2017 Volume 65 Issue 4 Pages 365-372
2017 Volume 65 Issue 4 Pages 365-372
In this report, we describe a new method for the synthesis of densely functionalized 2(1H)-pyrazinones. Treatment of mesoionic 1,3-oxazolium-5-olates with carbanions derived from activated methylene isocyanides (p-toluenesulfonylmethyl isocyanide (TosMIC) and ethyl isocyanoacetate) causes a novel ring transformation affording 2(1H)-pyrazinones in moderate to high yields. The cytotoxicity and antibacterial activity of some of the obtained products were studied and some of the products exhibited tumor-specific cytotoxicity.
The 2(1H)-pyrazinone is an important privileged scaffold in medicinal chemistry, as well as in several alkaloids with diverse biological activity, including antitumor and antiviral properties.1–6) Therefore, several methods for the synthesis of 2(1H)-pyrazinones have been described in the literature: (a) condensation of α-aminoamides with α-diketones,7,8) (b) condensation of α-aminonitrile with oxalyl halides,6,9,10) and (c) Ugi four-component reactions.11) Whereas these methods have proven very useful for the synthesis of 2(1H)-pyrazinones, they generally involve multistep synthetic operations and/or a hazardous cyanide source that limit the scope of these reactions. Indeed, it is still desirable to develop an efficient method for the synthesis of 2(1H)-pyrazinones.
As a continuation of our studies on the synthetic potential of mesoionic 4-trifluoroacetyl-1,3-oxazolium-5-olates (1) for heterocyclic synthesis,12) we herein report a reaction of mesoionic oxazoles (1) with carbanions derived from activated methylene isocyanides (p-toluenesulfonylmethyl isocyanide (TosMIC) and ethyl isocyanoacetate). The mesoionic oxazoles (1) are easily prepared from N-acyl-N-alkylglycines (2) in a one step through the cyclodehydration by trifluoroacetic anhydride followed by trifluoroacetylation at C-4 position of an intermediary mesoionic 1,3-oxazolium-5-olates12) (Chart 1).

TosMIC is a versatile, widely applicable reagent bearing an active methylene group and readily forms a stabilized, nucleophilic carbanion, which will react with a variety of electrophiles, such as aldehydes, ketones and imines.13) This class of reagent has most commonly been used in heterocyclic ring construction, in particular, of oxazole, pyrrole and imidazole moieties.
We have preliminarily reported that the unexpected reaction of mesoionic oxazoles (1) with TosMIC proceeds via an initial attack of TosMIC anions on the C-2 position of the ring followed by autooxidation to afford 2(1H)-pyrazinones in good yields.14) The origin of C-2 carbonyl oxygen in the 2(1H)-pyrazinones was molecular oxygen.
We report here our detailed studies of the reactions of 1 with carbanions derived from activated methylene isocyanides such as TosMIC and ethyl isocyanoacetate, and the behavior of 1 with TosMIC from the comparison with the reaction of the related mesoionic 1,3-thiazolium-5-olates (7) with TosMIC in order to elucidate the presence of 4-trifluoroacetyl group essential for the reaction; herein, efficient syntheses of multiply substituted 2(1H)-pyrazinones and their anti-bacterial and tumor-cytotoxic activities are reported.
In our previous communication,14) we examined the reaction of 1a with TosMIC under varying reaction conditions to determine the optimum conditions and reported the isolated yields of 3a–i which were shown in Table 1. The best conditions to obtain the 2-pyrazinones 3a–i were achieved using 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as the base in N,N-dimethylformamide (DMF) at 0°C under O2 atmosphere with bubbling by O2 throughout the reaction. It was found that the reaction was very sensitive to the dioxygen and the yield of 3a increased to 72% in the presence of dioxygen. The pathway of the reaction was elucidated to conduct the reaction under an atmosphere of 18O2 and the carbonyl group of amide in 3a contained more than 67% of the 18O label.14)
 | |||||
|---|---|---|---|---|---|
| Entry | 1 | R1 | R2 | Conditions | Products (Yield, %)b) |
| 1 | a | Me | Ph | 0°C, 3 h | 3a (72), 4a (16) |
| 2 | b | Ph | Ph | 0°C, 3 h | 3b (65), 4b (10) |
| 3 | c | Ph | Me | −40°C, 12 h | 3c (42), 4c (3) |
| 4 | d | Me | 4-BrC6H4 | 0°C, 1 h | 3d (70), 4d (20) |
| 5 | e | Me | 4-MeOC6H4 | 0°C, 1 h | 3e (78), 4e (5) |
| 6 | f | C6H5CH2 | Ph | 0°C, 2 h | 3f (55), 4f (36) |
| 7 | g | 4-MeOC6H4CH2 | Ph | 0°C, 2 h | 3g (53), 4g (34) |
| 8 | h | C6H5CH2 | 4-MeOC6H4 | 0°C, 2 h | 3h (58), 4h (35) |
| 9 | i | Me | t-Bu | 0°C, 5 h | 3i (11), 4i (0) |
| 10 | j | Ph | 4-NO2C6H4 | 0°C, 3 h | 3j (25), 4j (44) |
| 11 | k | Ph | 4-MeOC6H4 | 0°C, 1 h | 3k (35), 4k (36) |
a) Reactions were carried out using 1 (0.50 mmol), TosMIC (0.75 mmol) and DBU (1.00 mmol) in DMF (3 mL) under atmosphere of oxygen. b) Isolated yield.
In this study, two new substrates 1j and k were subjected to the reaction (Table 1, entries 10 and 11). In addition, imidazo[1,5-a]pyrazin-8(7H)-one derivatives (4a–h, j, k) were also isolated as a side product as shown in Table 1. The reaction of TosMIC with aldimines to form imidazoles is known as the van Leusen imidazole synthesis.15) The imidazo[1,5-a]pyrazine core was reported to be synthesized via condensation with TosMIC with pyrazin-2-one derivative.16) Therefore, the formation of imidazo[1,5-a]pyrazin-8(7H)-one derivatives (4) could be explained by the further reaction of the 3 with excessive TosMIC. Indeed, the reaction of 3a with TosMIC was reported to give 4a in high yield.17) The C3-position is elctrophilically reactive position in the pyrazinone core18–20) and could undergo nucleophilic attack by nucleophiles such as being part of an imine system.
On the basis of the above results, we tried to react 1a–h with ethyl isocyanoacetate, using the same conditions for the reaction with TosMIC.14) Gratifying, we found that the ring transformation reaction could be efficiently occurred in high yields. Thus, the substituents on C2 and N3 of the mesoionic ring on the reaction were investigated and the results are shown in Table 2. In the reaction of ethyl isocyanoacetate, an imidazo[1,5-a]pyrazin-8(7H)-one derivative was not obtained. In separate experiment, the reaction of 5a with ethyl isocyanoacetate failed to afford the expected imidazo[1,5-a]pyrazin-8(7H)-one derivative under the comparable reaction conditions. Also, the reaction of 3a with ethyl isocyanoacetate failed to afford the corresponding imidazo[1,5-a]pyrazine derivative. On the other hand, the reaction of 5a with TosMIC gave 6 in 65% yield (Chart 2). Overall, the yields of the 2-pyrazinone products are generally better for the reaction with ethyl isocyanoacetate than the reaction with TosMIC (Tables 1, 2).
 | |||||
|---|---|---|---|---|---|
| Entry | 1 | R1 | R2 | Conditions | Products (Yield, %)b) |
| 1 | a | Me | Ph | 0°C, 3 h | 5a (92) |
| 2 | b | Ph | Ph | 0°C, 3 h | 5b (88) |
| 3 | c | Ph | Me | −40°C, 12 h | 5c (52) |
| 4 | d | Me | 4-BrC6H4 | 0°C, 1 h | 5d (85) |
| 5 | e | Me | 4-MeOC6H4 | 0°C, 1 h | 5e (85) |
| 6 | f | C6H5CH2 | Ph | 0°C, 2 h | 5f (90) |
| 7 | g | 4-MeOC6H4CH2 | Ph | 0°C, 2 h | 5g (91) |
| 8 | h | C6H5CH2 | 4-MeOC6H4 | 0°C, 2 h | 5h (91) |
a) Reactions were carried out using 1 (0.50 mmol), TosMIC (0.75 mmol) and DBU (1.00 mmol) in DMF (3 mL) under atmosphere of oxygen. b) Isolated yield.

We next examined the effect of 4-trifluoroacetyl group in mesoionic oxazoles 1. In our laboratory, we have synthesized 4-trifluoroacetyl- (7a), 4-acetyl- (7b)21) and 4-formyl-1,3-thiazolium-5-olates (7c). Notably, there is much less information about the reactivity of 1,3-thiazolium-5-olates in the literature compared to 1,3-oxazolium-5-olates. A comparative study of the suitability of 4-acyl group in the reaction of 7a–c with TosMIC under the same reaction conditions with 1 was undertaken. The results were shown in Table 3. The trifluoroacetyl derivative 7a worked well (entry 1). On the other hand, 4-acetyl (7b) and 4-formyl (7c) derivatives afforded poor conversion (entries 2 and 3).
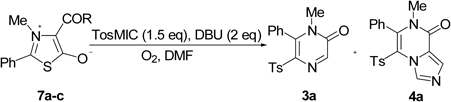 | |||||
|---|---|---|---|---|---|
| Entry | 7 | R | Conditions | Products (Yield, %)b) | |
| 3a | 4a | ||||
| 1 | a | CF3 | 0°C, 2 h | 68 | 2 |
| 2 | b | CH3 | 0°C, 12 h | 10 | 11 |
| 3 | c | H | 0°C, 12 h | 13 | 15 |
a) Reactions were carried out using 1 (0.50 mmol), TosMIC (0.75 mmol) and DBU (1.00 mmol) in DMF (3 mL) under atmosphere of oxygen. b) Isolated yield.
The molecular structures of 3a14) and 4a17) were previously confirmed by X-ray diffraction study of single crystals. A tentative mechanism which accounts for the formation of 3 is described in the communication.14) Thus, initial nucleophilic attack by the TosMIC anion on C-2 of 1 gave rise to an adduct which is converted to the 2-pyrazineones (3) via a ring closure followed by auto-oxidation.
The anti-microbial activities of new pyrazinones (3a–j and 5a–h) for six Gram-negative bacteria and six Gram-positive bacteria (listed in Experimental section) were measured by disc diffusion method.22) All the tested pyrazinones (3a–j and 5a–h) showed no inhibition zone same as that of solvent control, whereas Gentamicin and Kanamycin showed inhibition zone (≥11 mm) against tested Gram-negative bacteria except for Pseudomonas aeruginosa 01 and Vancomycin showed inhibition zone (≥10 mm) against Gram-positive bacteria (data not shown). The tested compounds (3a–j and 5a–h) were not active against the all tested microorganisms. Additional compounds in this series will be prepared to try to improve activity.
The compounds 3a–j and 5a–h were evaluated for their cytotoxicity against human oral squamous cell carcinoma cell lines (Ca9–22 derived from gingival tissue, and HSC-2, HSC-3 and HSC-4 derived from tongue), and human normal oral cells (HGF gingival fibroblasts, HPLF periodontal ligament fibroblasts and HPC pulp cells), using 5-fluorouracil (5-FU) and melphalan as the positive controls. These data for the selected compounds are presented in Table 4. Among ten 3 series and eight 5 series, 3g showed the highest cytotoxicity, followed by 3f, 5g, and 3j, whereas the other compounds showed much lower cytotoxicity. Out of these four compounds, 3g showed the highest tumor-specifity (TS=4.7), followed by 3f (TS=4.0). When tumor-selectivity was calculated by Ca9–22 and HGF cells, both derived from gingival tissues, 3g showed higher tumor-specifity [TS=7.3 (358/49)], as compared with melphalan [TS=1.9 (111/57)] (Table 4). The rest of the assayed compounds did not show any cytotoxicities against the studied tumor cells (CC50>400 µM). The compounds in 3 series are more potent than the analogs in 5 series in which the same substituents are present at 1 and 6 positions (data not shown). Compounds 3g, f and 5g, with 1-(4-methoxyphenyl)methyl or 1-benzyl and 6-phenyl groups, were found to be the derivatives with the highest cytotoxic activity of the two series. 3j with 4-nitrophenyl group showed moderate cyctotoxicity against Ca9–22 and HSC-2 cells. These results suggest that further modification of these scaffolds might lead to compounds with enhanced cytotoxic activities.
| Compounds | Human oral squamous cell carcinoma cell lines | Human oral normal cells | SIb) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca9–22 | HSC-2 | HSC-3 | HSC-4 | Mean (A)c) | HGF | HPLF | HPC | Mean (B)d) | B/A | |
| 3f | 70 | 93 | 95 | 85 | 86±11 | 275 | 362 | >400 | >346 | >4.0 |
| 3g | 49 | 47 | 180 | 50 | 82±66 | 358 | >400 | >400 | >386 | >4.7 |
| 3j | 75 | 71 | 199 | 101 | 112±60 | 173 | 138 | 62 | 124±57 | 1.1 |
| 5g | 163 | 87 | 95 | 87 | 108±37 | 228 | 225 | 216 | 223±6 | 2.1 |
| 5-FU | 80 | 9.5 | 7.8 | <7.8 | 26±36 | >1000 | >1000 | >1000 | >1000 | >38.5 |
| Melphalan | 57 | 11 | 12 | 9.8 | 22±23 | 111 | 192 | 145 | 149±41 | 6.8 |
a) The CC50 figure is the concentration of compound required to kill 50% of the cells and is the average of two independent determinations. b) The letter SI refer to the selectivity index. c) Mean A is average CC50 value of the compound toward Ca9–22, HSC-2, HSC-3, and HSC-4 squamous cell carcinoma cell lines. d) Mean B is average CC50 value of the compound toward human non-malignant HGF gingival fibroblasts, HPLF periodontal ligament fibroblasts and HPC pulp cells.
We described herein a novel type of ring transformation of mesoionic oxazoles 1 into 2(1H)-pyrazinones 3 and 5 via an initial attack of TosMIC or ethyl isocyanoacetate on the C-2 position of the ring, opening of the oxazole ring, subsequent cyclization, and autoxidation. This unique ring transformation reaction of mesoionic oxazoles with TosMIC was not anticipated but the reaction is general as evidenced by the examples indicated in Tables 1 and 2. The method appears to be useful and convenient in terms of the ready accessibility of the starting materials, operational simplicity and mild condition. In addition, the 2(1H)-pyrazinones showed promising anticancer potency through preliminary biological studies.
Melting points (mp) were measured with a Yanaco MP-J3 melting point apparatus and are uncorrected. NMR spectra were recorded on a Bruker AVANCE500 (500 MHz for 1H and 126 MHz for 13C) with tetramethylsilane (Me4Si) as an internal reference and CDCl3 as the solvent. 1H- and 13C-NMR spectral data are reported in parts per million (δ) relative to Me4Si. IR spectra were recorded on a JASCO FT/IR-4100 spectrometer. Mass spectra were recorded on JEOL JMS-GC mate II spectrometer with a direct inlet system at 70 eV and a Bruker micrOTOF-Q mass spectrometer with methanol as the solvent. Elemental analyses were carried out on a Yanaco CHN Corder MT-5 at the Integrated Center for Science, Ehime University. Standard work-up means that the organic layers were finally dried over anhyd. Na2SO4, filtered, and concentrated in vacuo below 37°C using a rotary evaporator.
MaterialsThe following compounds were prepared by employing the reported method.
N-Benzoyl-N-methylglycine (2a)mp 101–104°C (mp23) 102–104°C).
N-Benzoyl-N-phenylglycine (2b)mp 126–128°C (mp23) 127–129°C).
N-Acetyl-N-phenylglycine (2c)mp 196–198°C (mp24) 193–195°C).
N-(4-Bromobenzoyl)-N-methylglycine (2d)mp 143–145°C (mp25) 147–150°C).
N-(4-Methoxybenzoyl)-N-methylglycine (2e)mp 151–154°C (mp23) 155–160°C).
N-Benzoyl-N-benzylglycine (2f)mp 103–104°C (mp26) 106–107°C).
N-Benzoyl-N-(4-methoxyphenylmethyl)glycine (2g)mp 157–158°C (mp27) 157–158°C).
N-Benzyl-N-(4-methoxybenzoyl)glycine (2h)mp 149–151°C (mp25) 149–151°C).
N-Methyl-N-pivaloylglycine (2i)mp 65–68°C (mp28) 75–76°C).
N-(4-Nitrobenzoyl)-N-phenylglycine (2j)mp 178–180°C (mp23) 172–175°C).
N-(4-Methoxybenzoyl)-N-phenylglycine (2k)mp 135–137°C (mp23) 158–163°C).
4-Trifluoroacetyl-3-methyl-2-phenyl-1,3-oxazolium-5-olate (1a)Pale yellow crystals, 95% yield. mp 161–163°C (mp29) 162–163°C).
4-Trifluoroacetyl-2,3-diphenyl-1,3-oxazolium-5-olate (1b)Yellow crystals, 94% yield. mp 194–196°C (mp29) 194–196°C).
4-Trifluoroacetyl-2-methyl-3-phenyl-1,3-oxazolium-5-olate (1c)White crystals, 90% yield. mp 200–203°C (mp29) 211–212°C).
2-(4-Bromophenyl)-4-trifluoroacetyl-3-methyl-1,3-oxazolium-5-olate (1d)White crystals, 90% yield. mp 188–191°C (mp25) 188–191°C).
4-Trifluoroacetyl-2-(4-methoxyphenyl)-3-methyl-1,3-oxazolium-5-olate (1e)Pale yellow crystals, 82% yield. mp 142–143°C (ethyl acetate/hexane). (mp17) 142–143°C).
3-Benzyl-4-trifluoroacetyl-2-phenyl-1,3-oxazolium-5-olate (1f)White crystals, 83% yield. mp 143–145°C (mp27) 143–145°C).
4-Trifluoroacetyl-3-(4-methoxybenyl)-2-phenyl-1,3-oxazolium-5-olate (1g)White crystals, 75% yield. mp 147–148°C (mp27) 147–148°C).
3-Benzyl-4-trifluoroacetyl-2-(4-methoxyphenyl)-1,3-oxazolium-5-olate (1h)White crystals, 89% yield. mp 158–160°C (mp26) 158–160°C).
2-tert-Butyl-4-trifluoroacetyl-3-methyl-1,3-oxazolium-5-olate (1i)White crystals, 67% yield. mp 120–121°C (mp28) 120–121°C).
4-Trifluoroacetyl-2-(4-nitrophenyl)-3-phenyl-1,3-oxazolium-5-olate (1j)Yellow crystals, 70% yield. mp 175–178°C (ethyl acetate/hexane). (mp17) 175–178°C).
4-Trifluoroacetyl-2-(4-methoxyphenyl)-3-phenyl-1,3-oxazolium-5-olate (1k)Pale yellow crystals, 93% yield. mp 203–205°C (mp25) 182–185°C).
General Procedure for Synthesis of 2(1H)-Pyrazinones (3) and Imidazo[1,5-a]pyrazin-8(7H)-ones (4)To a stirred solution of TosMIC (146 mg, 0.75 mmol) in DMF (3 mL) was added DBU (152 mg, 1.00 mmol) at 0°C, and the mixture was stirred for 1 h under atmosphere of oxygen. To the mixture was added 4-trifluoroacetyl-1,3-oxazolium-5-olate 1 (0.50 mmol), and the whole was stirred at 0°C for an additional several hours. After workup with aq. Na2CO3, the mixture was extracted with AcOEt (×3). The combined organic layers were washed with brine, dried over anhyd. Na2SO4, and evaporated. The residue was purified by column chromatography (silica gel, hexane–AcOEt=4 : 1 to 1 : 1) to give the products 3 and 4.
1-Methyl-5-[(4-methylphenyl)sulfonyl]-6-phenylpyrazin-2(1H)-one (3a)White crystals, 72% yield. mp 147–149°C (CHCl3–hexane). IR (KBr) cm−1: 3057, 1674, 1662, 1487, 1322, 575. 1H-NMR (500 MHz, CDCl3) δ: 2.41 (s, 3H, ArCH3), 3.13 (s, 3H, NCH3), 7.21–7.27 (m, 4H, ArH), 7.51–7.59 (m, 5H, ArH), 8.13 (s, 1H, H-3). 13C-NMR (126 MHz, CDCl3) δ: 21.5 (CH3), 33.3 (NCH3), 128.3 (CH), 128.6 (CH), 128.9 (CH), 129.0 (C), 129.4 (CH), 130.6 (CH), 134.0 (C-5), 137.3 (C), 144.3 (C), 145.0 (C-6), 145.8 (C-3), 155.5 (CO). MS m/z: 313 (M+, 66), 58 (100). Anal. Calcd for C18H16N2O3S: C, 63.51; H, 4.74; N, 8.23. Found: C, 63.32; H, 5.03; N, 8.09.
5-[(4-Methylphenyl)sulfonyl]-1,6-diphenylpyrazin-2(1H)-one (3b)Yellow crystals, 65% yield. mp 228–230°C (CHCl3–hexane). IR (KBr) cm−1: 3059, 3050, 1685, 1485, 1328, 1214, 1169, 1160, 806, 692, 681, 651, 585, 553. 1H-NMR (500 MHz, CDCl3) δ: 2.43 (s, 3H, ArCH3), 6.89–6.91 (m, 2H, ArH), 7.02–7.04 (m, 2H, ArH), 7.17–7.27 (m, 8H, ArH), 7.62 (d, J=8.0 Hz, 2H, ArH), 8.24 (s, 1H, H-3). 13C-NMR (126 MHz, CDCl3) δ: 21.7 (CH3), 127.7 (CH), 128.3 (CH), 128.6 (CH), 128.7 (C), 129.2 (C), 129.2 (CH), 129.5 (CH), 129.8 (CH), 130.0 (CH), 134.0 (C), 135.3 (C), 137.3 (C), 144.5 (CH), 144.8 (C), 147.6 (C-3), 155.2 (CO). MS m/z: 402 (M+, 5.6), 180 (100). Anal. Calcd for C23H18N2O3S·0.5H2O: C, 67.14; H, 4.65; N, 6.81. Found: C, 66.80; H, 4.73; N, 6.75.
6-Methyl-5-[(4-methylphenyl)sulfonyl]-1-phenylpyrazin-2(1H)-one (3c)Orange crystals, 42% yield. mp 212–215°C (CHCl3–hexane). IR (KBr) cm−1: 2926, 1680, 1508, 1491, 1305, 1159, 696, 675, 586, 556. 1H-NMR (500 MHz, CDCl3) δ: 2.46 (s, 6H, CH3 and ArCH3), 7.13 (d, J=7.5 Hz, 2H, ArH), 7.37 (d, J=8.0 Hz, 2H, ArH), 7.52–7.59 (m, 3H, ArH), 7.91 (d, J=8.5 Hz, 2H, ArH), 8.03 (s, 1H, H-3). 13C-NMR (126 MHz, CDCl3) δ: 17.3 (CH3), 21.7 (CH3), 127.2 (CH), 128.5 (CH), 129.8 (CH), 130.2 (C), 130.5 (CH), 133.7 (C), 135.5 (C), 137.1 (C), 143.6 (C), 144.8 (C), 145.4 (C-3), 155.5 (CO). MS m/z: 340 (M+, 11.7), 118 (100). Anal. Calcd for C18H16N2O3S·0.5H2O: C, 61.87; H, 4.90; N, 8.02. Found: C, 62.21; H, 4.61; N, 8.24.
6-(4-Bromophenyl)-1-methyl-5-[(4-methylphenyl)sulfonyl]pyrazin-2(1H)-one (3d)Pale yellow crystals, 70% yield. mp 178–180°C (CHCl3–hexane). IR (KBr) cm−1: 3050, 2953, 2922, 1676, 1593, 1483, 1323, 1136, 1010, 850, 655, 582. 1H-NMR (500 MHz, CDCl3) δ: 2.42 (s, 3H, ArCH3), 3.14 (s, 3H, NCH3), 7.13 (dd, J=8.4, 1.9 Hz, 2H, ArH), 7.26–7.27 (m, 2H, ArH), 7.60 (dd, J=8.3, 1.7 Hz, 2H ArH), 7.69 (dd, J=8.5, 1.9 Hz, 2H, ArH), 8.13 (s, 1H, H-3). 13C-NMR (126 MHz, CDCl3) δ: 21.7 (CH3), 33.4 (NCH3), 125.3 (C), 127.9 (C), 128.3 (CH), 129.6 (CH), 130.3 (CH), 132.3 (CH), 134.0 (C), 137.1 (C), 143.8 (C), 144.7 (C), 146.2 (C-3), 155.4 (CO). MS m/z: 418 (4.2)+420 (4.3) (M+, 1 : 1), 196 (100). Anal. Calcd for C18H15BrN2O3S·0.5H2O: C, 50.48; H, 3.77; N, 6.54. Found: C, 50.47; H, 3.77; N, 6.68.
6-(4-Methoxyphenyl)-1-methyl-5-[(4-methylphenyl)sulfonyl]pyrazin-2(1H)-one (3e)Pale yellow crystals, 78% yield. mp 135–138°C (CHCl3–hexane). IR (KBr) cm−1: 2955, 2833, 1671, 1497, 1257, 1178, 1152, 848, 658. 1H-NMR (500 MHz, CDCl3) δ: 2.40 (s, 3H, ArCH3), 3.15 (s, 3H, NCH3), 3.90 (s, 3H, OCH3), 7.02 (d, J=8.7 Hz, 2H, ArH), 7.13 (d, J=8.6 Hz, 2H, ArH), 7.23 (d, J=8.2 Hz, 2H, ArH), 7.57 (d, J=8.2 Hz, 2H, ArH), 8.11 (s, 1H, H-3). 13C-NMR (126 MHz, CDCl3) δ: 21.6 (CH3), 33.3 (NCH3), 55.4 (OCH3), 114.3 (CH), 120.8 (C), 128.2 (CH), 129.4 (CH), 130.2 (CH), 134.4 (C), 137.5 (C), 144.3 (C), 145.3 (C), 145.5 (C-3), 155.8 (CO), 161.2 (C). MS m/z: 370 (M+, 5.1), 148 (100). Anal. Calcd for C19H18N2O4S·0.5H2O: C, 60.14; H, 5.05; N, 7.38. Found: C, 60.02; H, 5.06; N, 7.35.
1-Benzyl-5-[(4-methylphenyl)sulfonyl]-6-phenylpyrazin-2(1H)-one (3f)White crystals, 55% yield. mp 164–165°C (CHCl3–hexane). IR (KBr) cm−1: 3063, 3046, 3030, 1668, 1327, 1162, 704, 654, 576. 1H-NMR (500 MHz, CDCl3) δ: 2.41 (s, 3H, ArCH3), 4.95 (s, 2H, NCH2Ar), 6.75 (d, J=7.0 Hz, 2H, ArH), 7.00 (d, J=7.0 Hz, 2H, ArH), 7.05–7.30 (m, 5H, ArH), 7.37 (t, J=8.0 Hz, 2H, ArH), 7.54 (m, 3H, ArH), 8.21 (s, 1H, H-3). 13C-NMR (126 MHz, CDCl3) δ: 21.6 (CH3), 48.7 (NCH2), 127.3 (CH), 128.0 (CH), 128.1 (C), 128.3 (CH), 128.4 (CH), 128.5 (CH), 129.4 (CH), 129.5 (CH), 130.6 (CH), 134.4 (C), 134.7 (C), 137.3 (C), 144.5 (C), 145.0 (C), 146.8 (C-3), 155.6 (CO). MS m/z: 416 (M+, 22.8), 91 (100). Anal. Calcd for C24H20N2O3S: C, 69.21; H, 4.84; N, 6.73. Found: C, 69.21; H, 4.79; N, 6.86.
1-(4-Methoxyphenylmethyl)-5-[(4-methylphenyl)sulfonyl]-6-phenylpyrazin-2(1H)-one (3g)Pale yellow oil, 53% yield. IR (neat) cm−1: 3062, 2957, 3014, 1674, 1514, 1327, 1250, 1162, 795. 1H-NMR (500 MHz, CDCl3) δ: 2.40 (s, 3H, ArCH3), 3.75 (s, 3H, NCH3), 4.89 (s, 2H, NCH2Ar), 6.69 (s, 4H, ArH), 7.03 (d, J=8.3 Hz, 2H, ArH), 7.22 (d, J=8.1 Hz, 2H, ArH), 7.41 (t, J=8.1 Hz, 2H, ArH), 7.53–7.56 (m, 3H, ArH), 8.19 (s, 1H, H-3). 13C-NMR (126 MHz, CDCl3) δ: 21.6 (CH3), 48.3 (NCH2), 55.3 (OCH3), 113.8 (CH), 126.7 (C), 128.1 (C), 128.3 (CH), 128.4 (CH), 129.1 (CH), 129.5 (CH), 129.5 (CH), 130.6 (C), 134.3 (C), 137.3 (C), 144.5 (CH), 145.0 (C), 146.7 (C-3), 155.7 (CO), 159.3 (C). MS m/z: 446 (M+, 15.4), 121 (100). HR-MS (EI) for C25H22N2O4S (M+): Calcd, 446.1300. Found, 446.1307.
1-Benzyl-6-(4-methoxyphenyl)-5-[(4-methylphenyl)sulfonyl]pyrazin-2(1H)-one (3h)Yellow oil, 58% yield. IR (neat) cm−1: 3063, 3030, 2964, 1695, 1681, 1651. 1H-NMR (500 MHz, CDCl3) δ: 2.40 (s, 3H, ArCH3), 3.89 (s, 3H, ArOCH3), 4.96 (s, 2H, NCH2Ar), 6.79 (d, J=8.2 Hz, 2H, ArH), 6.85–6.93 (m, 4H, ArH), 7.17–7.26 (m, 5H, ArH), 7.55 (d, J=8.3 Hz, 2H, ArH), 8.19 (s, 1H, ArH). 13C-NMR (126 MHz, CDCl3) δ: 21.6 (CH3), 48.7 (NCH2), 55.4 (OCH3), 113.7 (CH), 120.1 (C), 127.3 (CH), 128.0 (C), 128.4 (CH), 128.6 (CH), 129.5 (CH), 130.9 (CH), 134.8 (CH), 134.9 (C), 137.3 (C), 144.4 (C), 145.2 (C), 146.5 (C-3), 155.8 (CO), 161.2 (C). MS m/z: 446 (M+, 40.4), 91 (100). HR-MS (EI) for C25H22N2O4S (M+): Calcd, 446.1281. Found, 446.1300.
6-tert-Butyl-1-methyl-5-[(4-methylphenyl)sulfonyl]pyrazin-2(1H)-one (3i)Pale yellow crystals, 11% yield. mp 128–130°C (CHCl3–hexane). IR (KBr) cm−1: 2981, 1668, 1482, 1301, 1162, 658. 1H-NMR (500 MHz, CDCl3) δ: 1.80 (s, 9H, C(CH3)3), 2.43 (s, 3H, ArCH3), 3.64 (s, 3H, NCH3), 7.29 (d, J=8.0 Hz, 2H, ArH), 7.62 (s, 1H, H-3), 7.77 (d, J=8.0 Hz, 2H, ArH). 13C-NMR (126 MHz, CDCl3) δ: 21.6 (CH3), 31.3 (C(CH3)), 38.3 (C(CH3)), 39.6 (NCH3), 129.2 (CH), 129.2 (CH), 138.0 (C), 139.0 (C), 139.8 (CH), 144.2 (C), 156.6 (C), 156.8 (C). MS m/z: 320 (M+, 1.6), 240 (100). Anal. Calcd for C16H20N2O3S·0.5H2O: C, 58.34; H, 6.43; N, 8.50. Found: C, 58.03; H, 6.14; N, 8.32.
5-[(4-Methylphenyl)sulfonyl]-6-(4-nitrophenyl)-1-phenylpyrazin-2(1H)-one (3j)Pale orange crystals, 25% yield. mp >300°C (dec.) (CHCl3–hexane). IR (KBr) cm−1: 3065, 1693, 1520, 1489, 1350, 1329, 1166, 862, 701. 1H-NMR (500 MHz, CDCl3) δ: 2.45 (s, 3H, ArCH3), 6.93 (dd, J=8.0, 2.2 Hz, 2H, ArH), 7.25–7.34 (m, 7H, ArH), 7.69 (d, J=8.3 Hz, 2H ArH), 8.09 (d, J=8.7 Hz, 2H, ArH), 8.25 (s, 1H, H-3). 13C-NMR (126 MHz, CDCl3) δ: 21.7 (CH3), 122.9 (CH), 128.1 (CH), 128.7 (CH), 129.7 (CH), 129.8 (CH), 129.9 (C), 131.1 (CH), 133.8 (C), 134.7 (C), 135.2 (C), 136.6 (C), 142.1 (C), 145.2 (CH), 148.2 (C), 148.7 (C-3), 154.6 (CO). MS m/z: 447 (M+, 1.5), 77 (100). Anal. Calcd for C23H17N3O5S·0.33H2O: C, 61.10; H, 4.33; N, 9.32. Found: C, 61.14; H, 4.22; N, 9.26.
6-(4-Methoxyphenyl)-5-[(4-methylphenyl)sulfonyl]-1-phenylpyrazin-2(1H)-one (3k)Pale orange crystals, 35% yield. mp 202–206°C (dec.) (CHCl3–hexane). IR (KBr) cm−1: 3026, 1676, 1596, 1446, 1359, 1340, 1258, 1162, 1087, 885, 810, 789, 658. 1H-NMR (500 MHz, CDCl3) δ: 2.42 (s, 3H, ArCH3), 3.75 (s, 3H, OCH3), 6.69 (d, J=8.7 Hz, 2H, ArH), 6.90 (dd, J=8.3, 1.7 Hz, 2H, ArH), 6.93 (d, J=8.7 Hz, 2H, ArH), 7.22–7.27 (m, 5H, ArH), 7.62 (d, J=8.3 Hz, 2H, ArH), 8.21 (s, 1H, H-3). 13C-NMR (126 MHz, CDCl3) δ: 21.6 (CH3), 55.2 (OCH3), 113.2 (CH), 120.7 (C), 128.2 (CH), 128.5 (CH), 129.1 (C), 129.3 (CH), 129.5 (CH), 131.5 (CH), 134.4 (C), 135.4 (C), 137.4 (C), 144.4 (CH), 144.9 (C), 147.2 (C-3), 155.4 (CO), 160.5 (C). MS m/z: 432 (M+, 7.8), 210 (100). Anal. Calcd for C24H20N2O4S·0.25H2O: C, 65.96; H, 4.73; N, 6.41. Found: C, 65.95; H, 4.64; N, 6.46.
7-Methyl-5-[(4-methylphenyl)sulfonyl]-6-phenylimidazo[1,5-a]pyrazin-8(7H)-one (4a)Pale yellow crystals, 16% yield. mp 178–180°C (CHCl3–hexane).17)
5-[(4-Methylphenyl)sulfonyl]-6,7-diphenylimidazo[1,5-a]pyrazin-8(7H)-one (4b)Pale yellow crystals, 10% yield. mp 263–266°C (dec.) (CHCl3–hexane).17)
6-Methyl-5-[(4-methylphenyl)sulfonyl]-7-phenylimidazo[1,5-a]pyrazin-8(7H)-one (4c)A yellow solid, 3% yield. mp 232–235°C (dec.) (CHCl3–hexane).17)
6-(4-Bromophenyl)-7-methyl-5-[(4-methylphenyl)sulfonyl]imidazo[1,5-a]pyrazin-8(7H)-one (4d)White crystals, 20% yield. mp 180–183°C (acetone–hexane).21)
6-(4-Methoxyphenyl)-7-methyl-5-[(4-methylphenyl)sulfonyl]imidazo[1,5-a]pyrazin-8(7H)-one (4e)Ocher crystals, 5% yield. mp 173–176°C (CHCl3–hexane).17)
7-Benzyl-5-[(4-methylphenyl)sulfonyl]-6-phenylimidazo[1,5-a]pyrazin-8(7H)-one (4f)Pale yellow crystals, 36% yield. mp 232–235°C (dec.) (CHCl3–hexane).17)
7-(4-Methoxyphenylmethyl)-5-[(4-methylphenyl)sulfonyl]-6-phenylimidazo[1,5-a]pyrazin-8(7H)-one (4g)Pale yellow crystals, 34% yield. mp 183–185°C (CHCl3–hexane).17)
7-Benzyl-6-(4-methoxyphenyl)-5-[(4-methylphenyl)sulfonyl]imidazo[1,5-a]pyrazin-8(7H)-one (4h)A pale yellow oil, 35% yield.17)
5-[(4-Methylphenyl)sulfonyl]-6-(4-nitrophenyl)-7-phenylimidazo[1,5-a]pyrazin-8(7H)-one (4j)Yellow crystals, 44% yield. mp 244–245°C (CHCl3–hexane).17)
6-(4-Methoxyphenyl)-5-[(4-methylphenyl)sulfonyl]-7-phenylimidazo[1,5-a]pyrazin-8(7H)-one (4k)Pale yellow crystals, 36% yield. mp 253–256°C (dec.) (CHCl3–hexane).17)
General Procedure for Synthesis of Ethyl 5-Oxo-3-phenylpyrazine-2-carboxylates (5)To a stirred solution of ethyl isocyanoacetate (85 mg, 0.75 mmol) in DMF (3 mL) was added DBU (152 mg, 1.00 mmol) at 0°C, and the mixture was stirred for 1 h under atmosphere of oxygen. To the mixture was added 4-trifluoroacetyl-1,3-oxazolium-5-olate 1 (0.50 mmol), and the whole was stirred at 0°C for an additional several hours. After workup with aq. Na2CO3, the mixture was extracted with AcOEt (×3). The combined organic layers were washed with brine, dried over anhyd. Na2SO4, and evaporated. The residue was purified by column chromatography (silica gel, hexane–AcOEt=4 : 1 to 1 : 1) to give the product 5.
Ethyl 4,5-Dihydro-4-methyl-5-oxo-3-phenylpyrazine-2-carboxylate (5a)Yellow crystals, 92% yield. mp 111–113°C (CHCl3–hexane). IR (KBr) cm−1: 2986, 1713, 1671, 1491, 1376, 1317, 1137, 1025, 866, 760, 711. 1H-NMR (500 MHz, CDCl3) δ: 1.05 (t, J=7.0 Hz, 3H, CH2CH3), 3.23 (s, 3H, NCH3), 4.09 (q, J=7.0 Hz, 2H, OCH2), 7.26–7.28 (m, 2H, ArH), 7.52–7.55 (m, 3H, ArH), 8.24 (s, 1H, H-6). 13C-NMR (126 MHz, CDCl3) δ: 13.8 (CH2CH3), 33.5 (NCH3), 61.3 (OCH2), 124.4 (C), 127.9 (CH), 129.0 (CH), 129.9 (CH), 131.9 (C), 146.1 (C-3), 146.2 (C), 156.1 (CO), 164.0 (CO). MS m/z: 258 (M+, 99), 185 (100). HR-MS (EI) for C14H14N2O3: Calcd, 258.1004. Found: 258.1006. Anal. Calcd for C14H14N2O3: C, 65.11; H, 5.46; N, 10.85. Found: C, 65.14; H, 5.43; N, 10.79.
Ethyl 4,5-Dihydro-5-oxo-3,4-diphenylpyrazine-2-carboxylate (5b)Yellow crystals, 88% yield. mp 198–201°C (CHCl3–hexane). IR (KBr) cm−1: 3001, 2983, 1712, 1681, 1569, 1484, 1389, 1378, 1323, 1126, 1004, 928, 754, 710. 1H-NMR (500 MHz, CDCl3) δ: 1.03 (t, J=7.0 Hz, 3H, CH2CH3), 4.11 (q, J=7.0 Hz, 2H, OCH2), 6.96–6.98 (m, 2H, ArH), 7.02–7.07 (m, 2H, ArH), 7.17–7.28 (m, 6H, ArH), 8.36 (s, 1H, H-6). 13C-NMR (126 MHz, CDCl3) δ: 13.8 (CH2CH3), 61.4 (OCH2), 124.5 (C), 127.8 (CH), 128.4 (CH), 129.0 (CH), 129.1 (CH), 129.2 (CH), 129.2 (CH), 131.5 (C), 135.8 (C), 145.7 (C), 147.8 (C-3), 155.7 (CO), 164.3 (CO). MS m/z: 320 (M+, 100). HR-MS (EI) for C19H16N2O3: Calcd, 320.1161. Found: 320.1149. Anal. Calcd for C19H16N2O3·0.33H2O: C, 69.94; H, 5.15; N, 8.59. Found: C, 69.95; H, 4.98; N, 8.41.
Ethyl 4,5-Dihydro-3-methyl-5-oxo-4-phenylpyrazine-2-carboxylate (5c)Yellow crystals, 52% yield. mp 150–153°C (CHCl3–hexane). IR (KBr) cm−1: 3060, 2976, 1705, 1671, 1517, 1385, 1306, 1230, 1095, 776, 709. 1H-NMR (500 MHz, CDCl3) δ: 1.43 (t, J=7.0 Hz, 3H, CH2CH3), 2.40 (s, 3H, 6-CH3), 4.43 (q, J=7.0 Hz, 2H, OCH2), 7.16–7.18 (m, 2H, ArH), 7.52–7.60 (m, 3H, ArH), 8.20 (s, 1H, H-6). 13C-NMR (126 MHz, CDCl3) δ: 14.4 (CH2CH3), 18.2 (CH3), 61.7 (OCH2), 123.7 (C), 127.2 (CH), 129.9 (CH), 130.3 (CH), 136.1 (C), 145.3 (C-3), 146.0 (C), 156.0 (CO), 165.0 (CO). MS m/z: 258 (M+, 100). HR-MS (EI) for C14H14N2O3: Calcd, 258.1004. Found: 258.1006. Anal. Calcd for C14H14N2O3·0.33H2O: C, 63.64; H, 5.59; N, 10.60. Found: C, 63.73; H, 5.42; N, 10.26.
Ethyl 3-(4-Bromophenyl)-4,5-dihydro-4-methyl-5-oxopyrazine-2-carboxylate (5d)Crystals, 85% yield. mp 183–185°C (CHCl3–hexane). IR (KBr) cm−1: 2982, 2901, 1723, 1662, 1485, 1423, 1379, 1313, 1177, 1013, 876, 833, 803, 677. 1H-NMR (500 MHz, CDCl3) δ: 1.12 (t, J=7.0 Hz, 3H, CH2CH3), 3.23 (s, 3H, NCH3), 4.13 (q, J=7.0 Hz, 2H, OCH2), 7.16 (d, J=8.5 Hz, 2H, ArH), 7.69 (d, J=8.5 Hz, 2H, ArH), 8.24 (s, 1H, H-6). 13C-NMR (126 MHz, CDCl3) δ: 13.9 (CH2CH3), 33.5 (NCH3), 61.5 (OCH2), 124.2 (C), 124.5 (C), 129.5 (CH), 130.7 (C), 132.4 (CH), 145.2 (C), 146.5 (C-3), 155.9 (CO), 163.8 (CO). MS m/z: 338 (M+, 55.7), 263 (100). HR-MS (EI) for C14H13BrN2O3: Calcd, 336.0110. Found: 336.0131. Anal. Calcd for C14H13BrN2O3: C, 49.87; H, 3.89; N, 8.31. Found: C, 49.94; H, 3.89; N, 8.55.
Ethyl 4,5-Dihydro-3-(4-methoxyphenyl)-4-methyl-5-oxopyrazine-2-carboxylate (5e)Crystals, 85% yield. mp 148–150°C (CHCl3–hexane). IR (KBr) cm−1: 2983, 2931, 2836, 1714, 1669, 1504, 1315, 1247, 1182, 1029, 871, 816, 694. 1H-NMR (500 MHz, CDCl3) δ: 1.11 (t, J=7.0 Hz, 3H, CH2CH3), 3.25 (s, 3H, NCH3), 3.88 (s, 3H, OCH3), 4.13 (q, J=7.0 Hz, 2H, OCH2), 7.04 (d, J=8.5 Hz, 2H, ArH), 7.19 (d, J=9.0 Hz, 2H, ArH), 8.21 (s, 1H, H-6). 13C-NMR (126 MHz, CDCl3) δ: 13.9 (CH2CH3), 33.4 (NCH3), 55.4 (OCH3), 61.3 (OCH2), 114.5 (CH), 123.8 (C), 124.9 (C), 129.4 (CH), 145.9 (C-3), 146.1 (C), 156.3 (CO), 160.7 (C), 164.2 (CO). MS m/z: 288 (M+, 75.8), 215 (100). HR-MS (EI) for C15H16N2O4: Calcd, 288.1110. Found: 288.1112. Anal. Calcd for C15H16N2O4·0.33H2O: C, 61.23; H, 5.71; N, 9.52. Found: C, 61.24; H, 5.38; N, 9.54.
Ethyl 4-Benzyl-4,5-dihydro-5-oxo-3-phenylpyrazine-2-carboxylate (5f)Yellow crystals, 90% yield. mp 116–119°C (CHCl3–hexane). IR (KBr) cm−1: 3032, 2984, 1715, 1664, 1567, 1519, 1398, 1307, 1197, 1130, 1020, 905, 767, 700. 1H-NMR (500 MHz, CDCl3) δ: 1.01 (t, J=7.0 Hz, 3H, CH2CH3), 4.06 (q, J=7.0 Hz, 2H, OCH2), 5.04 (s, 2H, NCH2Ar), 6.78–6.80 (m, 2H, ArH), 7.03–7.05 (m, 2H, ArH), 7.17–7.19 (m, 3H, ArH), 7.35–7.38 (m, 2H, ArH), 7.46–7.49 (m, 1H, ArH), 8.33 (s, 1H, H-6). 13C-NMR (126 MHz, CDCl3) δ: 13.7 (CH2CH3), 48.7 (NCH2), 61.3 (OCH2), 124.9 (C), 127.1 (CH), 127.8 (CH), 128.4 (CH), 128.5 (CH), 129.9 (CH), 131.0 (C), 135.1 (C), 146.0 (C), 147.0 (C-3), 156.0 (CO), 164.0 (CO). MS m/z: 334 (M+, 82.3), 91 (100). HR-MS (EI) for C20H18N2O3: Calcd, 334.1317. Found: 334.1312. Anal. Calcd for C20H18N2O3: C, 71.84; H, 5.43; N, 8.38. Found: C, 71.54; H, 5.40; N, 8.27.
Ethyl 4,5-Dihydro-4-(4-methoxyphenylmethyl)-5-oxo-3-phenylpyrazine-2-carboxylate (5g)Crystals, 91% yield. mp 75–77°C (CHCl3–hexane). IR (KBr) cm−1: 2966, 2935, 2837, 1723, 1661, 1510, 1297, 1250, 1132, 1028, 805, 764, 712. 1H-NMR (500 MHz, CDCl3) δ: 0.99 (t, J=7.2 Hz, 3H, CH2CH3), 3.74 (s, 3H, OCH3), 4.04 (q, J=7.2 Hz, 2H, OCH2), 4.98 (s, 2H, NCH2Ar), 6.39–6.74 (m, 4H, ArH), 7.07 (d, J=7.2 Hz, 2H, ArH), 7.40 (t, J=7.5 Hz, 2H, ArH), 7.49 (t, J=7.5 Hz, 1H, ArH), 8.29 (s, 1H, H-6). 13C-NMR (126 MHz, CDCl3) δ: 13.7 (CH2CH3), 48.2 (NCH2), 55.2 (OCH3), 61.2 (OCH2), 113.8 (CH), 124.9 (C), 127.2 (C), 128.4 (CH), 128.7 (CH), 128.8 (CH), 129.8 (CH), 131.1 (C), 145.9 (C), 147.0 (C-3), 156.0 (CO), 159.2 (C), 164.0 (CO). MS m/z: 364 (M+, 60.5), 121 (100). HR-MS (EI) for C21H20N2O4: Calcd, 364.1423. Found: 364.1411. Anal. Calcd for C21H20N2O4: C, 69.22; H, 5.53; N, 7.69. Found: C, 68.99; H, 5.42; N, 7.87.
Ethyl 4-Benzyl-4,5-dihydro-3-(4-methoxyphenyl)-5-oxopyrazine-2-carboxylate (5h)Crystals, 91% yield. mp 78–81°C (CHCl3–hexane). IR (KBr) cm−1: 2979, 2843, 1727, 1666, 1609, 1504, 1311, 1250, 1129, 1023, 857, 729, 695. 1H-NMR (500 MHz, CDCl3) δ: 1.07 (t, J=7.1 Hz, 3H, CH2CH3), 3.84 (s, 3H, OCH3), 4.09 (q, J=7.1 Hz, 2H, OCH2), 5.06 (s, 2H, NCH2Ar), 6.83–6.84 (m, 2H, ArH), 6.88 (d, J=8.8 Hz, 2H, ArH), 6.96 (d, J=8.5 Hz, 2H, ArH), 7.20–7.22 (m, 3H, ArH), 8.30 (s, 1H, H-6). 13C-NMR (126 MHz, CDCl3) δ: 13.9 (CH2CH3), 48.6 (NCH2), 55.3 (OCH3), 61.3 (OCH2), 113.8 (CH), 123.1 (C), 125.4 (C), 127.1 (CH), 127.8 (CH), 128.5 (CH), 130.0 (CH), 135.3 (C), 145.9 (C), 146.9 (C-3), 156.2 (CO), 160.7 (C), 164.2 (CO). MS m/z: 364 (M+, 63), 135 (100). HR-MS (EI) for C21H20N2O4: Calcd, 364.1423. Found: 364.1411. Anal. Calcd for C21H20N2O4: C, 69.22; H, 5.53; N, 7.69. Found: C, 69.01; H, 5.60; N, 7.59.
Conversion of 2(1H)-Pyrazinone (5a) to Imidazo[1,5-a]pyrazin-8(7H)-one (6)To a stirred solution of TosMIC (57 mg, 0.290 mmol) in DMF (1 mL) was added DBU (88 mg, 0.581 mmol) at 0°C, and the mixture was stirred for 1 h under atmosphere of argon. To the mixture was added 5a (50 mg, 0.194 mmol), and the whole was stirred at room temperature (r.t.) for an additional 24 h. After workup with aq. Na2CO3, the mixture was extracted with AcOEt (×3). The combined organic layers were washed with brine, dried over anhyd. Na2SO4, and evaporated. The residue was purified by column chromatography (silica gel, hexane–AcOEt=1 : 1) to give ethyl 7,8-dihydro-7-methyl-8-oxo-6-phenylimidazo[1,5-a]pyrazine-5-carboxylate 6 as colorless crystals, 65% yield. mp 163–165°C (CHCl3–hexane). IR (KBr) cm−1: 3146, 2979, 2934, 1709, 1667, 1336, 1321, 1207, 1065, 719. 1H-NMR (500 MHz, CDCl3) δ: 0.78 (t, J=7.2 Hz, 2H, CH2CH3), 3.11 (s, 3H, NCH3), 3.96 (q, J=7.2 Hz, 2H, OCH2), 7.30–7.32 (m, 2H, ArH), 7.49–7.54 (m, 3H, ArH), 8.06 (s, 1H, H-1), 8.83 (s, 1H, H-3). 13C-NMR (126 MHz, CDCl3) δ: 13.2 (CH2CH3), 32.2 (NCH3), 61.5 (OCH2), 109.0 (C), 122.0 (C), 128.8 (CH), 129.6 (CH), 131.4 (CH), 133.2 (CH), 135.0 (CH), 139.2 (C), 155.6 (CO), 161.9 (CO). MS m/z: 297 (M+, 100). HR-MS (EI) for C16H15N3O3: Calcd, 297.1113. Found: 297.1104. Anal. Calcd for C16H15N3O3: C, 64.64; H, 5.09; N, 14.13. Found: C, 64.66; H, 5.07; N, 13.88.
Procedures for Synthesis of 1,3-Thiazolium-5-olates (7a–c)4-Trifluoroacetyl-3-methyl-2-phenyl-1,3-thiazolium-5-olate (7a)To a stirred suspension of N-methyl-N-thiobenzoylglycine (1.00 g, 4.78 mmol) in tert butyl methyl ether (10 mL) was added trifluoroacetic anhydride (TFAA) (2 mL, 14.3 mmol) at 0°C, and the solution was stirred at 0°C for 3 h. After workup with aq. Na2CO3, the mixture was extracted with AcOEt (×3). The combined organic layers were washed with brine, dried over anhyd. Na2SO4, and evaporated. The residue was washed with diethyl ether, and recrystallized from hexane–ethyl acetate to give the product 7a as yellow crystals (1.17 g, 85% yield). mp 131–133°C (AcOEt–hexane). IR (KBr) cm−1: 3062, 3044, 2970, 1673, 1620, 1451, 1396, 1327, 1310, 1261, 1237, 1081, 1049, 1025, 997, 946, 791, 766, 736, 700. 1H-NMR (500 MHz, CDCl3) δ: 4.04 (s, 3H, NCH3), 7.50 (d, J=7.5 Hz, 2H, ArH), 7.59 (t, J=7.5 Hz, 2H, ArH), 7.63–7.66 (m, 1H, ArH). 13C-NMR (126 MHz, CDCl3) δ: 42.5 (NCH3), 115.5 (C), 116.9 (q, 1J=288.5 Hz, CF3), 127.1 (C), 129.3 (CH), 129.7 (CH), 132.6 (CH), 158.5 (C), 168.6 (q, 2J=38.1 Hz, CCF3), 174.2 (C). MS m/z: 287 (M+, 67.5), 121 (100). HR-MS (EI) for C12H8F3NO2S: Calcd, 287.0228. Found: 287.0217.
4-Acetyl-3-methyl-2-phenyl-1,3-thiazolium-5-olate (7b)A mixture of N-methyl-N-thiobenzoylglycine (1.00 g, 4.78 mmol) and acetic anhydride (15 mL) was heated at 120°C for 4 h. After workup with aq. Na2CO3, the mixture was extracted with AcOEt (×3). The combined organic layers were washed with brine, dried over anhyd. Na2SO4, and evaporated. The residue was purified by column chromatography (silica gel, hexane–AcOEt=4 : 1 to 1 : 1) to give the product 7b as yellow crystals (0.903 g, 81% yield). mp 131–132°C (AcOEt–hexane) (mp21) 133–134°C). IR (KBr) cm−1: 3057, 3000, 2920, 1648, 1622, 1375, 1341, 1252, 1173, 1083, 946, 764, 695. 1H-NMR (500 MHz, CDCl3) δ: 2.53 (s, 3H, CCH3), 4.06 (s, 3H, NCH3), 7.46 (dd, J=8.0, 1.5 Hz, 2H, ArH), 7.53–7.60 (m, 3H, ArH). 13C-NMR (126 MHz, CDCl3) δ: 29.7 (CH3), 42.1 (NCH3), 119.0 (C), 127.7 (C), 129.4 (CH), 129.5 (CH), 131.7 (CH), 151.3 (C), 176.5 (C), 187.7 (C). MS m/z: 233 (M+, 36.7), 118 (100). HR-MS (EI) for C12H11NO2S: Calcd, 233.0510. Found: 233.0504.
4-Formyl-3-methyl-2-phenyl-1,3-thiazolium-5-olate (7c)To a mixture of pyridine (0.805 mL, 10 mmol) and DMF (1.925 mL, 25 mmol) in toluene (15 mL) was added phosphoryl chloride (1.2 mL, 12.5 mmol) at 0°C, and the solution was stirred at 0°C for 1 h. To the mixture was added N-methyl-N-thiobenzoylglycine (0.522 g, 2.5 mmol) and the whole was heated at 80°C for an additional 3 h. After workup with aq. Na2CO3, the mixture was extracted with AcOEt (×3). The combined organic layers were washed with brine, dried over anhyd. Na2SO4, and evaporated. The residue was recrystallized from hexane–ethyl acetate to give 7c as amber crystals (0.318 g, 58% yield). mp 160–162°C (AcOEt–hexane). IR (KBr) cm−1: 3056, 3027, 2829, 2782, 2757, 1670, 1604, 1365, 1326, 1307, 1239, 1152, 1051, 859, 774, 759, 697. 1H-NMR (500 MHz, CDCl3) δ: 4.11 (s, 3H, NCH3), 7.47–7.49 (m, 2H, ArH), 7.55–7.63 (m, 3H, ArH), 9.57 (s, 1H, CHO). 13C-NMR (126 MHz, CDCl3) δ: 40.2 (NCH3), 118.1 (C), 127.1 (C), 129.4 (CH), 129.6 (CH), 132.1 (CH), 152.0 (C), 176.2 (C), 178.4 (C). MS m/z: 219 (M+, 30.8), 118 (100). HR-MS (EI) for C11H9NO2S: Calcd, 219.0354. Found: 219.0346.
BiologyAnti-microbial ActivityThe preliminary anti-microbial activies of new compounds were measured in a concentration of 50 mg/L by disc diffusion method.22) Gram-negative bacteria of Acinetobacter baumannii ATC C17978, Escherichia coli TG1, Klebsiella pneumonia IID5209, Pseudomonas aeruginosa 01, Serratia marcescens (clinical isolate), and Vibrio parahaemolyticus RIMD221051 and Gram-positive bacteria of Bacillus subtilis ATC C6633, Enterococcus faecalis IID622, Staphylococcus aureus FDA209P, methicillin-resistant Staphylococcus aureus (MRSA) (clinical isolate), Streptococcus pneumonia IID555, and S. pyogenes 124 were used. Briefly, 100 µL of the middle-logarithmic phase bacteria cells (108 CFU/mL) were spread on surface of the Muller–Hinton agar plate and discs containing 5 µg of new compounds were put on the agar plate, and then incubated at 37°C. The inhibition zone were measured in millimeters at the end of an incubation period of 18–24 h. Dimethyl sulfoxide (DMSO) was used as solvent control and Gentamicin, Kanamycin and Vancomycin were used as standard drugs.
Cytotoxicity DeterminationsA literature procedure was employed when examining the lethal effects of series 3 and 5 compounds on Ca9–22, HSC-2, HSC-3, HSC-4 (purchased from Riken Cell Bank, Tsukuba, Japan), HGF, HPLF and HPC cells (established in Meikai University School of Dentistry according to the guideline of intramural ethic committee) except the time of incubation was extended from 24 to 48 h.30) In brief, different concentrations of test compounds or positive control such as 5-FU (Wako Pure Chemical Industries, Ltd., Osaka, Japan) or melphalan (Sigma-Aldrich Inc., St. Louis, MO, U.S.A.) were incubated at 37°C with the cells in Dulbecco’s modified Eagle’s medium (DMEM) medium supplemented with 10% heat-inactivated fetal bovine serum. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method was used in determining cell viability.30)
The authors gratefully acknowledge Ms. Ayaka Uematsu and Mr. Daichi Kameda for conducting in vitro antibacterial assay. This work was partially supported by KAKENHI from the Japan Society for the Promotion of Science (JSPS) (Challenging Exploratory Research 25670897; Scientific Research (C) 16K11519).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.