2017 Volume 65 Issue 2 Pages 143-150
2017 Volume 65 Issue 2 Pages 143-150
A series of pyrazolo[3,4-b]pyridines were prepared by a microwave-assisted aza-Diels–Alder reaction between pyrazolylformimidamides 1 and β-nitrostyrenes 2 in toluene as the solvent. This procedure provides a simple one-step and environmentally friendly methodology with good yields for the synthesis of these compounds. All compounds were tested for antifungal activity against two clinically important fungi Candida albicans and Cryptococcus neoformans. Within the compounds of the series bearing a –CH3 group on the carbon C-3 of the azole ring (3a–e), the compound without a substituent on the p′-phenyl ring (3a), showed the best activity against both fungi, followed by the p′-Br-phenyl (3c). Within the compounds of the series bearing a tert-butyl group in the carbon C-3 of the azole ring (3f–j), the non-substituted p′-compound (3f) was the most active one, followed by (3h) (p′-Br substituted) that showed the best activity against both fungi. The remaining compounds of this sub-series (3g, i, j) showed similar moderate activities. The antifungal activity of the compounds of the series was found to be correlated with a higher log P and a lower dipole moment in the more active compounds.
Hetero-Diels–Alder (H-DA) reactions are being extensively used in the synthesis of bioactive, natural and synthetic heterocyclic compounds1); actually, the H-DA methods have proven to be efficient under affordable conditions, when the diene and dienophile are electronically well matched, may go through procedures without solvent or catalyst and are being diastereo- and/or regioselectives reactions.2,3) To obtain six-membered nitrogen-containing and/or pyridine fused polyheterocyclic compounds, the aza-Diels–Alder (A-DA) strategy is a powerful synthetic tool and mechanistically the cyclization may occur in a concerted manner or by step-wise reaction between the azadiene and dienophile.4,5)
According to our experience, the microwave assisted organic synthesis (MAOS) is a technique to simplify and improve classic organic synthesis because it allows cleaner and shorter reactions with higher yields and reducing byproducts.6,7) Recently we have developed several MAOS methodologies to obtain pyrazolo[3,4-b]pyridines,2,8–13) since they are an important group of nitrogen-containing fused heterocycles useful for medicinal and organic chemistry with a wide spectrum of biological and pharmacological activities14–16) as antibacterial, antidepressant, anti-hyperglycemic, anti-inflammatory, antitumor, and anxiolytic agents and are being used in the treatment of Alzheimer’s diseases, drugs addiction, and infertility.7,17–23) To continue within the framework of our interest to develop synthetic strategies to obtain functionalized and biologically interesting pyrazolo[3,4-b]pyridines, we wish to report an efficient MAOS method between N,N-dimethyl-N′-pyrazolylformimidamides2,10,24,25) and β-nitrostyrenes to obtain 5-nitro-1-phenyl-pyrazolo[3,4-b]pyridines.26,27)
Considering that this type of compounds have previously shown good antimicrobial activities, including antifungal properties against Saccharomyces cerevisiae and Candida albicans,28–33) we tested the new series of 5-nitro-1-phenyl-pyrazolo[3,4-b]pyridines for antifungal activities against two clinically important fungi C. albicans and Cryptococcus neoformans. C. albicans was selected because it is among the most common causes of opportunistic fungal infections in immune compromised hosts worldwide.34) In turn, C. neoformans was chosen because it produces the most important human immunodeficiency virus (HIV)-related fatal opportunistic mycosis in the whole world.35) Although the incidence of this infection tends to decline in countries with highly active anti-retroviral therapy, the outcome of infection is influenced by a variety of factors including antifungal resistance.36)
In an initial study of the A-DA cycloaddition, we tested the reaction between the pyrazolylformimidamide 1a and the β-nitrostyrene 2e (Chart 1) under different reaction conditions in order to find the optimal ones (Table 1).

| Entry | Time (min) | Reaction conditions | Yield (%) |
|---|---|---|---|
| 1 | 120 | Ethanol, reflux | — |
| 2 | 120 | Acetonitrile, reflux | — |
| 3 | 120 | Toluene, reflux | 35 |
| 4 | 4 | DMF, MWI | 28 |
| 5 | 4 | Toluene, MWI | 73 |
| 6 | 4 | DMSO, MWI | 67 |
At first, we performed the experiment varying the solvent under reflux conditions but the reaction either did not take place (entries 1, 2) or the yield was low (35%, entry 3). Under microwave irradiation (MWI) the time reaction and yield product improve when we used high boiling points solvents (dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF) and toluene) and when the reaction was conducted under MWI. In toluene the desired product 3e was obtained in higher yield (entry 5).
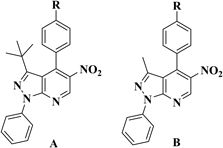
The established reaction conditions for 3e were extrapolated to obtain the series of compounds 3a–j. The structures of the obtained products are shown in Table 2.
 |
All the structures 3a–j were characterized by proton- and carbon-NMR (1H-NMR, 13C-NMR), MS and elemental analyses. Formation of the pyrazolo[3,4-b]pyridine was unequivocally established by NMR data of the products. The chemical shifts and multiplicities of the protons were in accordance with the expected values. For example, signals for the protons of the phenyl and pyridine rings of compounds 3 were found between 7.05 and 9.20 ppm. The signal of CH3 appears as a singlet between 2.0 and 2.3 ppm and the signal of the H-6 proton appears as a singlet between 8.92 and 9.26 ppm.
The observed high regioselectivity in the obtained products is in complete agreement with the calculated atomic coefficients in the frontier molecular orbital of the pyrazolylformimidamide 2a and the β-nitrostyrene 2e25,27) (Fig. 1).

According to the calculated atomic coefficients and the previous reports,2,5) the possible mechanistic route to explain the formation of pyrazolo[3,4-b]pyridine involves in the initial step the cycloaddition between the formimidamide 1a and the β-nitrostyrene 2 with the corresponding formation of the Diels–Alder adduct 3′. This eliminates a dimethylamine molecule and is oxidized by air under thermal conditions to obtain the aromatic product corresponding 5-nitro-1-phenyl-pyrazolo[3,4-b]pyridine 32,5,11) (Chart 2).

All these compounds were tested for antifungal properties against C. albicans ATCC 10231 and C. neoformans ATCC 32264 with the standardized microbroth dilution method M-27A3 for yeasts of the Clinical and Laboratory Standards Institute (CLSI, 2008). The percentages of growth inhibition of each fungus by compounds 3a–j were determined in the range 250–0.98 µg/mL, and the results are recorded in Table 3.
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | R | Inhibition percentages (%) of C. albicans ATCC 10231 by the compounds 3a–j at different concentrations | ||||||||
| Compd. | 250 µg/mL | 125 µg/mL | 62.5 µg/mL | 31.2 µg/mL | 15.6 µg/mL | 7.81 µg/mL | 3.9 µg/mL | MIC50 | ||
| A | H | 3a | 100 | 89.1±1.6 | 51.8±1.0 | 28.6±1.0 | 26.3±1.6 | 21.8±0.9 | 11.3±0.7 | 62.5 |
| A | Cl | 3b | 54.91±1.22 | 52.7±0.6 | 22.8±1.0 | 13.3±0.5 | 12.5±0.8 | 11.4±0.6 | 0 | 125.0 |
| A | Br | 3c | 63.69±1.94 | 52.6±1.8 | 41.0±1.2 | 31.2±0.2 | 29.5±1.7 | 27.2±0.4 | 26.8±0.7 | 125.0 |
| A | CH3 | 3d | 18.28±1.01 | 4.7±0.8 | 3.8±0.4 | 0.1±0.0 | 0 | 0 | 0 | >250 |
| A | OCH3 | 3e | 34.92±0.73 | 18.2±1.2 | 10.2±0.3 | 10.1±0.9 | 5.4±1.3 | 1.5±0.9 | 0 | >250 |
| B | H | 3f | 100 | 100 | 100 | 51.8±1.0 | 11.4±0.8 | 8.8±0.2 | 2.4±0.6 | 31.2 |
| B | Cl | 3g | 45.4±1.7 | 20.4±0.7 | 10.2±0.3 | 6.2±0.4 | 5.7±1.00 | 4.3±0.3 | 2.8±0.6 | >250 |
| B | Br | 3h | 100 | 79.6±1.1 | 34.9±1.2 | 15.3±1.3 | 0 | 0 | 0 | 100.0 |
| B | CH3 | 3i | 40.4±1.4 | 25.1±0.4 | 2.9±1.0 | 0 | 0 | 0 | 0 | >250 |
| B | OCH3 | 3j | 47.9±1.0 | 26.0±0.9 | 12.3±0.4 | 4.0±1.7 | 2.9±0.5 | 0 | 0 | >250 |
| Inhibition percentages (%) of C. neoformans ATCC 32264 by the compounds 3a–j at different concentrations | ||||||||||
| Compd. | 250 µg/mL | 125 µg/mL | 62.5 µg/mL | 31.2 µg/mL | 15.6 µg/mL | 7.8 µg/mL | 3.9 µg/mL | |||
| A | H | 3a | 100 | 100 | 100 | 100 | 68.01±1.60 | 49.06±1.69 | 0 | 7.8 |
| A | Cl | 3b | 32.5±1.4 | 31.9±0.5 | 17.1±0.6 | 14.9±0.7 | 0 | 0 | 0 | >250 |
| A | Br | 3c | 67.3±1.4 | 46.1±1.0 | 35.4±0.0 | 25.8±1.4 | 4.9±0.2 | 0 | 0 | 125 |
| A | CH3 | 3d | 11.8±1.8 | 6.7±0.9 | 6.5±0.5 | 0 | 0 | 0 | 0 | >250 |
| A | OCH3 | 3e | 33.6±0.2 | 23.0±1.3 | 4.4±0.1 | 0 | 0 | 0 | 0 | >250 |
| B | H | 3f | 100 | 100 | 100 | 100 | 72.9±1.9 | 34.6±1.1 | 22.5±1.1 | 12 |
| B | Cl | 3g | 35.4±1.1 | 18.1±0.9 | 14.6±1.1 | 12.5±0.2 | 0 | 0 | 0 | >250 |
| B | Br | 3h | 100 | 92.5±1.2 | 87.5±0.2 | 55.1±0.7 | 42.5±0.9 | 21.1±0.6 | 0 | 31.2 |
| B | CH3 | 3i | 42.6±1.7 | 39.2±1.2 | 22.1±0.7 | 21.3±0.6 | 16.0±0.4 | 14.8±0.3 | 0 | >250 |
| B | OCH3 | 3j | 44.7±2.5 | 31.3±1.2 | 25.9±0.8 | 19.8±0.3 | 0 | 0 | 0 | >250 |
| Amph B | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 1 | ||
For a more comprehensive analysis of the results, we grouped the whole series of compounds (3a–j) in two series (I, II) that differ one each other only in the position of the substituent on C-3 of azole ring: (I) includes compounds 3a–e with a CH3 on the 3-position of azole ring; (II) includes compounds 3f–j with a tert-butyl on the same position. The five compounds of each sub-series possess different R substituent (H, Cl, Br, CH3, OCH3) in the p′-position (Chart 1).
In order to understand the role of the p′-R substituent in each sub-series, we compare the activities of sub-series (I) and (II) against C. albicans and C. neoformans in the dose–responses curves of Figs. 2 and 3, respectively.


Figure 2 (left) shows that compound 3a (non p′-substituted) displayed the best activity against C. albicans, followed by 3c (with p′-Br) and 3b (with p′-Cl) while compounds 3d and e (possessing the donor substituents p′-CH3 and p′-OCH3) showed lower activities. Against C. neoformans, 3a showed also the best activity, followed by 3c. Unlike the behavior against C. albicans, 3b showed low activity, similar to those compounds possessing a donor p′-phenyl substituent.
Figure 3 left and right show that compound 3f (non p′-substituted) showed the best activity against C. albicans and C. neoformans, followed by 3h (with p′-Br), while compounds 3g, i and j (possessing the attractant substituent Cl or the donor substituents CH3 and OCH3) showed much lower activities.
Although the activities showed by the whole series of compounds do not reach the high activity of Amphotericin B, it is worth to take into account that 3a and f possess minimal inhibitory concentrations required to inhibit the growth of 50% (MIC50) of C. neoformans as low as 7.8 and 12 µg/mL, respectively, thus being considered highly active.37)
With the purpose of correlating the structure–activity relationship described above with quantitative parameters, we calculated the log P and dipole moment for all studied pyrazolo[3,4-b]pyridines (3a–j), and attempted to find a relationship between these values and the inhibition percentages of the compounds at 250 µg/mL against both fungi (Table 4). For the calculation of log P and dipole moment, we used theoretical calculations at semi-empirical level using Gaussian interface, with the parametric method 3 (PM3). The molecular modeling were prepared using CS ChemOffice Software version 9.0 (Cambridge software).38) The models were minimized until the root mean square (RMS) gradient value reached a value smaller than 0.0001 kcal/mol. The lowest energy structure was used for each molecule to calculate log P values and dipole, as lipophilicity measures.
| Compound | Log P | Dipole (D) | Inh. (%) C.a. at 250 µg/mL | Inh. (%) C.n. at 250 µg/mL |
|---|---|---|---|---|
| 3a | 6.48 | 2.212 | 100 | 100 |
| 3b | 3.13 | 4.542 | 54.9 | 32.5 |
| 3c | 5.23 | 4.624 | 63.6 | 67.3 |
| 3d | 2.98 | 6.135 | 18.2 | 11.8 |
| 3e | 3.49 | 3.889 | 34.9 | 33.6 |
| 3f | 6.80 | 2.143 | 100 | 100 |
| 3g | 3.52 | 4.369 | 45.4 | 35.4 |
| 3h | 6.67 | 2.737 | 100 | 100 |
| 3i | 4.30 | 3.994 | 40.4 | 42.6 |
| 3j | 4.81 | 3.925 | 47.9 | 44.7 |
a) Two hundred fifty micrograms/milliliters doses of compounds 3a–j.
From Table 4 it is clear that the most active compounds (3a, f, h) that reach 100% inhibition at 250 µg/mL have the highest log P and the lower dipole moments values. Compound 3c that follows them in activity (63.6, 67.3% against C. albicans and C. neoformans, respectively) has a log P value of 5.23 although the dipole moment is not concomitantly low. For the remaining compounds (3b, d, e, g) that have lower antifungal activities, the log P ranges from 3.13 to 4.81. Since log P describes the macroscopic hydrophobicity of a molecule, which is a factor that determines its ability to penetrate fungal cell membranes and to reach the interacting sites,39,40) these results suggest that the antifungal activity of compounds 3 would be related to their hydrophobicity being the less-polar compounds more active against to the fungi tested; such relationship is depicted in Figs. 4 and 5.


So, the theoretical calculated log P and dipole moment values are in agreement with the antifungal activity of the new described compounds 3a–j (of which 3a, f, h showed the highest activities due to their low dipoles and polarizabilities). It is important to highlight that the dipolar moment of the derivatives described above is addressed mainly by the characteristics of the p′-substituent in the aryl group and this dipolar moment is located in the fused rings plane (Fig. 6). As observed, the dipole vector orientation is not a key factor in antifungal activity of compounds 3 compared to their scalar quantity. Since 3a has similar dipole orientation with respect to 3b, but different magnitude value in the 2.212 D for 3a in contrast with 4.542 D for 3b.

In this article we described the microwave-assisted synthesis of novel pyrazolo[3,4-b]pyridines by the aza-Diels–Alder reaction between pyrazolilformimidamides 1 and β-nitrostyrenes 2 in toluene as the solvent. The presented synthetic procedure is environmentally friendly and simple process for the preparation of compounds 3. The whole series of compounds 3 were tested against standardized strains of the clinically important fungi C. albicans and C. neoformans. The compounds 3a, c, f, h showed the best activity against both fungi, and appear as good models for the development of new analogues with improved activity.
Commercially available starting materials, reagents and solvents were used as supplied. The TLC analysis was performed on Merck TLC-plates aluminum silica gel 60 F254. Melting point was measured using a Büchi melting point apparatus and was uncorrected. Microwave reactions were performed in glass vessels (10 mL) using a CEM Focused Microwave Synthesis SystemTM apparatus, Model Discover, with power output from 0 to 300 W. 1H- and 13C-NMR spectra were run on a Bruker DPX 400 spectrometer operating at 400 and 100 MHz, respectively, using CDCl3 as solvent and tetramethylsilane as internal standard. Mass spectra were obtained from Shimadzu GCMS-QP 2010 spectrometer (equipped with a direct inlet probe) operating at 70 eV
General Procedure for Preparation of Pyrazolo[3,4-b]pyridines 3All experiments were carried out using a focused microwave reactor (CEM Discover TM). A mixture of the pyrazolylformimidamide (1 mmol) and the β-nitrostyrenes (1 mmol) in toluene (1 mL) was exposed to microwave irradiation during 4 min at 230°C (200 W). Purification of products was performed using column chromatography in a mixture CH2Cl2–hexane (2 : 1) as eluent.
3-Methyl-5-nitro-1,4-diphenyl-1H-pyrazolo[3,4-b]pyridine (3a C19H14N4O2)Yellow oil; yield 65%; 1H-NMR (400 MHz, CDCl3) δ: 2.19 (s, 3H, CH3), 7.21–7.29 (m, 4H), 7.43–7.51 (m, 4H), 8.21 (d, J=7.8 Hz, 2H), 9.46 (s, 1H) ppm; 13C-NMR (100 MHz, CDCl3) δ: 14.4 (CH3), 115.6 (CH), 121.0 (C), 124.3 (C), 125.4 (CH), 128.2 (CH), 128.4 (CH), 128.6 (CH), 129.1 (CH), 136.4 (C), 139.6 (C), 142.5 (C), 144.2 (CH), 150.1 (C), 150.8 (C) ppm. Electron ionization (EI)-MS (70 eV): m/z: 330 (M+, 100), 283 (20); Anal. Calcd for C19H14N4O2: C, 69.08; H, 4.27; N, 16.96. Found: C, 69.26; H, 4.36; N, 16.78.
4-(4-Chlorophenyl)-3-methyl-5-nitro-1-phenyl-1H-pyrazolo[3,4-b]pyridine (3b C19H13ClN4O2)Yellow solid; yield 68%; mp: 134–136°C; 1H-NMR (400 MHz, CDCl3) δ: 2.06 (s, 3H, CH3), 7.31–7.42 (m, 3H), 7.55–7.58 (m, 4H), 8.22 (d, J=7.7 Hz, 2H), 9.26 (s, 1H) ppm; 13C-NMR (100 MHz, CDCl3) δ: 14.6 (CH3), 115.3 (C), 121.6 (CH), 127.0 (CH), 128.8 (CH), 129.3 (CH), 129.3 (CH), 130.8 (C), 135.7 (C), 138.3 (C), 139.5 (C), 140.6 (C), 145.3 (C), 145.7 (CH), 150.4 (C) ppm. EI-MS (70 eV): m/z: 364/366 (M+, 22/7), 329 (8), 69 (100). Anal. Calcd for C19H13ClN4O2: C, 69.56; H, 3.59; N, 15.36. Found: C, 69.45; H, 3.63; N, 15.45.
4-(4-Bromophenyl)-3-methyl-5-nitro-1-phenyl-1H-pyrazolo-[3,4-b]pyridine (3c C19H13BrN4O2)Yellow solid; yield 70%; mp: 150–152°C; 1H-NMR (400 MHz, CDCl3) δ: 2.07 (s, 3H, CH3), 7.28 (d, J=8.4 Hz, 2H), 7.40 (t, J=8.0 Hz, 1H), 7.58 (t, J=7.9 Hz, 2H), 7.72 (d, J=8.4 Hz, 2H), 8.21 (d, J=8.0 Hz, 2H), 9.26 (s, 1H) ppm; 13C-NMR (100 MHz, CDCl3) δ: 14.6 (CH3), 115.2 (C), 121.6 (CH), 123.8 (CH), 127.04 (CH), 129.3 (CH), 129.5 (CH), 131.4 (C), 131.8 (C), 138.3 (C), 139.5 (C), 142.1 (C), 145.3 (C), 145.8 (CH), 150.5 (C) ppm. EI-MS (70 eV): m/z: 408/410 (M+, 100/97), 363 (21), 282 (24). Anal. Calcd for C19H13BrN4O2: C, 55.76; H, 3.20; N, 13.69. Found: C, 55.56; H, 3.29; N, 13.57.
3-Methyl-5-nitro-1-phenyl-4-(p-tolyl)-1H-pyrazolo[3,4-b]pyridine (3d C20H16N4O2)Yellow solid; yield 62%; mp: 157–159°C; 1H-NMR (400 MHz, CDCl3) δ: 2.07 (s, 3H, CH3), 2.51 (s, 3H, CH3), 7.28 (d, J=8.0 Hz, 2H), 7.37 (d, J=8.0 Hz, 2H), 7.40 (t, J=7.9 Hz, 1H), 7.58 (t, J=7.9 Hz, 2H), 8.22 (d, J=7.9 Hz, 2H), 9.22 (s, 1H) ppm; 13C-NMR (100 MHz, CDCl3) δ: 14.4 (CH3), 21.7 (CH3), 115.5 (C), 121.6 (CH), 126.8 (CH), 127.8 (CH), 129.2 (CH), 129.3 (CH), 136.4 (C), 138.5 (C), 139.4 (C), 140.1 (C), 142.1 (C), 145.2 (CH), 145.6 (C), 150.4 (C) ppm. EI-MS (70 eV): m/z: 344 (M+, 100), 299 (47), 69 (75). Anal. Calcd for C20H16N4O2: C, 69.76; H, 4.68; N, 16.27. Found: C, 69.81; H, 4.81; N, 16.22.
4-(4-Methoxyphenyl)-3-methyl-5-nitro-1-phenyl-1H-pyrazolo[3,4-b]pyridine (3e C20H16N4O3)Yellow solid; yield 73%; mp: 164–166°C; 1H-NMR (400 MHz, CDCl3) δ: 2.11 (s, 3H, CH3), 3.94 (s, 3H, OCH3), 7.09 (d, J=8.7 Hz, 2H), 7.33 (d, J=8.7 Hz, 2H), 7.40 (t, J=7.9 Hz 1H), 7.58 (t, J=8.0 Hz, 2H), 8.22 (d, J=8.0 Hz, 2H), 9.20 (s, 1H) ppm; 13C-NMR (100 MHz, CDCl3) δ: 14.6 (CH3), 55.4 (OCH3), 114.0 (CH), 115.7 (C), 121.6 (CH), 124.1 (C), 126.8 (CH), 129.3 (CH), 129.5 (CH), 138.5 (C), 140.4 (C), 141.8 (C), 145.5 (CH), 145.6 (C), 150.4 (C), 160.5 (C) ppm. EI-MS (70 eV): m/z: 360 (M+, 32), 315 (40). Anal. Calcd for C20H16N4O3: C, 66.66; H, 4.48; N, 15.55. Found: C, 66.55; H, 4.57; N, 15.43.
3-(tert-Butyl)-5-nitro-1,4-diphenyl-1H-pyrazolo[3,4-b]pyridine (3f C22H20N4O2)Yellow oil; yield 68%; 1H-NMR (400 MHz, CDCl3) δ: 1.15 (s, 9H, CH3), 7.44 (d, J=8.0 Hz, 2H), 7.50–7.62 (m, 6H), 8.27 (d, J=8.1 Hz, 2H), 9.02 (s, 1H) ppm; 13C-NMR (100 MHz, CDCl3) δ: 30.2 (CH3), 34.5 (C), 114.3 (C), 122.1 (CH), 126.8 (CH), 128.1 (C), 129.2 (CH), 129.4 (CH), 129.7 (CH), 134.1 (C), 138.7 (C), 140.7 (C), 142.9 (C), 143.6 (CH), 150.8 (C), 155.9 (C) ppm. EI-MS (70 eV): m/z: 372 (M+, 52), 357 (79), 85 (100). Anal. Calcd for C22H20N4O2: C, 70.95; H, 5.41; N, 15.04. Found: C, 70.84; H, 5.34; N, 15.20.
3-(tert-Butyl)-4-(4-chlorophenyl)-5-nitro-1-phenyl-1H-pyrazolo[3,4-b]pyridine (3g C22H19ClN4O2)Yellow solid; yield 74%; mp: 178–179°C; 1H-NMR (400 MHz, CDCl3) δ: 1.18 (s, 9H, CH3), 7.38–7.43 (m, 3H), 7.53 (d, J=8.0 Hz, 2H), 7.59 (t, J=7.9 Hz, 2H), 8.26 (d, J=7.9 Hz, 2H), 9.03 (s, 1H) ppm; 13C-NMR (100 MHz, CDCl3) δ: 30.3 (CH3), 34.5 (C), 114.0 (C), 122.1 (CH), 126.9 (CH), 128.5 (CH), 129.2 (CH), 130.8 (CH), 132.6 (C), 135.9 (C), 138.5 (C), 139.5 (C), 142.7 (CH), 143.7 (C), 150.8 (C), 155.8 (C) ppm. EI-MS (70 eV): m/z: 406/408 (M+, 22/8), 391 (41), 346 (25), 83 (100). Anal. Calcd for C22H19ClN4O2: C, 64.95; H, 4.71; N, 13.77. Found C, 64.76; H, 4.85; N, 13.66.
4-(4-Bromophenyl)-3-(tert-butyl)-5-nitro-1-phenyl-1H-pyrazolo-[3,4-b]pyridine (3h C22H19BrN4O2)Yellow oil; yield 77%; 1H-NMR (400 MHz, CDCl3) δ: 1.18 (s, 9H, CH3),7.32 (d, J=8.3 Hz, 2H), 7.41 (t, J=8.0 Hz, 1H), 7.59 (t, J=8.0 Hz, 2H), 7.68 (d, J=8.3 Hz, 2H), 8.25 (d, J=8.0 Hz, 2H), 9.03 (s, 1H) ppm; 13C-NMR (100 MHz, CDCl3) δ: 30.3 (CH3), 34.5 (C), 114.0 (C), 122.1 (CH), 124.1 (CH), 126.9 (CH), 129.2 (CH), 131.1 (C), 131.5 (CH), 133.1 (C), 136.4 (C), 138.5 (C), 139.4 (C), 143.7 (CH), 150.8 (C), 155.8 (C) ppm. EI-MS (70 eV): m/z: 450/452 (M+, 21/21), 435/437 (32/37), 69 (100). Anal. Calcd for C22H19BrN4O2: C, 58.55; H, 4.24; N, 12.41. Found: C, 58.63; H, 4.31; N, 12.49.
3-(tert-Butyl)-5-nitro-1-phenyl-4-(p-toluyl)-1H-pyrazolo[3,4-b]pyridine (3i C23H22N4O2)Yellow solid; yield 77%; mp: 157–159°C; 1H-NMR (400 MHz, CDCl3) δ: 1.11 (s, 9H, CH3), 1.16 (s, 3H, CH3), 7.23–7.27 (m, 4H), 7.34 (t, J=7.6 Hz, 1H), 7.53 (t, J=8.0 Hz, 2H), 8.20 (d, J=7.9 Hz, 2H), 8.92 (s, 1H) ppm; 13C-NMR (100 MHz, CDCl3) δ: 21.5 (CH3), 30.3 (CH3), 34.5 (C), 114.5 (C), 121.7 (C), 122.1 (CH), 126.8 (CH), 128.6 (C), 128.8 (C), 129.1 (CH), 129.3 (CH), 131.01(C), 139.6 (C), 140.9 (C), 143.4 (CH), 150.8 (C), 156.01 (C) ppm. EI-MS (70 eV): m/z: 386 (M+, 13), 326 (85), 281 (34), 57 (100). Anal. Calcd for C23H22N4O2: C, 71.48; H, 5.74; N, 14.50. Found: C, 71.41; H, 5.63; N, 14.57.
3-(tert-Butyl)-4-(4-methoxyphenyl)-5-nitro-1-phenyl-1H-pyrazolo[3,4-b]pyridine (3j C23H22N4O3)Yellow oil; yield 73%; 1H-NMR (400 MHz, CDCl3) δ: 1.19 (s, 9H, CH3), 3.93 (s, 3H, OCH3), 7.0 (d, J=8.8 Hz, 2H), 7.34 (d, J=8.8 Hz, 2H), 7.40 (t, J=8.0 Hz, 1H), 7.55 (t, J=8.0 Hz, 2H), 8.26 (d, J=7.9 Hz, 2H), 8.96 (s, 1H) ppm; 13C-NMR (100 MHz, CDCl3) δ: 30.27 (CH3), 34.52 (C), 56.39 (OCH3), 113.63 (CH), 114.72 (C), 122.07 (CH), 125.93 (C), 126.76 (C), 129.14 (CH), 130.74 (CH), 136.42 (C), 138.70 (C), 140.63 (C), 143.32 (C), 146.80 (C), 156.01 (C), 160.58 (C) ppm. EI-MS (70 eV): m/z: 402 (M+, 17.6), 387 (25), 149 (48), 228 (64). Anal. Calcd for C23H22N4O3: C, 68.64; H, 5.51; N, 13.92. Found: C, 68.49; H, 5.45; N, 13.83.
Antifungal ActivityMicroorganisms and MediaFor the antifungal evaluation, strains from the American Type Culture Collection (ATCC), Rockville, MD, U.S.A., C. albicans ATCC 10231 and C. neoformans ATCC 32264, were used. Strains were grown on Sabouraud-chloramphenicol agar slants for 48 h at 30°C, maintained on slopes of Sabouraud-dextrose agar (SDA, Oxoid) and sub-cultured every 15 d to prevent pleomorphic transformations. Inocula were obtained according to reported procedures (CLSI, 2008)41) and adjusted to 1–5×103 cells with colony forming units (CFU)/mL.
Fungal Growth Inhibition Percentage DeterminationYeasts broth microdilution technique M27-A3 of CLSI (2008) were performed in 96-well microplates. For the assay, compound test-wells (CTWs) were prepared with stock solutions of each compound in DMSO (maximum concentration ≤1%), diluted with RPMI-1640, to final concentrations of 250–3.9 µg/mL. An inoculum suspension (100 µL) was added to each well (final volume in the well=200 µL). A growth control well (GCW) (containing medium, inoculum, and the same amount of DMSO used in a CTW, but compound-free) and a sterility control well (SCW) (sample, medium, and sterile water instead of inoculum) were included for each fungus tested. Microtiter trays were incubated in a moist, dark chamber at 30°C for 48 h for both yeasts. Microtiter plates were read in a VERSA Max microplate reader (Molecular Devices, Sunnyvale, CA, U.S.A.). Amphotericin B was used as positive control. Tests were performed in triplicate. Reduction of growth for each compound concentration was calculated as follows: % of inhibition=100−[(OD 405 CTW−OD 405 SCW)/(OD 405 GCW−OD 405 SCW)]. The mean±standard deviations (S.D.) were used for constructing the dose–response curves representing % inhibition vs. concentration of each compound. Dose–response curves were constructed with SigmaPlot 11.0 software. MIC50 was defined as the lowest concentration of a compound that inhibited 50% of the growth control.
Authors wish to thank the Universidad del Valle, COLCIENCIAS and the Science, Technology and Innovation Fund-General Royalties System (FCTeI-SGR) under contract BPIN 2013000100007 for financial support. Susana Zacchino acknowledges ANPCyT PICT 2014-1170 and the National University of Rosario for Grant number 19/0522.
The authors declare no conflict of interest.