2017 年 65 巻 11 号 p. 1004-1010
2017 年 65 巻 11 号 p. 1004-1010
Tong-Qiao-Huo-Xue Decoction (TQHXD) is a classical prescription in traditional Chinese medicine treating blood stagnation in the head and facial channels, especially cerebral ischemia. We investigate the effect of TQHXD on the expressions of related proteins of the blood–brain barrier (BBB) and analysis of constituents in the cerebrospinal fluid (CSF) on cerebral ischemic model rats. Here, we demonstrate that TQHXD protected the hippocampus neurons, reduced the opening of tight junction (TJ) and decreased the permeability of BBB by up-regulating ZO-1, occludin, claudin-5 expressions, down-regulating aquaporin-4 (AQP-4) and matrix metalloproteinase-9 (MMP-9) expressions. Meanwhile, we detected Muscone, ligustilide and hydroxysafflor yellow A in CSF on cerebral ischemic model rats. These compounds could be identified as the main active ingredients of TQHXD on protecting the damaged BBB. These results suggest that TQHXD could act as a potential neuroprotective agent against BBB damage for cerebral ischemia.
Ischemia brain damage is one of the most challenging central nervous system (CNS) diseases which threaten human life and health due to its high rates of disability and mortality.1,2) The blood–brain barrier (BBB) injury is an important performance in ischemia brain damage.3) BBB is a highly specialized structure in CNS that comprises arachnoid membranes, cerebral capillary endothelial cells, and the choroid plexus epithelium.4,5) These layered cell structures construct the so-called tight junction (TJ) and the TJ-associated protein of Claudin-5, Occludin and ZO-1, which contribute to the integrity of the BBB, and the changes of its composition and the expression of relative ingredients are closely associated with BBB permeability.6–8) These proteins are the major structural protein of TJ, their expressions levels are relevant to the degree of cerebral microvascular permeability and cerebral edema.
In recent years, experimental reports and clinical applications of traditional Chinese medicine (TCM) against cerebral ischemia injury had shown superiority because of its effects of multiple targeting and multi-directional regulations.9) Tong-Qiao-Huo-Xue Decoction (TQHXD) is a classical prescription in traditional Chinese medicines, prescribed by Qingren Wang, a distinguished doctor of the Qing Dynasty, comprising seven Chinese herbs (Table 1). TQHXD promotes blood circulation by removing blood stasis, and induces resuscitation by dredging the channels. It has remarkable efficacy on the treatment of stagnation of blood in the head and facial channels,10) especially cerebral ischemia. Our previous experiments in vivo and vitro showed that TQHXD had beneficial effects in cerebral ischemia.11–16) However, we just investigated the effect of TQHXD on cerebral ischemia from the perspective of pharmacodynamics. Moreover, the model of animal used in the experiment was a cerebral ischemic model established by bilateral carotid artery occlusion but the mortality of animal was high. In this experiment, we used middle cerebral artery occlusion (MCAO) to establish cerebral ischemic model of rats; this model was similar to the clinical pathogenesis of cerebral ischemia and the mortality of animal was low.
| Chinese medicine | Family | Weight (g) | |
|---|---|---|---|
| 1 | She Xiang (Moschus berezovskii Flerov) | Cervidae | 0.15 g |
| 2 | Hong Hua (Carthamus tinctorius L.) | Asteraceae | 9 g |
| 3 | Chuan Xiong (Ligusticum chuanxiong Hort.) | Apiaceae | 3 g |
| 4 | Tao Ren (Prunus persica (L.) Batsch) | Rosaceae | 9 g |
| 5 | Chi Shao (Paeonia veitchii Lynch) | Ranunculaceae | 9 g |
| 6 | Da Zao (Ziziphus jujube Mill.) | Rhamnaceae | 5 g |
| 7 | Cong (Scallion) | Liliaceae | 3 g |
Researches also had shown that ligustilide, hydroxysafflor yellow A and muscone played a therapeutic role in brain diseases.17–19) Meanwhile, the three compounds were the main active ingredients of TQHXD.16–20) However, the underlying therapeutic mechanisms of TQHXD on cerebral ischemia are still unclear and whether ligustilide, hydroxysafflor yellow A and muscone in TQHXD could pass through BBB and whether they are the therapeutic material basis of TQHXD remain to be elucidated. On basis of our previous work, the changes of expression of proteins on BBB such as TJ proteins (Claudin-5, Occludin, ZO-1), aquaporin-4 (AQP-4) and matrix metalloproteinase-9 (MMP-9), which can affect drugs penetrating BBB will be investigated.
The constitutions of TQHXD were purchased from He Yi Tang Traditional Chinese Medicines Group Corporation (Hefei, China). Their species were kindly authenticated by Prof. Shoujin Liu (Professor of Pharmacognosy, Anhui University of Chinese Medicine). Ten times of 75% ethanol was added to 10 times of weight of TQHXD. These herbs were soaked for 2 h and then extracted twice, each time lasting 2 h; the solution was filtered and condensed to 2 g/mL.16)
Nimodipine was purchased from Bayer (Germany). Naomaitai was obtained from Guilin Sanjin Pharmaceutical Co., Ltd., China. Anti-ZO-1, Occludin antibodies were purchased from Proteintech Group, U.S.A. Anti-MMP-9, Claudin-5 antibodies were purchased from Bioworld Technology, U.S.A. Rabbit anti-AQP-4 antibody was purchased from Santa Cruz Biotechnology, U.S.A. Mouse Anti-beta Actin monoclonal antibody was purchased from Zhongshan Goldenbridge Biotechnology Co., China. Three reference standards, ligustilide, hydroxysafflor yellow A and muscone (Fig. 8) were purchased from Mansite Bio-Technology Co., Ltd., Chengdu, China. The purities of these reference standards were no less than 98.5%. All other chemicals were commercial products of analytical grade.
Animals and Cerebral Ischemic ModelMale Sprague-Dawley rats (SD) (Certificate No. SCXK2010-001) weighing 230–270 g, were purchased from Experimental Animal Center of Anhui Medical University (Anhui, China). The animals were housed under controlled temperature (24–27°C) and a 12h/12h light/dark cycle with food and water available. All animal experiments were approved by the Anhui University of Chinese Medicine on Ethics in the Care and Use of Laboratory Animals. The animals were subjected to MCAO as described by Longa et al.21) Briefly, rats were anesthetized with 3.5% chloral hydrate (350 mg/kg, intraperitoneal (i.p.) injection). The left common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA) were exposed and then separated. The proximal parts of the ECA and CCA were ligated with nylon suture and then a diameter of 0.285 mm nylon thread coated with silicon was inserted from the common carotid artery into the internal carotid artery until the origin of the middle cerebral artery (MCA). Following the surgery, the rats were placed in an intensive care incubator where the temperature was maintained at 37±0.5°C until the animals regained consciousness. The neurological deficits of each group at 24 h after being awake had been taken through blind through observation.
Drug AdministrationAll drugs were given by oral administration with the volume of 1 mL/100 g after onset of ischemia for 15 d. Fifty-six rats were randomly divided into seven groups as follows: (A) sham group, the external carotid artery was surgically prepared for insertion of the filament as described above, but the filament was not inserted; (B) MCAO group; (C) Nimodipine (3.6 mg/kg) group; (D) Nao Maitai (0.3 g/kg) group, C and D were set as positive drug control groups; (E) TQHXD treated groups, which were drenched with 3, 6 and 12 g/kg of TQHXD, respectively. Sham and MCAO group were given saline. Thirty minutes after the last administration, all the animals were anesthetized with 3.5% chloral hydrate (350 mg/kg, i.p.) and sacrificed.
Neurological Defect ScoringThe behavioral testing was assessed according to the method described by Longa et al.21) The neurological deficit was scored on a 5-point scale. 0=no neurologic defect, 1=failure to extend left forepaw fully on lifting whole body by tail, 2=circling to right, 3=falling to right, 4=did not walk spontaneously and had depressed levels of consciousness.
Transmission Electron Microscopy (TEM)Two rats in each group were anesthetized and perfused transcardially by normal saline followed by cold 4% paraformaldehyde solution. The brain tissues were removed and set in 4% paraformaldehyde solution. The hippocampus tissues and frontoparietal cortex of ischemia brain tissues were divided into some pieces of 1 mm3, embedded into 2.5% glutaraldehyde at 4°C. Then the tissues were placed in 2% osmium for 1.5 h, dehydrated, and embedded in Epon 812 Resin. Ultrathin sections were prepared and stained with uranyl acetate and lead citrate, and then observed by TEM.
Hematoxylin–Eosin (HE) StainingTwo rats in each group were anesthetized and perfused transcardially by normal saline followed by cold 4% paraformaldehyde solution. The brain tissues were removed and set in 4% paraformaldehyde solution. The coronal sections (5 µm thick) were taken from brain and stained with hematoxylin and eosin and observed under a microscope.
Western Blotting AnalysisSoluble proteins were harvested from rat’s ischemic cerebral hemispheres using lysis buffer. Lysed protein samples were loaded. Samples were separated on 6–12% sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to polyvinylidene difluoridemembranes. The membranes were blocked using 5% skim milk. Then, incubated overnight at 4°C with primary rabbit monoclonal antibodies respectively (ZO-1, 1 : 1000; Occludin, 1 : 800; Claudin-5, 1 : 500; AQP-4, 1 : 300; MMP-9, 1 : 600; Mouse anti-beta actin monoclonal antibody, 1 : 1000). The membranes were incubated with the secondary antibody conjugated with horseradish-peroxidase after washing with PBST three times for 10 min each. The targeted antigens were detected by enhanced chemiluminescence analysis. The proteins bands were scanned with Chemi Imager 5500 V2.03 software and the IDV were calculated using a computerized image analysis system and normalized with that of β-actin.
Samples of CSF Preparation for AnalysisAdult male SD rats (16) were randomly divided into four groups: (a) sham animals, received distilled water; (b) MCAO animals, also received distilled water; (c) sham animals, received TQHXD (12 g/kg); (d) MCAO animals, TQHXD (12 g/kg) was administered intragestrically twice a day for 4 d. One hour after the last administration, rats were anesthetized by 3.5% chloral hydrate solution (1 mL/100 g). Hold the rat’s head downward, cropped its parietal hair, the longitudinal incision was made in the skin and subcutaneous tissue incision along the frontal midline to the occipital protuberance. The operating point was fixed at about 0.2 to 0.3 cm in the midline of occipital protuberance. Used a syringe needle to obtain CSF and stored at −20°C. Five hundred microliters CSF was placed in a tube with 1.5 mL methanol, overtaxing for 1 min and centrifugation at 10000 rpm for 5 min at 4°C twice. The organic layers were combined and freeze-dried following stored at −80°C. After this process, the residues dissolved in 100 µL of methanol and filtered through a 0.45 µm membrane, the solution was injected into GC and HPLC.
Analytical Conditions of Ligustilide and Hydroxysafflor Yellow AAnalysis was performed with Waters 1525 HPLC system (Waters Corporation, U.S.A.) with UV detector. The analytical column employed was a Phenomenex luna C18 column (4.6×250 mm, 5 µm, Agilent Corporation). The mobile phase used for separation ligustilide was methanol–water (78 : 22) and hydroxysafflor yellow A was methanol–acetonitrile–water containing 0.7% phosphoric acid (26 : 2 : 72), at a flow rate of 1.0 mL/min. The injection volume was 20 µL. The detection wavelength of ligustilide was set at 320 nm and hydroxysafflor yellow A was 403 nm.
Analytical Conditions of MusconeAnalysis was performed using a Shimadzu GC2014 gas chromatograph equipped with a FID detector. The conditions were as follows: an initial temperature of 180°C held for 2 min, ramped to 230°C at 10°C/min and held for 8 min. The injection port temperature was 220°C and the detector temperature was 240°C. The carrier gas was nitrogen and flow rate was 2.5 mL/min.
Statistical AnalysisData are presented as mean±standard deviation (S.D.) Statistical comparisons were made using analysis of Kruskal–Wallis test. One-way ANOVA with a Bonferroni correction of the SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, U.S.A.) was used for significance analysis. p<0.05 was considered significant.
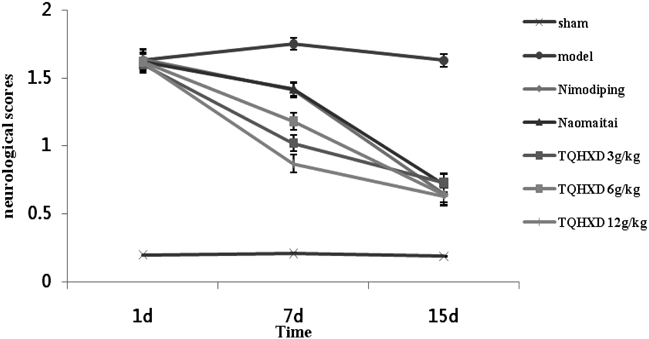
To determine the protective effects of TQHXD on cerebral ischemic rats, the neurological scored respectively before, during and after the administration (Fig. 1), the morphology of hippocampal neurons (Fig. 2) and the histological changes (Fig. 3) were measured firstly. The results showed that neurological deficit scores were significantly increased in the MCAO group (Fig. 1), the edge of the nucleus appears vacuolization, a small number of mitochondria also vacuolar degeneration (Fig. 2B), and vascular endothelial cells damaged, there was congestion in the central of infarction and inflammatory cells (Fig. 3B). However, neurological deficit scores and hippocampal neurons disruption were alleviated to varying degrees by the treatment of TQHXD after MCAO. At the same time, Nimodiping and Naomaitai also had a protective effect on these damages.
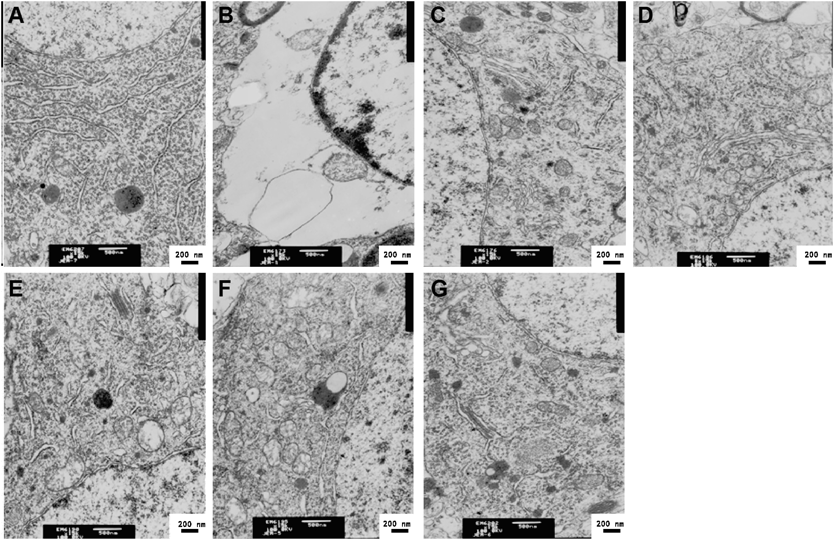
A: Sham group; B: MCAO group; (C) Nimodipine (3.6 mg/kg) group; (D) Nao Maitai (0.3 g/kg) group; (E) TQHXD (3 g/kg) group; (F) TQHXD (6 g/kg) group and (G) TQHXD (12 g/kg) group.

A: Sham group; B: MCAO group; (C) Nimodipine (3.6 mg/kg) group; (D) Nao Maitai (0.3 g/kg) group; (E) TQHXD (3 g/kg) group; (F) TQHXD (6 g/kg) group and (G) TQHXD (12 g/kg) group.
In addition, BBB, which is necessary for the function of central nervous system, plays an important role in the maintenance of brain homeostasis.22) BBB damage, which is induced by cerebral ischemia, is associated with the multiple impairment such as TJ opening between microvascular endothelial cells (BMECs), neurons disruption and so on.23) Therefore, we also detected the opening of TJ by TEM (Fig. 4) Arrows as shown in figure, a series of electronically dense bands were exhibited in TJ, which are located between adjacent plasma membranes and enclose cell cleavage. Part of BMECs tight junctions were in the open state in model rats (Fig. 4B). However, the reduction of openness of TJ was observed in the Nimodiping, Naomaitai and TQHXD groups, indicating that drugs might repair the postischemia TJ integrity.
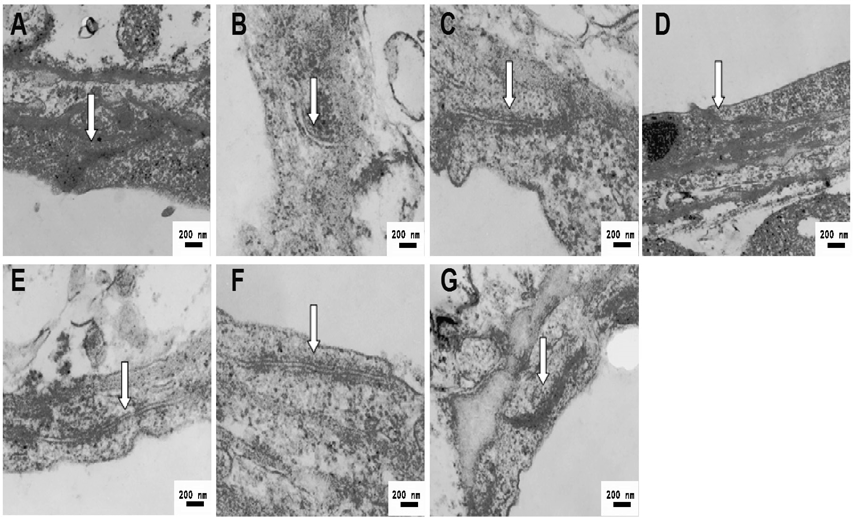
A: Sham group; B: MCAO group; (C) Nimodipine (3.6 mg/kg) group; (D) Nao Maitai (0.3 g/kg) group; (E) TQHXD (3 g/kg) group; (F) TQHXD (6 g/kg) group and (G) TQHXD (12 g/kg) group.
Next, effect of TQHXD on claudin-5, occludin, and ZO-1 proteins expressions were obtained. The results verified that TQHXD significantly increased the expressions of claudin-5 (p<0.01 at 6, 12 g/kg), occludin (p<0.01 at 3, 6, 12 g/kg) and ZO-1 (p<0.05 at 3, 6, 12 g/kg) (Fig. 5), which indicated the protective effects of TQHXD against the ischemia BBB leakage. Among them, the expression of occludin was the highest in TQHXD group (3 g/kg) and the expression of claudin-5 in TQHXD group (6 g/kg). The author considered that it may be difficult to form a dose–effect relationship due to the interaction of the multi-component in traditional Chinese medicine compound. Of course, TQHXD itself may have toxicity. In addition, Nimodiping and Naomaitai could also inhibit the down-regulation of these proteins expressions (p<0.01, p<0.05).
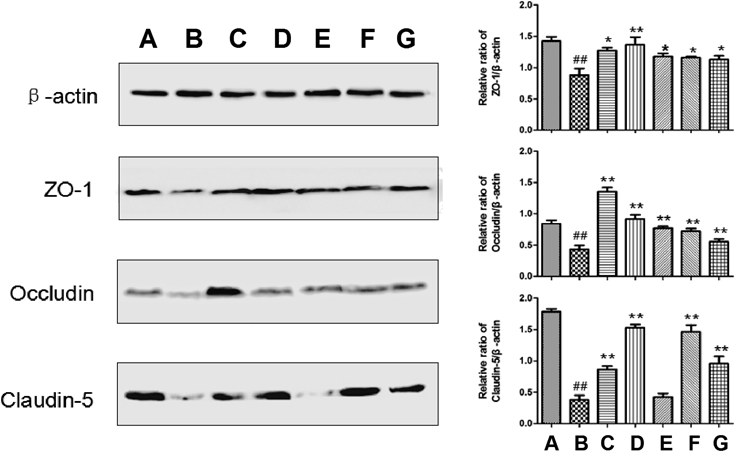
(A) Sham group; (B) MCAO group; (C) Nimodipine (3.6 mg/kg) group; (D) Nao Maitai (0.3 g/kg) group; (E) TQHXD (3 g/kg) group; (F) TQHXD (6 g/kg) group and (G) TQHXD (12 g/kg) group. (x̄±S, n=4). ##p<0.01 vs. sham-operated group; ** p<0.01 vs. model group.
BBB disturbances can also cause the decrease of the expression of the major structural proteins of the TJs including claudin-5, occludin and ZO-1, increased expression of AQP-4 and MMP-9 and so on.24–26) Claudin-5, occludin and ZO-1 are the major structural proteins of the TJ, and affect the paracellular permeability of BBB and brain edema.27) Reducing the TJ proteins expressions and increasing the MMP-9 expression can directly affect BBB integrity and increase BBB permeability after cerebral ischemic injury.28,29) AQP-4 is unique because it has polarized expression on the BBB. This property leads to an important water transport regulatory role in brain parenchymal tissue, most likely by enhancing transmembrane water flux in astrocytes.30) Recent studies demonstrated that after ischemic stroke, there was a rapidly marked increase on the expression of MMP-9 at the site of ischemia. The formation of vasogenic edema has been linked to the activation of MMPs, which are associated with BBB degradation and increased permeability and leakage.31,32) So we also measured the expressions of AQP-4 and MMP-9, the results showed that TQHXD significantly reduced expressions of AQP-4 (Fig. 6) and MMP-9 (Fig. 7). Likewise, Nimodiping and Naomaitai could also inhibit the up-regulation of AQP-4 expression (p<0.01); Nimodiping could reduced the expression of MMP-9 (p<0.05), but Naomaitai did not. The reasons may be that Nimodipine and Naomaitai, as positive drugs in this experiment, are the recognized drugs to treat ischemic cerebrovascular disease in clinical, but Naomaitai is a traditional Chinese medicine compound, which has a relatively slow and moderate effect. Moreover, it is possible that the drug does not reach an effective dose to inhibit MMP-9 expression.
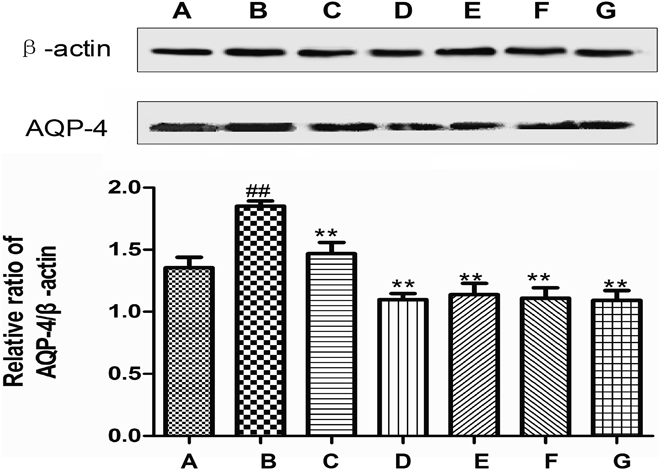
(A) Sham group; (B) MCAO group; (C) Nimodipine (3.6 mg/kg) group; (D) Nao Maitai (0.3 g/kg) group; (E) TQHXD (3 g/kg) group; (F) TQHXD (6 g/kg) group and (G) TQHXD (12 g/kg) group. (x̄±S, n=4). ## p<0.01 vs. sham-operated group; ** p<0.01 vs. model group.

(A) Sham group; (B) MCAO group; (C) Nimodipine (3.6 mg/kg) group; (D) Nao Maitai (0.3 g/kg) group; (E) TQHXD (3 g/kg) group; (F) TQHXD (6 g/kg) group and (G) TQHXD (12 g/kg) group. (x̄±S, n=4). ## p<0.01 vs. sham-operated group; * p<0.05, ** p<0.01 vs. model group.
We have studied the ingredients of TQHXD by the chromatographic fingerprints.33) In view of the fact that the neuroprotective effect of traditional Chinese medicine treating CNS diseases was bound up with its ingredients penetrating BBB, so the analysis of pharmaceutical ingredients in CSF of rats being intragastric administration by TQHXD had been examined (Fig. 8). In the validation procedure, only linearity (Table 2), precision, stability and recovery were evaluated. The results of precision, stability and recovery were showed in Table 3. We could summarize that analytical methods were reliable, stable and repeatable.
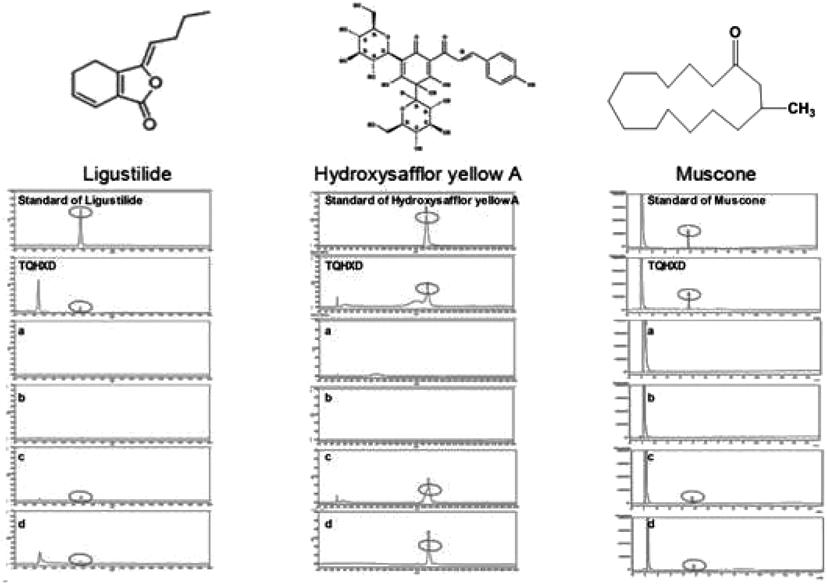
Ligustilide and hydroxysafflor yellow A were detected by HPLC and muscone was detected by GC and a: The CSF of sham rats, received distilled water was; b: The CSF of MCAO rats, received distilled water; c: The CSF of sham rats, received TQHXD (12 g/kg); d: The CSF of MCAO rats, received TQHXD (12 g/kg).
| Compounds | Regression equation | Correlation coefficient (r2) | Liner range (µg/mL) |
|---|---|---|---|
| Ligustilide | y=5.2E+6x+6.5E+5 | 0.9992 | 0.84–1.078×104 |
| Hydroxysafflor yellow A | y=2.3E+7x−80721 | 0.9993 | 0.1–3.2 |
| Muscone | y=9.0E+6x−8211.1 | 0.9999 | 0.13×10−3–13 |
| Compounds | Nominal concentration (µg/mL) | Precision concentration found (µg/mL) | Precision (%, RSD) | Nominal concentration (µg/mL) | Stability concentration found (µg/mL) | Precision (%, RSD) | Nominal concentration (µg/mL) | Recovery concentration found (µg/mL) | Precision (%, RSD) |
|---|---|---|---|---|---|---|---|---|---|
| Ligustilide | 0.84 | 0.848±0.016 | 1.98 | 17.5 | 16.42±0.582 | 3.54 | 1.71 | 98.45±0.932 | 0.95 |
| Hydroxysafflor yellow A | 0.13 | 0.129±0.004 | 1.65 | 0.27 | 0.265±0.005 | 2.13 | 0.53 | 98.76±1.909 | 1.93 |
| Muscone | 1.31 | 1.319±0.019 | 1.46 | 1.67 | 1.654±0.032 | 1.96 | 0.22 | 98.39±1.156 | 1.17 |
Surprisingly, ligustilide, hydroxysafflor yellow A and muscone were detected in CSF. And, in the d group, the content of ligustilide, hydroxysafflor yellow A and muscone was 10.73, 0.19 and 1.28 µg/mL, respectively. However, in the c group, the content of ligustilide, hydroxysafflor yellow A and muscone was 18.80, 0.27 and 1.67 µg/mL, respectively (Table 4). We speculated that the reason may be the opening of tight junction had been restored and the tight junction proteins expression had been up-regulated, MMP-9 and AQP-4 had been down-regulated simultaneously, thus the content of the compounds permeated into the CSF was reduced. We have done some pharmacological experiments on these compounds.34,35) These findings will increase our understanding of the effective substances of TQHXD. But the shortcoming of this paper is we do not isolate these compounds and prove their effects in comparison with the decoction.
| Compounds | Sham-group (c) (µg/mL) | Model-group (d) (µg/mL) |
|---|---|---|
| Ligustilide | 18.80 | 10.73 |
| Hydroxysafflor yellow A | 0.27 | 0.19 |
| Muscone | 1.67 | 1.28 |
Therefore, the protective effects of TQHXD on damaged BBB may be concerned with the up-regulation of ZO-1, occludin and claudin-5 expressions and down-regulation of the expressions of AQP-4 and MMP-9. The neuroprotective ingredients of TQHXD may be related to Muscone, ligustilide and hydroxysafflor yellow A.
The current study was mainly supported by National Natural Science Foundation of China (81374005, 81773933); “Twelfth Five Year” National Science and Technology Support Program (No. 2012BAI26B03); Academic Project of Outstanding Talents in Disciplines (Specialties) of Universities and Colleges in Anhui (2017, jxbjZD15).
The authors declare no conflict of interest.