2017 Volume 65 Issue 11 Pages 1093-1097
2017 Volume 65 Issue 11 Pages 1093-1097
Various 3-arylmethyl-2-oxindole derivatives were synthesized by the Knoevenagel condensation of oxindole and aromatic aldehydes followed by palladium-mediated hydrogenation or hydride-reduction. Further substituted derivatives at C-3 and/or N-1 of the oxindole skeleton were prepared from the condensation products. Their protective effect against neuronal cell death induced by oxidative stress was evaluated by lactate dehydrogenase assay. A structure–activity relationship study revealed that compounds with any of the dialkylamino, nitro or hydroxy groups on the 3-arylmethyl moieties elicit a superior potency to suppress cell death, while others are ineffective. Substitutions with less polar functional groups on the benzene or lactam ring of the oxindole skeleton positively, but not remarkably, affect the potency. In addition, the stereochemistry at C-3 of the oxindole core was not a crucial factor for the neuroprotective activity of the compounds.
Loss of neurons in specific areas of the brain due to neuronal cell death is a common feature of neurodegenerative disorders such as Parkinson’s and Alzheimer’s diseases.1,2) Intracellular oxidative stress and endoplasmic reticulum (ER) stress are generally considered to play important roles in the death of neuronal cells.3–6) In this context, the agents alleviating the oxidative and ER stresses and restore cellular homeostasis are expected to be potential therapeutic candidates for neurodegenerative diseases.
In our continuous studies to elaborate small molecules with neuroprotective activities, we previously reported the design, synthesis and action mechanisms of artificial cyclopentenone-type prostaglandin analogs 1 and 27–10) (Fig. 1). These compounds exert their effects through covalent bond formation between the electrophilic conjugated-enone moiety and a cysteine residue of the target proteins, such as Keap1.7,10) Unfortunately, these compounds induced cell death at higher doses presumably due to non-specific bindings to various proteins.9) Strongly electrophilic compounds are known to inhibit the function of several proteins having cysteine residues and contribute to cell death. One possible approach to avoid this cytotoxic effect of the electrophilic compounds is to synthesize molecules of moderate electrophilicity. Based on this idea, we designed an α,β-unsaturated oxindole structure 3 (Fig. 2) in which the variable substituent Z can modulate the electrophilicity of the double bond. A preliminary study showed that some of the compounds 3, as expected, suppressed the oxidative stress-induced neuronal cell death without any cytotoxic effect.11) However, further study has been suspended, because such compounds were already known as potent inhibitors for receptor tyrosine kinases.12–14)

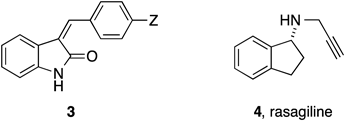
Meanwhile, recent studies reported that rasagiline 4, a therapeutic agent for Parkinson’s disease, exhibits a neuroprotective activity through not yet fully solved mechanism,15,16) besides its primary activity for monoamine oxidase-B inhibition.17) Compound 4 has no electrophilic moiety to form a covalent bond with thiol groups, suggesting a non-covalent mechanism for the activity. Consideration of the structural features of 3 and 4 inspired us to attempt to assess the effect of the saturated analog of 3 on neuronal cell death. Fortunately, we found that such a compound also suppressed the death of neuronal cells induced by oxidative stress. In addition, saturated-type oxindoles are reportedly poor inhibitors of kinases, thus avoiding unnecessary side effects.18) In this paper, we describe the design, synthesis and structure–activity relationship (SAR) studies of 3-arylmethyl-2-oxindole derivatives and related compounds regarding the suppression of neuronal cell death.
First, we designed several 3-arylmethyl-2-oxindole derivatives 5 (Figs. 3, 4) and synthesized them by the sequence described in Chart 1. Thus, the Knoevenagel reaction between 2-oxindole and substituted benzaldehydes afforded the condensation products 3 as E/Z mixtures.12,19) Subsequent reduction of 3 produced the desired saturated products 5 in racemic forms. Related 3-heteroarylmethyl analogs 6–10 were likewise prepared using the corresponding heteroaromatic aldehydes at the condensation stage.



Reagents and conditions: (i) piperidine, methanol; (ii) H2, Pd–C, methanol, or NaBH4, methanol–DMF.
For further SAR studies, we designed oxindole derivatives 11–13 bearing a fixed substituent Z=NMe2 on the phenyl ring of the 3-arylmethyl moiety and substituents of various types on the oxindole core (Fig. 5). The compounds 11 were generally synthesized according to the sequence depicted in Chart 2 (upper part) using substituted oxindoles. Exceptional is the preparation of 11f; palladium-catalyzed hydrogenation of 16 (Y1=NO2) and subsequent acetylation of the resulting 5-amino intermediate with acetyl chloride afforded the desired product 11f.

a Compounds 12b and c are enantiopure antipodes of each other. b 3-[3-(Dimethylamino)benzyl]-3-methyl-2-oxindole. c 3-[2-(Dimethylamino)benzyl]-3-methyl-2-oxindole.

Reagents and conditions: (i) piperidine, methanol; (ii) H2, Pd–C, methanol, or NaBH4, methanol–DMF; (iii) Boc2O, 4-(dimethylamino)pyridine, CH3CN; (iv) H2, Pd–C, CH3OH, or NaBH4, methanol–DMF, (v) NaH, MeI (N-fluorobenzenesulfonimide for 12b/c; HC≡CCH2Br for 12d), DMF; (vi) CF3COOH, CH2Cl2; (vii) NaH, Y3–Br (MeI for 13a; Ac2O for 13d), DMF.
The lower part of Chart 2 illustrates the synthetic pathways leading to 12 and 13. Briefly, N-tert-butoxycarbonyl (Boc) protection of 16 and subsequent hydrogenation produced the saturated intermediates 17. The treatment of 17 with sodium hydride generated amide enolates that were then alkylated with alkyl halides to afford 18. N-Boc deprotection of 18 completed the preparation of the 3-alkylated products 12. The reaction of the amide enolate from 17 (Y1=H) with N-fluorobenzenesulfonimide followed by N-Boc deprotection produced the 3-fluorinated derivative 12b/c as a racemic mixture. The additional compounds 14 and 15 in Fig. 5 were also prepared in this sequence. The N-alkylated derivatives 13b and c were prepared by the alkylation of the amidate generated from 12a. The reaction of the amidate with acetic anhydride provided 13d. Compound 13a was synthesized by direct alkylation of the amidate of 16 (Y1=H) with methyl iodide and subsequent hydrogenation. The N-aryl derivative 13e was synthesized in a manner similar to that for 5 using N-(4-methoxyphenyl)-2-oxindole as the starting substrate.
The designed oxindole derivatives have an asymmetric carbon at C-3 of the lactam ring, but they were synthesized in a racemic form in this study. In general, the absolute configurations of the drug molecules are known to play an important role in the interaction with target proteins. Therefore, it is worth investigating the effect of the stereochemistry on the activity. As a representative example, we separated the racemic mixture of the 3-fluoro derivatives 12b and c into two pure enantiomers by HPLC using a chiral column (Daicel Chiralcel OD-H). The isomer 12b has a shorter retention time during the HPLC compared to 12c. The absolute configurations of both enantiomers were not assigned.
Biological Activity and SARWe evaluated the effect of the compounds on the oxidative stress-induced neuronal cell death using HT22, an immortalized mouse hippocampal cell line.20) In these cells, a high concentration of extracellular glutamate inhibits the glutamate-cystine antiporter leading to a depletion of intracellular glutathione and excess reactive oxygen species (ROS) production. The cells were treated with each compound at 10 and 25 µM with 10 mM glutamate. After incubation for 24 h, the extent of cell death was determined by measuring lactate dehydrogenase (LDH), a marker of injured cells, released into the culture medium using a cytotoxicity detection kit (LDH assay). Compound 2 prevented the cell death by 70% at 10 µM in this assay system, as reported earlier,21) while rasagiline (4) suppressed the cell death by only ca. 25% at 50 µM.
Figures 6–8 illustrate the suppressive effects of the oxindole derivatives on the oxidative stress-induced cell death in HT22 cells. All but one of the compounds alone exerts no severe cytotoxic effects on HT22 cells up to 100 µM (data not shown). Only compound 11c caused a minor cytotoxicity (ca. 30% of the glutamate toxicity) to the cells at 100 µM. A general survey of the data shows that the derivatives with specific types of substituents on the benzene ring of the arylmethyl group in 5 exhibited a suppressive effect on the cell death induced by oxidative stress. Thus 5b, f and j intensely, and 5a moderately suppressed the cell death, while the others did not (Fig. 6). The estimated IC50 values for 5b was 7.1 µM. Phase-contrast microphotographs of glutamate-treated HT22 cells showed that most of the cells survived in the presence of 5b (data not shown). There is no consistency in the electronic character and polarity of the substituents Z on the activity: The dialkylamino groups of 5b and j have strong electron-donating nature, while the nitro of 5f is a less polar but strong electron-withdrawing group. The hydroxy of 5a is a polar electron-donating group. The other functional groups did not confer any significant activity on the compounds. Moreover, 5k and l, analogs of 5j, having an extra heteroatom in the azacycloalkane ring, together with all the heteroaryl-type derivatives 6–10 were completely inactive. These results may also exclude the possible advantageous hydrogen-bonding or ionic interactions with the putative target proteins. Although compound 5a with a hydroxy group, a hydrogen-bond donor, suppressed the cell death, the potency was far less than that of 5b and 5f. Thus, hydrogen-bonding interaction, if any, is considered not to be significant. Alternatively, the antioxidant effect of the phenolic moiety might be involved in the activity of 5a.22) At present, it is difficult to provide a unifying explanation for the above SAR, but we consider that hydrophobic substituents Z with adequate spatial size are crucial for eliciting the suppressive effect of the oxindole derivatives 5 on the oxidative stress-induced cell death.

HT22 cells were incubated with each compound (open bar, 0; dot, 10 µM; check, 25 µM) and glutamate (10 mM) for 24 h, and cell death was determined by measuring LDH released into the culture medium. The LDH level of the control cultures (c) was set at 100%.

HT22 cells were incubated with each compound (open bar, 0; dot, 10 µM; check, 25 µM) and glutamate (10 mM) for 24 h, and cell death was determined by measuring LDH released into the culture medium. The LDH level of the control cultures (c) was set at 100%.

HT22 cells were incubated with each compound (open bar, 0; dot, 10 µM; check, 25 µM) and glutamate (10 mM) for 24 h, and cell death was determined by measuring LDH released into the culture medium. The LDH level of the control cultures (c) was set at 100%.
Next, we analyzed the SAR of the 5b-based analogs 11, 12 and 13, containing substituents at various positions of the oxindole skeleton. For the analogs 11 bearing a substituent Y1 at C-5 of the oxindole moiety, all but 11f had an equivalent or superior activity to the reference compound 5b (Fig. 7). This means that the activity seems to be tolerant to substitution more at the phenyl ring of the oxindole skeleton than the side-chain arylmethyl group. However, substitution with the acetamide group (11f) resulted in an intensely reduced activity, suggesting a tendency to avoid polar interactions even around the oxindole skeleton.
The derivatives 12 with the second substituent Y2 at C-3 also retained an activity in general (Fig. 8, left half). These data indicated that the benzylic hydrogen at C-3 is unnecessary, and thus substitution with a variety of alkyl groups at C-3 seems to be permissible. Most remarkable is that both enantiomeric compounds 12b and c exhibited a comparable activity, indicating that the stereochemistry at C-3 of the compounds is not a crucial factor for the neuroprotective activity. This implies that the compounds need not be synthesized in an enantiopure form, at least for use in the present assay system. Additionally, the related analogs 14 and 15, the position isomers of 12a, provided contrasting results, in which the former was active, but the latter inactive. Thus, the location of the dimethylamino group on the side-chain aromatic ring is important for the interaction with the target. Presumably, the position at C-4′ (para) is most desirable. The sole exception is 12g. The structural features of 12g meet the criteria stated above, but it has two methoxy groups on the phenyl ring of the oxindole skeleton. It is currently unclear what factors contribute to this reduction in the activity of 12g. Finally, methylation (13a), propargylation (13b), benzylation (13c), arylation (13e) and even acetylation (13d) at N-1 retained their activity (Fig. 8, right half). These data may exclude the familiar mechanism that the amide N–H interacts with the target molecule by hydrogen-bonding to exert the effect. Taken together, substitutions at the lactam ring of the oxindole skeleton positively, but not remarkably, affect the activity of the compounds. This allows further modifications of the compounds to improve their potency, water-solubility, and bioavailability. Applications to molecular probes, such as affinity labeling, fluorescent and radiochemical probes, may also be possible, because the propargyl group, if introduced in advance in the compounds, is easily accessible for click chemistry.23)
We synthesized thirty-eight 3-arylmethyl-2-oxindole derivatives and evaluated their suppressive activity against oxidative stress-induced neuronal cell death. Among the compounds, the 3-[4-(dimethylamino)benzyl]-2-oxindole derivatives exhibited a significant suppressive activity against oxidative stress-induced neuronal cell death. As regards the substituents on the 3-arylmethyl moieties, the nitro or hydroxy groups also elicit a potency, while others are ineffective. Substitutions with less polar functional groups on the benzene or lactam ring of the oxindole skeleton positively, but not remarkably, affect the potency. Furthermore, the stereochemistry at C-3 of the oxindole core was not a crucial factor for the neuroprotective activity of the compounds. The oxindole derivatives described here could be potential candidates for treatment of neurodegenerative disorders. Assessment of the in vivo effects on the Parkinson’s disease model and elucidation of the underlying molecular mechanisms for neuroprotective actions of the compounds including identification of the target proteins are currently in progress.
ExperimentalThe details of the experimental procedures are provided in the supplementary materials, which can be found as attachment.
This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.