2017 Volume 65 Issue 7 Pages 629-636
2017 Volume 65 Issue 7 Pages 629-636
Photodynamic therapy is achieved by the combination of photosensitizers, harmless visible or near-infrared (NIR) light, and molecular oxygen (O2). Photosensitizers transfer their absorbed light energy to O2 to generate a major active species in photodynamic therapy, singlet oxygen. In this review, I will discuss the possibility of single-walled carbon nanotubes as NIR photosensitizers, while explaining the general photophysics and photochemistry underlying photodynamic therapy as well as summarizing recent advances in the purification technologies for single-walled carbon nanotubes to reduce their toxicity concerns.
Carbon nanotubes were discovered and reported by Iijima in 1991.1) This was just serendipity. At that time, graphitic filaments, consisting of elemental carbon in the sp2 state, were known to be produced by thermal decomposition of hydrocarbon species. During his electron microscopy (EM) observation of carbon electrodes used in the direct-current arc-discharge evaporation of carbon in an argon-filled vessel, Iijima happened to discover graphitic carbon “needles” with diameters in the range of 4–30 nm and lengths up to 1 µm. He showed the high-resolution EM images of three carbon nanotubes consisting of two, five, or seven graphitic sheets. These are now called double-walled or multi-walled carbon nanotubes (MWNTs) (Fig. 1). Two years after this first report, single-walled carbon nanotubes (SWNTs) were reported independently by Iijima and Ichihashi2) and Bethune et al.3) (Fig. 1). These carbon nanotubes possess very high mechanical strength as well as very high heat and electrical conductivities, and have revolutionized the fields of Materials Science and Engineering.

In the biomedical fields, SWNTs have been explored as drug delivery carriers, photoresponsive agents, etc.4–14) Various bioactive molecules, such as anticancer drugs and small interfering RNAs, can be bound to the surface of SWNTs and thereby delivered to, and even inside, target cells. A major motivation for the latter would be the ability of SWNTs to absorb near-infrared (NIR) light efficiently. This is because NIR light is the least invasive to the human body. The scattering and absorption of incident light are the major determinants of the efficiency of light delivery to photoresponsive agents, and they are minimal in the human body. Anderson and Parrish reported that the degree of scattering in skin was inversely correlated with the wavelength of the incident light (i.e., lower scattering, longer wavelength) and suggested the existence of an optical “window” between 600 and 1300 nm.15) Hemoglobin (and its oxygenated forms) and water are the major absorbers of visible and IR light, respectively, and their absorption coefficients are lowest in the NIR region around 650–900 nm.16) As shown in Fig. 2, SWNTs have intense NIR absorption in this wavelength region. These NIR absorption peaks are characteristic of the “intact” graphene sheets of SWNTs, and they are significantly decreased with an increase of the degree of chemical functionalization, which is discussed later in the text.

NIR window region (650–900 nm) is indicated in gray.
The incident light energies absorbed by photoresponsive agents in a stable singlet excited state can be utilized for emission and chemical reactions with surrounding molecules. In this review, I will focus on the chemical reactions of photoexcited SWNTs with molecular oxygen (O2); that is, the photodynamic action of SWNTs. Additionally, the aqueous dispersion, purification, and safety of SWNTs are discussed.
The discovery of the photodynamic effect was also serendipitous. Oscar Raab, who was a German medical student at the time, observed that Paramecium cells could sometimes be killed when treated with acridine orange (Fig. 3) for staining the DNA, and then he found that a combination of light and the dye could induce cell death.17) Later, this phenomenon was found to be inhibited in the absence of O2, and was named “photodynamic action” by Tappeiner and Jodlbauer.18) Photoresponsive agents (e.g., acridine orange) that mediate this action are called photosensitizers (PSs). To date, various types of PSs have been used clinically in photodynamic therapy (PDT) against cancers, pre-cancerous diseases, and age-related macular degeneration.19)

The photophysics and photochemistry of the photodynamic action of PSs can be well documented in a Jablonski diagram (Fig. 4). A PS has two electrons with opposite spins in the ground state (singlet state). Upon absorption of light by the PS, one of the electrons is excited to a high-energy level while keeping the spin state. This singlet excited state of the PS is very short-lived (ca. 10−9 s) and subsequently loses its energy via nonradiative decay (=heat generation), radiative decay (fluorescence emission), or intersystem crossing into the triplet excited state through inversion of the spin state. The triplet excited state is relatively long-lived owing to the fact that quenching to the singlet ground state, via nonradiative decay (=heat generation) or radiative decay (phosphorescence emission), is a spin-forbidden process. In oxygenated environments, therefore, the triplet excited state of the PS readily interacts with O2, which has a triplet ground state (3O2) and low-lying excited states.

In photodynamic action, the PS triplet excited state executes two types of reactions with 3O2; namely, Type I and Type II reactions (Fig. 4). The Type I reaction involves electron transfer from the PS triplet excited state to 3O2 to produce the superoxide anion (O2·−). This reaction is mediated by some reductants. O2·− by itself does not cause severe oxidative damage, but it can be further converted to the much more cytotoxic reactive oxygen species (ROS), hydroxyl radical (HO·), and hydrogen superoxide (HOO·; pKa=4.68). The oxidative damage caused by HO· is nearly diffusion rate-limited. HO· oxidizes some organic substrates, such as unsaturated lipids and DNA. Subsequently, chain reactions of the organic radical species with 3O2, named autooxidation, can proceed because 3O2 has two unpaired electrons (radicals).
Singlet oxygen (1O2), generated via the Type II reaction, has been recognized to be a major active species in PDT. In this reaction, the PS triplet excited state transfers its energy directly to 3O2 to produce 1O2 (Fig. 4). The energy required for this 1O2 generation is 22.5 kcal·mol−1, which corresponds to light energy at 1274 nm. 1O2 is an oxidizing agent that reacts directly with various biological molecules. Its lifetime has been determined by phosphorescence lifetime measurements to be 3.5–4.4 µs in H2O.20,21) Based on this lifetime, the diffusion radius of 1O2 is estimated to be 155 nm,22) which is quite short compared with the size of mammalian cells (a few tens of micrometers). Importantly, Hatz et al. also estimated the lifetime of 1O2 inside HeLa cells to be about 3 µs, concluding that 1O2 is a selective rather than reactive intermediate.23) What determines the preference for a Type I or Type II reaction? A few studies have suggested that it depends on the structure and concentration of the PS and the concentration of O2.24–26)
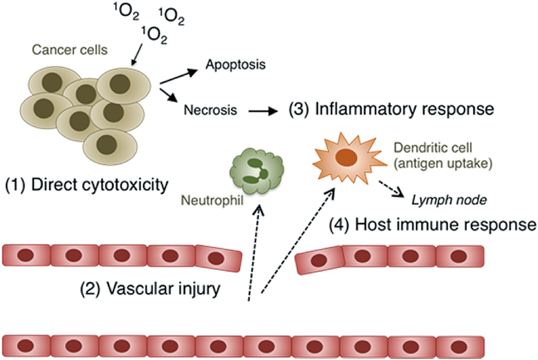
The effects of PDT on tumor tissues are now known to be due to four mechanisms: (1) direct cytotoxicity, (2) vascular injury, (3) inflammatory response, and (4) host immune response27,28) (Fig. 5). ROS generated via the photodynamic action of PSs can kill tumor cells as well as endothelial cells in tumor tissues. The latter case would cause hypoxia or anexia in the tumor tissue, leading to its destruction. The types of cells injured would depend on the location of the PS, which in the case of m-tetrahydroxyphenyl chlorin (temocene) is reported to be mainly endothelial cells in a relatively short time (15 min) after administration and then later tumor cells (24 h).29) Cell death induced by the photodynamic action of PSs also proceeds via apoptosis and/or necrosis (Fig. 5). The factors that determine the pathways are known to be the (1) dose of photodynamic action (PS concentration×light fluence), (2) subcellular location of the PS, (3) light fluence rate, (4) O2 concentration, and (5) cell type. Activation of the apoptotic pathway may be advantageous in terms of its lower requirement of the dose compared with induction of necrosis, whereas necrotic cell death seems to induce an immune response more effectively. Necrotic cancer cells spread their cytosolic constituents throughout the damaged plasma membrane, which could induce an inflammatory response, thereby recruiting leukocytes such as neutrophils and dendritic cells.
The most important subcellular target of PSs in PDT has been recognized to be the mitochondria. This is because many PSs tend to induce apoptosis via mitochondria damage upon illumination. Using a series of pyropheophorbide-a derivatives with different alkyl chain lengths, MacDonald et al. revealed the 50% lethal dose concentration to be inversely correlated with the degree of mitochondria localization.30) They also suggested the importance of the PS being in a monomer (not aggregated) state inside cancer cells, due to its higher photodynamic action than its aggregates.
SWNTs have single graphene sheets that are rolled up into cylinders (see Fig. 1) and are thus often illustrated as simple tubes. However, each single tube of SWNTs has a different chemical structure, which depends on the tube length, degree of chemical modification (i.e., defects and oxygenation), and, perhaps the most important, chirality. The chirality of SWNTs is defined as the direction of the roll-up of a graphene sheet. This direction is depicted as a roll vector (n,m) (Fig. 6). Importantly, the optical properties of SWNTs are defined by this chirality index if their graphene sheet is intact. SWNTs with n–m evenly divisible by 3 are metals or semimetals, whereas those that are not divisible by 3 are semiconductors (Fig. 6). Like many PSs, semiconducting SWNTs will fluoresce when illuminated at an appropriate wavelength, which will vary with the chirality index (n,m). The semiconducting SWNTs show the most intense NIR absorption values, which are derived from first (E11) and second (E22) van Hove transitions (Fig. 7). Such electronic transitions have been assigned for more than 30 chiral components,31) and are known to be a function of the diameter of the SWNT (Kataura plot).32,33)

(a) Honeycomb structure of a graphene sheet, with roll-up vectors (arrows) and different (n,m) indices (in each hexagon). (b) Schematic illustration of representative metallic and semiconducting SWNTs.

The selective acquisition of metallic and semiconducting SWNTs was first reported in 2001 by Collins et al., using a strategy of stepwise electrical breakdown of MWNTs into SWNTs.34) Since then, bulk separation methods for SWNT suspensions have been developed that apply electrophoresis, ultracentrifugation, ion-exchange chromatography, selective chemical binding, etc.35–38) This metallic–semiconducting SWNT separation efficiency was improved by Tanaka et al.39) In their method, SWNTs were extensively sonicated at a high-power level for a long time, in aqueous solution containing sodium dodecyl sulfate (SDS) as a dispersant, to yield individually isolated SWNTs. The suspension was then subjected to agarose gel electrophoresis, where only metallic SWNTs were electrophoresed, most likely due to binding of more SDS on the surface. Later, they advanced this method by using agarose gel columns, which enabled continuous metallic–semiconducting SWNT separation.40) In this method, metallic SWNTs were enriched in the flow-through fractions, where the bound fractions containing semiconducting SWNTs were easily eluted with sodium deoxycholate. Enrichment of the metallic and semiconducting SWNTs was confirmed by UV-Vis-NIR absorption and Raman spectroscopies.
Enrichment of single-chirality (n,m) species of semiconducting SWNTs has also been developed. These methods are categorized into three groups: density-gradient ultracentrifugation,41) aqueous two-phase extraction,42,43) and gel chromatography.44,45) Among these chirality enrichment methods, gel chromatography seems to be the most effective in terms of the purity achieved. In this method, individually isolated SWNTs in aqueous 2 wt% SDS solution were subjected to dextran-based gel chromatography, cooled at 10°C at first. Only (6,4)-SWNTs were trapped in the column and then eluted with 5 wt% SDS. When the flow-through fraction depleted of (6,4)-SWNTs was applied to a new column at a higher temperature (12°C), the trapped species were mainly (6,5)-SWNTs. In this way, by increasing the temperature stepwise from 10 to 28°C, 13 different (n,m) species could be fractionated at high purity. With the further modification methods reported recently by the same group, (5,4), (9,4), and (10,3)-SWNTs could be obtained at a purity higher than 90%.46,47)
Like the PSs used in PDT, SWNTs can fluoresce and/or generate ROS and heat upon illumination. Their NIR photoluminescence was first reported in 2002.31,48) In vivo NIR fluorescence imaging using SWNTs and other carbon nanomaterials, such as graphene, carbon dot, and nanodiamond, has been well summarized in recent reviews.5,7,49,50) Antaris et al. reported that (6,5)-SWNTs, which were sorted by density gradient ultracentrifugation and dispersed in water by physical adsorption of polyethylene glycol (PEG)-conjugated poly(maleic anhydride-alt-1-octadecene) (C18-PMH-mPEG), are capable of providing clear subcutaneous tumor imaging in mice (excitation, 808 nm; emission, 980 nm) after intravenous administration (ca. 4 µg per mouse=0.254 mg·kg−1).51) The NIR photoluminescence intensity was 6 times higher for (6,5)-SWNTs than for an unsorted mixture of SWNTs on a weight basis. More recently, in vivo vascular imaging in mice (excitation, 735–800 nm; emission, >1000 nm) was achieved through intravenous administration (0.17 µg per mouse) of (9,4)-SWNTs, which were enriched by a dextran-based gel chromatography and dispersed in water with a PEG–phospholipid (PL) conjugate (PEG–distearoylphosphatidylethanolamine).46) On the other hand, besides the sorting of species with specific chirality, sophisticated chemical modification of SWNTs is another strategy to control their photoluminescence properties.52–55) For example, Maeda et al. reported that reductive alkylation and subsequent thermal treatment of SWNTs yielded a red-shifted and more intense fluorescence signal, while not changing the excitation wavelength.55) This method is very useful in terms of its easiness and controllability.
The phototherapeutic modalities of SWNTs are heat and ROS generation by the photothermal and photodynamic actions, respectively. As shown in Fig. 4, heat generation from photoexcited PSs occurs through nonradiative decay (i.e., vibrational relaxation), while the subsequent energy/electron transfer to 3O2 results in ROS generation. Thus, these two actions are competitive, and the preference for either of them depends on the electronic structure of the PS (i.e., the energy band gap between triplet excited and ground states and the oxidation potential). In the case of SWNTs, the utility of photothermal action was first demonstrated for cancer therapies.14)
Photothermal cancer therapy is rationalized by the fact that tumor tissues have a relatively poor blood supply compared with their normal counterparts, which makes tumor cells more susceptible than normal cells to heat in each tissue.56) Basically, photothermal action on tissues results in the following three outcomes: hyperthermia, coagulation, and vaporization.57) The latter two outcomes necessitate intense heating at >50°C for several seconds, thereby killing cells indiscriminately, whereas hyperthermia can be achieved by more mild heating at 42–49°C for tens of minutes. Therefore, less-invasive photothermal cancer therapy should be based on hyperthermia.
The pioneering work of photothermal ablation of cancer cells with SWNTs is from Dai’s group, published in the paper by Kam et al.58) SWNTs were coated with a single-stranded DNA or a folate (FA)–PEG(2000)–PL conjugate (FA–PEG–PL). The SWNT concentration was determined on the basis of its absorbance at 808 nm (Hipco SWNTs, ε≈7.9×106 M−1·cm−1). After 40 s of illumination of the aqueous SWNT suspension (ca. 25 µg·mL−1) at 808 nm at 1.4 W·cm−2, the suspension temperature was increased to over 42°C. Next, FA receptor-positive HeLa cells (established by culture in a FA-free medium) and the parent HeLa cells were treated with the FA–PEG–PL-coated SWNTs for 12–18 h, washed, and then illuminated at 808 nm at 1.4 W·cm−1 for 2 min. Cell death was observed only for the FA receptor-positive cells. In a later study, the same group reported that chirality-sorted (6,5)-SWNTs showed >10-times higher photothermal heat generation than the parent SWNTs with the same mass, when illuminated at 980 nm at 0.6 W·cm−2.51)
In vivo studies were also performed with (6,5)-SWNTs on subcutaneous tumor-bearing mice. By detecting the intrinsic Raman G band of SWTNs after intravenous administration,59) the blood circulation half-life of (6,5)-SWNTs coated with C18-PMH-mPEG was determined to be 23.95±1.49 h.51) The authors also reported that the half-life of SWNTs was extendable up to 1 d with a branched PEG (7000) chain-PL conjugate.59) The tumor uptake of (6,5)-SWNTs was ca. 13% injected dose (ID)·g–1, which was comparable to the 17% ID·g–1 and 12% ID·g–1 for the liver and spleen, respectively. As a result, (6,5)-SWNTs were able to visualize tumor tissues in living mice (see above) and subsequently ablate them via photothermal heat generation. It was observed in another report that the photothermal action of SWNTs yielded heat shock protein 70 expression in tumor tissues.60)
The photogeneration of 1O2 by SWNTs was first reported in 2009 by Gandra et al.61) The 1O2 was detected by its emission signal at 1270 nm after excitation of SWNTs in aqueous solution at 532 nm via a two-photon absorption process. Importantly, the relative quantum yield of 1O2 generation (ΦΔ) was inversely correlated with the intactness of the SWNTs; that is, the degree of defects of the graphene sheet. The highest ΦΔ was 0.53 for iron-free, nonfunctionalized SWNTs suspended in D2O/H2O containing 10% poly(diallyldimethylammonium chloride). Before this report, one computational chemistry study of the triplet excited state of SWNTs had been reported in 2005.62) In 2011, Park et al. utilized pump-probe transient absorption spectroscopy to prove the existence of the triplet excited state of (6,5)-SWNTs.63) The E22 excitation (587–600 nm) of (6,5)-SWNTs suspended in D2O with an ionic polymer yielded a transient absorption signal at 1150 nm with the lifetime of ca. 15 µs. Based on the observation that the decay of this signal was significantly enhanced in the presence of 3O2, the authors concluded that the photoexcited state showing the transient absorption signal at 1150 nm possessed triplet character. Another research group also analyzed the photodynamics of (6,5)-SWNTs suspended in H2O with another polymer, to determine the triplet state lifetime (30±10 µs) and the quantum yield of triplet formation (5±2%).64)
Our group has revealed that semiconducting SWNTs of high purity are able to generate 1O2 and O2·− in H2O containing 1 wt% sodium deoxycholate under NIR illumination at 808 nm65) (Fig. 8). The wavelength of 808 nm was selected because an 808-nm laser was used for the photothermal study with SWNTs described above and has been approved for clinical use. Detection of 1O2 and O2·− was inhibited in the presence of ROS quenchers, sodium azide, and mannitol, respectively. In contrast, metallic-enriched SWNTs were found to show higher photothermal action than semiconducting ones (Fig. 8). It should be noted that significant ROS generation by the metallic-enriched SWNTs used in our study was not obvious, although they contained a significant amount of semiconducting species as impurities (45%). Careful comparison of their structures by atomic force microscopy suggested that, in addition to the purity, the degree of individual isolation of semiconducting SWNTs is a key factor to enable clear-cut detection of their photodynamic action. More concretely, semiconducting species in metallic SWNTs were slightly bundled; thus, their photoexcited states could be quenched via energy transfer within the bundles and, of course, to the surrounding metallic SWNTs. Similar NIR-photodynamic action has been reproduced by using SWNTs chemically modified with polyethylene imine (300 W tungsten-halogen lamp)66) and SWNTs coated with a protein–PEG–PL conjugate (pulse of 980 nm laser, 2 W·cm−2).67) Thus, one of the future studies would be exploration of single-chirality species with high photodynamic action, based on the optical band gaps31) and oxidation potentials68) determined or predicted for each chiral species of semiconducting SWNTs.

In the left panel, the amount of 1O2 generated is compared between metallic- and semiconducting SWNTs, designated as m-SWNTs and s-SWNTs in the panel, respectively (n≥3, average±S.D.). For s-SWNTs, 1O2 generation is increased with increasing laser power intensity at 808 nm and inhibited in the presence of sodium azide, a 1O2 quencher. In the right panel, the time dependent change in temperature of metallic and semiconducting SWNT suspensions under 808 nm illumination (n=3, average±S.D.). Images reprinted with permission from Murakami T., Nakatsuji H., Inada M., Matoba Y., Umeyama T., Tsujimoto M., Isoda S., Hashida M., Imahori H., J. Am. Chem. Soc., 134, 17862–17865 (2012). Copyright 2012 American Chemical Society.
SWNTs and MWNTs are reported to be biodegradable69–73) and excretable from mice.59,74) Allen et al. incubated SWNTs with horseradish peroxidase and hydrogen peroxide (40 µM) at 4°C for over 12 weeks and confirmed their degradation by EM.71) Kagan et al. successfully demonstrated that SWNTs taken up by human neutrophils were also biodegraded by myeloperoxidase and nicotinamide adenine dinucleotide phosphate oxidase.69) More importantly, the biodegraded SWNTs did not elicit a proinflammatory response in mice after pharyngeal aspiration, which was observed in previous studies for SWNTs75) and MWNTs.76)
With regard to SWNT excretion, Liu et al. used Raman microscopy to analyze the various organs of mice for up to 3 months after intravenous injection of SWNTs coated with PEG–PL conjugates.59) The Raman signal intensity in the liver and spleen gradually decreased to below the detection limit (0.2 µg·mL−1 SWNTs in tissues). The biliary and renal pathways were suggested to be responsible for the excretion because the Raman signals were detected in the intestine, feces, kidney, and bladder. Importantly, the blood half-life of SWNTs, which depended on the structure of PEG–PL conjugates, was inversely correlated with their excretion rate. With regard to chemically modified MWNTs, Lacerda et al. obtained impressive EM images of single tubes crossing the fenestrae of the renal glomerular endothelial cells in such a way that the tube longitudinal axes were perpendicular to the cell layers.74) These results suggest that the individually isolated state of SWNTs is a key determinant of its excretion rate.
Highly water-dispersed SWNTs seem to be well-tolerated in mice, at least in the short term.77) Although their biodegradation and excretion (as described above) would be possible, the rate and efficiency of both processes are insufficient, raising concerns about potential long-term toxicity.4,78) Thus, it is desirable to shorten the SWNT size to under 5–8 nm, the size of glomerular fenestrae, and/or to endow greater biodegradability in some way. Alternatively, SWNTs might in future be used clinically as they are, after better understanding of their clinical risks and benefits.
In this review, the recent advances in biomedical applications of SWNTs were briefly summarized from the viewpoint of their NIR photoresponsiveness. Chirality sorting technologies have greatly advanced our understanding of the intrinsic photoresponsiveness of SWNTs. As a result, it has been revealed that SWNTs are capable of generating ROS by illumination with clinically desired light in the NIR region. This NIR photodynamic action can be further enhanced through the utilization of putative photodynamically active single-chirality species and chemical/physical surface modification of SWNTs with some agents to improve their NIR light-harvesting capability and/or to enhance the energy/electron transfer from photoexcited SWNTs to 3O2.
This research was supported partly by JSPS KAKENHI to T.M. (No. 24300162).
The author declares no conflict of interest.