1640. The monolayers of 104 cells adhered at the bottom of the wells in a 96-well microtiter plate incubated for 24 h at 37°C in a humidified incubator with 5% CO2. The monolayers were then washed with sterile phosphate buffered saline (0.01 M pH 7.2) and simultaneously the cells were treated with 100 µL from different dilutions of tested sample in fresh maintenance medium and incubated at 37°C. A control of untreated cells was made in the absence of tested sample. Positive controls containing doxorubicin drug was also tested as reference drug for comparison. Six wells were used for each concentration of the test sample. Every 24 h the observation under the inverted microscope was made. The number of the surviving cells was determined by staining the cells with crystal violet27) followed by cell lysing using 33% glacial acetic acid and read the absorbance at 590 nm using microplate reader (SunRise, TECAN, Inc., U.S.A.) after well mixing. The absorbance values from untreated cells were considered as 100% proliferation. The number of viable cells was determined using microplate reader as previously mentioned before and the percentage of viability was calculated as [1−(ODt/ODc)]×100% where ODt is the mean optical density of wells treated with the tested sample and ODc is the mean optical density of untreated cells. The relation between surviving cells and drug concentration is plotted to get the survival curve of each tumor cell line after treatment with the specified compound. The IC50, the concentration required to cause toxic effects in 50% of intact cells, was estimated from graphic plots.
In this context, a series of novel thiadiazoles bearing imidazole moiety were synthesized by using 2-(5-oxo-4,4-diphenyl-4,5-dihydro-1H-imidazol-2-yl)-N-phenylhydrazinecarbothioamide as the starting compound. The structure of all the newly prepared products was established based on both elemental analysis and spectroscopic data and by alternative method wherever possible. Moreover, the mechanisms of formation of the title compounds were discussed. All the synthesized compounds were evaluated for their anticancer activity against the liver carcinoma cell line. Also, their SAR was studied. The results revealed that 1,3,4-thiadiazole derivatives 16c, 21c, 10g, 21b and 10h have promising antitumor activities (IC50=0.86, 1.02, 1.08, 1.17, 1.44 µM, respectively) against liver carcinoma cell line and most of the tested compounds showed moderate anticancer activities.
The authors declare no conflict of interest.