2018 Volume 66 Issue 11 Pages 1019-1022
2018 Volume 66 Issue 11 Pages 1019-1022
Upon single treatment against Staphylococus aureus, quercetin–pivaloxymethyl conjugate (Q-POM) had antibacterial activities with minimum inhibitory concentrations (MICs) of 16–32 mg/L. Q-POM showed MIC of 32 mg/L against vancomycin-resistant Enterococcus faceium (VRE), which is remarkably lower than other antibiotics investigated (≥256 mg/L). Under sub-MIC concentrations, Q-POM potentiated the activity of ampicillin, cefepime, and vancomycin against S. aureus and Enterococcus (including highly resistant strains such as hetero-resistant vancomycin-intermediate S. aureus (hVISA), vancomycin-intermediate S. aureus (VISA), and VRE), by decreasing the MICs of these antibiotics by 4–128 folds. Q-POM was found to be partially synergistic with ampicillin and cefepime against S. aureus and Enterococcus, while it was strongly synergistic with vancomycin. Q-POM at 5 mg/L inhibited the formation of biofilms of S. aureus by 24–83% and VRE by 70%. Additionally, Q-POM inhibited the hemolytic activity of S. aureus in a dose-dependent manner. Cytotoxic activity was evaluated in human liver epithelial cells (HepG2), and the 50% cytotoxicity concentration (CC50) value of Q-POM was higher than 50 mg/L. These results indicate the potential use of Q-POM in treatment of methicillin-resistant Staphylococcus aureus (MRSA) and VRE infections.
Multidrug resistance (MDR) bacteria contain diverse resistance phenotypes, which tend to be superimposed to develop complex resistance mechanisms.1) The continued evolution of such a complex array of antibiotic-resistance phenotypes presents a formidable challenge, and antibiotics with limited effective span have resulted in increase in morbidity and mortality.2) Therefore, MDR bacteria have triggered major global concern, raised new scientific challenges, and imposed enormous economic burdens. In particular, a global pandemic of methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE) has become one of the biggest threats to public health.3) Vancomycin has been the drug of choice for treatment of MRSA, but emergence of S. aureus with reduced susceptibility to vancomycin, such as vancomycin-resistant S. aureus (VRSA), vancomycin-intermediate S. aureus (VISA), and hetero-resistant VISA (hVISA), complicates the current therapy.4,5)
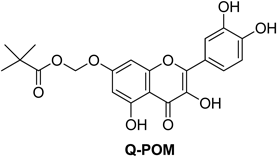
Thus, numerous efforts have been made over the past decade to minimize the emergence and impact of resistance and, among those, combination of antibiotic therapy drew specific attention. Antibiotic combination has shown synergistic antibacterial effects,6) and a recent U.S. Food and Drug Administration (FDA) approval of β-lactamase inhibitor avibactam in combination with a third-generation cephalosporin (ceftazidime) clearly shows the benefits of antibiotic combination approach.7) Previously, we reported that 7-O-pivaloxymethyl quercetin (Q-POM, Fig. 1) could serve to enhance the bactericidal activity of various antibiotics (EtBr, tetracycline, ciprofloxacin, and gentamycin) against methicillin-sensitive S. aureus (MSSA) by 2–8 folds.8) In this study, considering the urgent need for the compound to potentiate antibiotics against MRSA and VRE, we evaluated the bactericidal activity-enhancing activity of Q-POM upon combination with various antibiotics. Antibiotic resistance limits treatment options for S. aureus and Enterococcus, and biofilm formation ability of these organisms confers additional antibiotic resistance that limits treatment efficacy. Therefore, we also tested for anti-biofilm activity of Q-POM against S. aureus and Enterococcus strains.
Eight bacterial strains with different level of antibiotic resistance were used in this study (Table 1). Six S. aureus included a MSSA, three MRSA clinical strains that displayed different sequence types (ST5, ST72, and ST239), a hVISA, and a VISA. Two Enterococcus strains included a vancomycin-susceptible Enterococcus faecium (VSE) and a vancomycin-resistant Enterococcus faecalis.
| Bacteria | Sensitivity | Treatment | MIC (mg/L)f) | ||||
|---|---|---|---|---|---|---|---|
| Q-POM | AMPa) | CAZb) | FEPc) | VANd) | |||
| S. aureus | MSSA | Single | 32e) | 1 | 16 | 0.5 | 0.5 |
| +(Q-POM 4 mg/L) | 1 | 16 | 0.5 | 0.5 | |||
| +(Q-POM 8 mg/L) | — | 1 | 16 | 0.5 | 0.5 | ||
| +(Q-POM 16 mg/L) | — | 0.25 | 4 | 0.5 | 0.5 | ||
| +(Q-POM 32 mg/L)e) | — | <0.06 | <0.06 | <0.06 | <0.06 | ||
| MRSA (ST5) | Single | 32e) | 32 | >256 | >256 | 1 | |
| +(Q-POM 4 mg/L) | — | 32 | >256 | >256 | 1 | ||
| +(Q-POM 8 mg/L) | — | 32 | >256 | >256 | 1 | ||
| +(Q-POM 16 mg/L) | — | 32 | >256 | >256 | 1 | ||
| +(Q-POM 32 mg/L)e) | <0.06 | <0.06 | <0.06 | <0.06 | |||
| MRSA (ST72) | Single | 32e) | 8 | 128 | 64 | 1 | |
| +(Q-POM 4 mg/L) | — | 8 | 128 | 64 | 1 | ||
| +(Q-POM 8 mg/L) | — | 8 | 128 | 16 | 1 | ||
| +(Q-POM 16 mg/L) | — | 8 | 128 | 16 | 0.125 | ||
| +(Q-POM 32 mg/L)e) | <0.06 | <0.06 | <0.06 | <0.06 | |||
| MRSA (ST239) | Single | 16e) | 64 | 8 | 2 | 1 | |
| +(Q-POM 2 mg/L) | — | 32 | 8 | 2 | 1 | ||
| +(Q-POM 4 mg/L) | — | 8 | 8 | 1 | 1 | ||
| +(Q-POM 8 mg/L) | — | 8 | 8 | 0.016 | 0.06 | ||
| +(Q-POM 16 mg/L)e) | — | <0.06 | <0.06 | <0.06 | <0.06 | ||
| hVISA | Single | 32e) | 32 | >256 | >256 | 1 | |
| +(Q-POM 2 mg/L) | — | 32 | >256 | >256 | 1 | ||
| +(Q-POM 4 mg/L) | — | 16 | >256 | 128 | 1 | ||
| +(Q-POM 8 mg/L) | — | 8 | >256 | 64 | 1 | ||
| +(Q-POM 16 mg/L)e) | — | <0.06 | <0.06 | <0.06 | <0.06 | ||
| VISA | Single | 16e) | 4 | 256 | 256 | 4 | |
| +(Q-POM 2 mg) | — | 4 | 256 | 256 | 4 | ||
| +(Q-POM 4 mg) | — | 1 | 256 | 128 | 4 | ||
| +(Q-POM 8 mg)e) | — | <0.06 | <0.06 | <0.06 | <0.06 | ||
| Enterococcus species | VSE | Single | 8e) | 1 | 256 | 16 | 2 |
| +(Q-POM 1 mg) | — | 1 | 256 | 16 | 0.5 | ||
| +(Q-POM 2 mg) | — | 1 | 256 | 4 | 0.5 | ||
| +(Q-POM 4 mg) | — | 1 | 256 | 4 | 0.5 | ||
| +(Q-POM 8 mg)e) | — | <0.06 | <0.06 | <0.06 | <0.06 | ||
| VRE | Single | 32e) | 256 | >256 | >256 | 256 | |
| +(Q-POM 4 mg) | — | 256 | 256 | >256 | 64 | ||
| +(Q-POM 8 mg) | — | 256 | 256 | >256 | 64 | ||
| +(Q-POM 16 mg) | — | 16 | 128 | >256 | 64 | ||
| +(Q-POM 32 mg)e) | — | <0.06 | <0.06 | <0.06 | <0.06 | ||
a) Ampicillin; b) ceftazidime; c) cefepime; d) vancomycin; e) MIC concentrations of POM; f) gray zones denote decreases of MICs of antibiotics by at least 4 folds when they were combined with sub-MIC concentrations of Q-POM.
For evaluation of the potential synergism through combination with Q-POM, four different antibiotics belonging to different classes were investigated (Table 1): ampicillin (AMP, aminopenicillin), ceftazidime (CAZ, 3rd generation cephalosporin), cefepime (FEP, 4th generation cephalosporin), and vancomycin (VAN, glycopeptide). The antibiotic susceptibility test was performed by employing the broth microdilution checkerboard (CB) method to provide minimum inhibitory concentrations (MIC’s, Table 1) and, as a predictor of synergy between Q-POM and antibiotics, fractional inhibitory concentration (FIC) index was determined9) (Table 2).
| Bacteria | Sensitivity | FICa) upon combination with Q-POM | |||
|---|---|---|---|---|---|
| AMPb) | CAZc) | FEPd) | VANe) | ||
| S. aureus | MSSA | 0.75 | 0.75 | 1.00 | 1.00 |
| MRSA (ST5) | 1.00 | 1.00 | 1.00 | 1.00 | |
| MRSA (ST72) | 1.00 | 1.00 | 0.56 | 0.63 | |
| MRSA (ST239) | 0.63 | 1.00 | 0.51 | 0.38 | |
| hVISA | 0.75 | 1.00 | 0.75 | 1.00 | |
| VISA | 0.52 | 1.00 | 0.53 | 1.00 | |
| Enterococcus species | VSE | 1.00 | 1.00 | 0.50 | 0.38 |
| VRE | 0.56 | 1.00 | 1.00 | 1.00 | |
a) FIC=MIC (Q-POM in combination with antibiotics)/MIC (Q-POM alone)+MIC (antibiotics in combination with Q-POM)/MIC (antibiotics alone), where MIC is minimum inhibitory concentration. FIC values between 1 and 0.76 indicate additive effects, values between <0.76–0.5 indicate partial synergy and <0.5 indicate a synergistic effect; b) ampicillin; c) ceftazidime; d) cefepime; e) vancomycin.
First of all, Q-POM itself showed moderate antibacterial activity against all of the bacterial strains investigated with MIC values between 8 and 32 mg/L (Table 1). The MIC values of Q-POM is higher than those of the classical antibiotics, but it is worth to note that Q-POM is not cytotoxic up to 100 µM against normal human cells8) and it did not show significant decrease in bactericidal activity against the resistant strains (Table 1). Most strikingly, upon single treatment against VRE, Q-POM showed MIC of 32 mg/L, which is remarkably lower than other antibiotics investigated (≥256 mg/L, Table 1).
Upon combination with antibiotics, sub-MIC concentrations of Q-POM decreased the MICs of the antibiotics tested (Table 1). Q-POM decreased the MICs of ampicillin (4–64 folds), cefepime (4–128 folds), and vancomycin (4–16 folds).
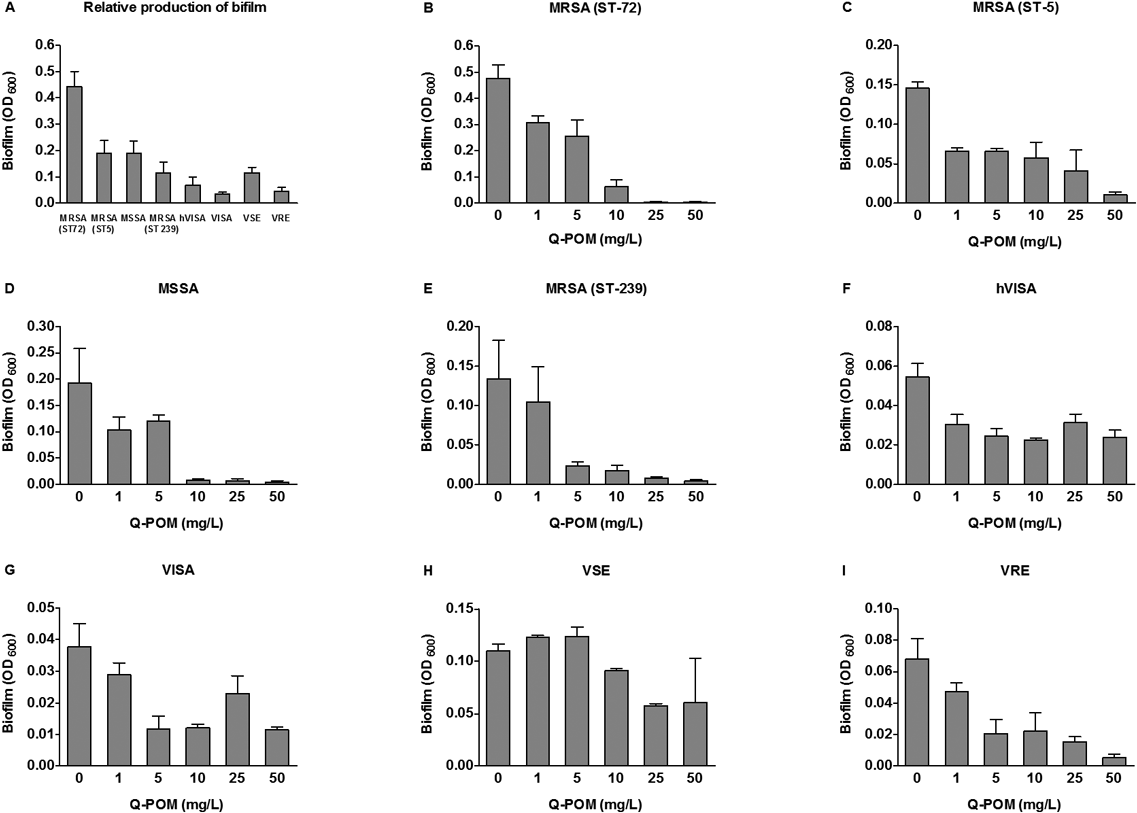
The in vitro synergistic effects of Q-POM and antibiotics combinations were determined by FIC index (Table 2). In combination with sub-MIC concentrations of Q-POM, antibiotic potency of ampicillin was enhanced in both susceptible and resistant strains. In particular, ampicillin/Q-POM combination showed partial synergy with FIC values of 0.52–0.75 against MRSA, hVISA, VISA, and VRE. By the same token, Q-POM potentiated cefepime with FIC values of 0.50–0.75 against MRSA, hVISA, VISA, and VSE, respectively. In contrast, combination of Q-POM with ceftazidime showed no interaction (FIC=1.00). Interestingly, however, upon combination with vancomycin, Q-POM strongly enhanced its antibiotic activity against MRSA (ST239) and VSE, and the FIC value reached up to 0.38. The formation of biofilm was tested against six S. aureus and two Enterococcus strains10) (Fig. 2). The formation of biofilm was inhibited by Q-POM at a dose dependent manner. Percent inhibition of biofilm formation was calculated at 5 mg/L of Q-POM because Q-POM at this concentration did not inhibit growth of organisms. Q-POM at 5 mg/L inhibited the formation of biofilms of S. aureus by 24–83%, and VRE by 70%.
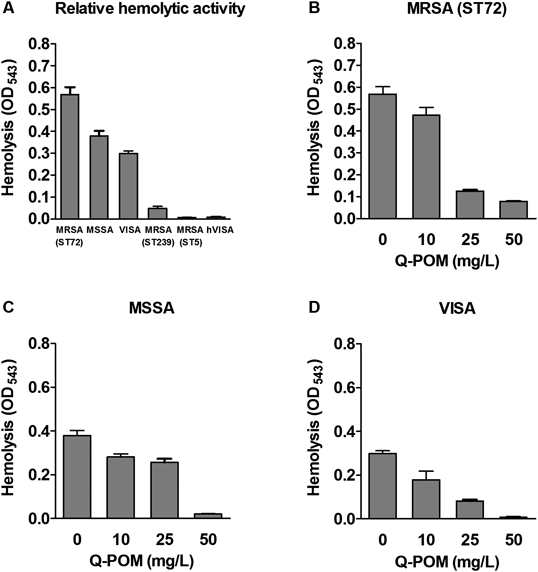
Since S. aureus produces α-toxin that cause hemolysis11) and contributes to biofilm formation,12) the effect of Q-POM on hemolytic activity by S. aureus were investigated. Of the six S. aureus strains tested, we selected three strains with hemolytic activity (MSSA, ST72-MRSA, and VISA). Q-POM inhibited the hemolysis by S. aureus in a dose-dependent manner (Fig. 3).
To demonstrate that Q-POM inhibits antibiotic activity and potentiates antibiotics against the antibiotic-resistant strains at nontoxic concentrations, 50% cytotoxicity concentration (CC50) values on human liver epithelial (HepG2) cells were measured. The CC50 value of Q-POM was higher than 50 mg/L and, at this concentration, more than 70% of the HepG2 cells were shown to be viable (data not shown). This indicated that Q-POM showed both antibiotic and antibiotic-potentiating effects against the resistance strains at concentrations significantly below its CC50.
The mechanism for antibacterial activity and antibiotic-enhancing effects of Q-POM is still elusive. However, we have previously shown that Q-POM inhibits NorA efflux pump of S. aureus, which is overexpressed in MRSA.8) Also, Q-POM was shown to be effective in permeabilizing bacterial membrane to induce leakage of intracellular components.13) This observation is in line with the previous reports that flavonoids including quercetin inflict damage to microbial cell wall and membrane.14–16) Thus, Q-POM, a prodrug of quercetin, is believed to induce cytoplasmic membrane damage, which results in the leakage of intracellular K+ and thereby loss of the integrity and viability of bacterial cells.17)
In conclusion, single treatment of Q-POM showed interesting antibacterial activity against MRSA, hVISA, VISA, and VRE with MIC values between 16 and 32 mg/L. In particular, upon single treatment against VRE, Q-POM showed MIC of 32 mg/L, which is remarkably lower than other antibiotics investigated (≥256 mg/L). On the other hand, sub-MIC concentrations of Q-POM potentiated the activity of ampicillin, cefepime, and vancomycin against S. aureus and Enterococcus (including highly resistant strains such as hVISA, VISA, and VRE). Q-POM decreased the MICs of these antibiotics by 4–128 folds. The in vitro synergistic effects of Q-POM/antibiotics combinations were determined by FIC index. Q-POM was found to be partially synergistic with ampicillin and cefepime against S. aureus and Enterococcus, while it was strongly synergistic with vancomycin. Taken together, along with the proven safety profiles, the antimicrobial activity, synergistic effect, and anti-biofilm activity represent Q-POM as a viable treatment option against the notorious antibiotic-resistant pathogens.
This study was supported by a Grant from the Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI17C0995), and by a Grant from the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0093824).
The authors declare no conflict of interest.