2018 Volume 66 Issue 12 Pages 1174-1180
2018 Volume 66 Issue 12 Pages 1174-1180
Polygala Root (the root of Polygala tenuifolia WILLDENOW; Japanese name “Onji”), a well-known crude drug, traditionally used as an expectorant and sedative, has been attracting increased interest in recent years owing to its newly found pharmacological effect related to neuroprotection. However, there is no specific method for identifying and estimating the quality of this crude drug in the Japanese Pharmacopoeia, 17th edition. Therefore, in order to develop a TLC-based simple and convenient identification method using characteristic chemical marker(s) for the drug and its extract products, UV-sensitive constituents of Polygala Root were first investigated. A total of 23 aromatic compounds were isolated and characterized. Two new compounds, namely, polygalaonjisides A (1) and B (2), were characterized as syringic acid 4-O-(2′-O-β-D-apiosyl)-β-D-glucoside and 2-O-(β-D-glucosyl)-3′-O-benzoylsucrose, respectively. Based on these phytochemical results, a TLC method focusing on three marker spots with Rf value of approximately 0.4–0.5 due to tenuifolisides A and B and 3,6′-di-O-sinapoylsucrose was proposed as a simple and convenient test to identify Polygala Root or its single-extract products on the market. The data presented in this paper could be useful in stipulating a confirmation test to identify Polygala Root.
Polygala Root (Japanese name: “Onji”) is described as the root or the root bark of Polygala tenuifolia WILLDENOW (Polygalaceae) in the 17th edition of the Japanese Phamacopoeia (JP17).1) It is an important crude drug commonly incorporated in Kampo prescriptions such as Kamikihito, Ninjinyoeito, and Kamiuntanto, which are used as an anti-neurosis agent, tonic, and sedative agent, respectively. Polygala Root has also been used as an expectorant—a substitute for Senega (the root of Polygala senega LINNÉ or Polygala senega LINNÉ var. latifolia TORREY et GRAY)—because these plants are closely related saponin-containing species belonging to the same genus. Additionally, Polygala Root has been reported to improve memory defect symptoms that occur in Alzheimer’s patients.2–4) Approval for the acquisition and manufacture of the drug as an agent for the improvement of middle-aged forgetfulness was obtained in 2015 following the release of guidelines from the Ministry of Health, Labour and Welfare in Japan.5) Since then, many single-extract products of Polygala Root have been marketed as drugs to mitigate middle-aged forgetfulness in Japan. Therefore, the demand for Polygala Root extracts is predicted to increase in the future.
The main constituents of the Polygala Root are saponins called onjisaponins.6,7) The foaming test and the Liebermann–Burchard reaction, indicative of the presence of saponins, have been adopted as the main identification tests for this crude drug in JP17. Naturally, these identification tests are not specific to Polygala Root. The pharmacopoeia has stipulated that TLC can be used as a simple and convenient identification method to detect indicator ingredients of several drugs, thereby confirming their specificity in the crude form. However, no TLC identification method for Polygala Root has been listed in JP17. Considering the anticipated future demand for crude Polygala Root, a well-define quality evaluation method using TLC for this drug is desirable to be included in the pharmacopoeia.
Xanthones,8–11) phenones,12) and oligosaccharides,13–17) have also been reported as the main constituents of Polygala Root in addition to abundant saponins. Polygala Root is listed as Polygala Radix in the Chinese Pharmacopoeia, and the standard values of three of its major components—tenuifolin, 3,6′-di-O-sinapoylsucrose, and polygalaxanthone III—have been stipulated based on HPLC quantification.18)
The aims of this study were to characterize UV-sensitive constituents of Polygala Root and establish a simple, convenient, and reliable TLC-based quality control test applicable to Polygala Root or its extract. Here, we report on the isolation and characterization of water-soluble aromatic constituents, including two newly found compounds from Polygala Root, and proposal a TLC-based simple identification test focusing on the marker compounds
A homogenate of Polygala Root in methanol (MeOH) was concentrated and defatted by extraction with n-hexane. The residual water extract was subjected to successive chromatographies on Diaion HP-20, YMC GEL ODS-AQ, and Chromatorex ODS with aqueous MeOH and preparative TLC to afford 23 compounds including two new glycosides designated as polygalaonjisides A (1) and B (2). The known compounds were identified as 4-hydroxybenzoic acid (3),19) monordicophenoide A (4),20) 4-hydroxy-3-methoxybenzoic acid (vanillic acid) (5),19) hemsleyanumoide (6),21) sibiricose A3 (7),22) 3,5-dimethoxy-4-hydroxybenzoic acid (syringic acid) (8),23) sibiricose A5 (9),22) sibiricose A6 (10),22) trans-ferulic acid (11),23) sibiricose A1 (12),22) glomeratose A (13),24) sibiricoxanthone B (14),11) tenuifoliside B (15),14) polygalaxanthone XI (16),11) polygalaxanthone III (17),9) 3,6′-di-O-sinapoylsucrose (18),25) tenuifoliside A (19),14) 1′-cinnamoyl-3′-benzoyl-(2-O-β-glucosyl)-sucrose (20),16) 6-(4‴-methoxybenzoyl)-3′-(3″,4″,5″-trimethoxycinnamoyl)-sucrose (21),16) 1,3,7-trihydroxyxanthone (22),26) and tenuifoliose J (23)15) (Fig. 1). The known compounds, 3–6, 8, and 11, as well as the novel compounds 1 and 2, were obtained from Polygala Root in this study.

Polygalaonjiside A (1) was isolated as a light brown amorphous powder. Its molecular formula was determined to be C20H28O14 based on high-resolution-electrospray ionization (HR-ESI)-MS data (m/z 491.1384 [M−H]−), and the 13C-NMR spectrum displayed twenty 13C signals. The UV spectrum showed absorption maxima at 208 and 252 nm. The 1H- and 13C-NMR spectra (Table 1) of 1 showed the signals corresponding to one aromatic 2H-singlet (δ 7.32) and 6H singlet (δ 3.86) due to two methoxy groups, six aromatic carbons, and one carbonyl carbon [δ 129.7, 108.6 (2C), 154.0 (2C), 139.1, 171.0] which are characteristic of syringic acid (4-hydroxy-3,5-dimethoxybenzoic acid) nucleus. The presence of a syringic acid unit was further supported by correlations in the heteronuclear single quantum coherence (HSQC) and heteronuclear multiple bond connectivity (HMBC) spectra as shown in Fig. 2. The chemical evidence for the aglycone of 1 was obtained by acid hydrolysis followed by HPLC analysis, which showed the production of syringic acid. The presence of two sugar units was identified based on two anomeric proton signals at δ 5.19 (d, J=7.0 Hz) and δ 5.47 (d, J=1.0 Hz), and 11 aliphatic carbon signals, including one quaternary (δ 80.9) and three methylene carbon resonances (δ 62.6, 66.3 and 75.6). These data along with the assignments of sugar proton signals by the 1H–1H correlation spectroscopy (COSY) spectrum (Table 1) were similar to those of the apiosylglucose moiety.17) D-Glucose and D-apiose as sugar units of 1 were confirmed by acid hydrolysis followed by HPLC analysis of derivatives prepared by a previously reported method involving a reaction between L-cysteine methyl ester and o-tolyl isothiocyanate.27) The linking position for each unit was determined using HMBC (Fig. 2) which showed three-bond correlations among the glucose H-1′ (δ 5.19)/C-4 (δ 139.1) of the syringic acid, apiose H-1″ (δ 5.47)/glucose C-2′ (δ 78.73), and glucose H-2′ (δ 3.692)/apiose C-1″ (δ 110.4)]. β-Glycosidic linkage in the glucose core was evidenced by large coupling constants (J=7.0 Hz) of the anomeric proton. The configuration at the anomeric center of the apiose molecule was similarly found to be β based on comparison of the 13C-NMR signal (δ 110.4) for C-1″ of 1 with those of α- and β-D-apiofuranoside.28) This was further confirmed by the fact that the coupling constant of H-1″ and 2″ of 1 was the same as the reported value for β-D-apiofuranoside.29) Therefore, polygalaonjiside A (1) was established as syringic acid 4-O-(2′-O-β-D-apiosyl)-β-D-glucoside.
| Position | 1 | Position | 2 | ||
|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | ||
| 1 | 129.7 | 1 | 93.3 | 5.65 (d, J=3.5) | |
| 2 | 108.6 | 7.32 (s) | 2 | 82.1 | 3.47 (dd, J=3.5, 10.0) |
| 3 | 154.0 | 3 | 73.7 | 3.75 (t, J=10.0) | |
| 4 | 139.1 | 4 | 70.9 | 3.44 (t, J=10.0) | |
| 5 | 154.0 | 5 | 74.4 | 3.93 (ddd, J=2.5, 4.5, 7.0) | |
| 6 | 108.6 | 7.32 (s) | 6 | 62.3 | 3.77–3.85a) |
| 7 | 171.0 | 3.77–3.85a) | |||
| OCH3-3,5 | 57.0 | 3.86 (6H, s) | 1′ | 64.4 | 3.61 (d, J=12.0) |
| 1′ | 102.4 | 5.19 (d, J=7.0) | 3.77 (d, J=12.0) | ||
| 2′ | 78.73 | 3.692 (dd, J=7.0, 9.0) | 2′ | 105.1 | |
| 3′ | 78.66 | 3.54 (t, J=9.0) | 3′ | 79.4 | 5.66 (d, J=8.0) |
| 4′ | 71.4 | 3.45 (t, J=9.0) | 4′ | 73.4 | 4.44 (d, J=8.0) |
| 5′ | 78.12 | 3.18 (ddd, J=2.0, 5.0, 9.0) | 5′ | 84.2 | 3.97 (ddd, J=3.0, 5.5, 8.5) |
| 6′ | 62.6 | 3.63 (dd, J=5.0, 12.0) | 6′ | 63.1 | 3.77–3.85 (2H)a) |
| 3.71 (dd, J=2.0, 12.0) | 1″ | 106.1 | 4.41 (d, J=8.0) | ||
| 1″ | 110.4 | 5.47 (d, J=1.0) | 2″ | 75.4 | 3.16 (dd, J=8.0, 9.5) |
| 2″ | 78.07 | 3.99 (d, J=1.0) | 3″ | 77.9 | 3.25–3.30b) |
| 3″ | 80.9 | 4″ | 71.7 | 3.21 (dd, J=9.0, 9.5) | |
| 4″ | 75.6 | 3.689 (d, J=9.5) | 5″ | 78.3 | 3.25–3.30b) |
| 4.03 (d, J=9.5) | 6″ | 62.9 | 3.66 (dd, J=6.0, 12.0) | ||
| 5″ | 66.3 | 3.58 (d, J=11.5) | 3.88 (dd, J=2.0, 12.0) | ||
| 3.688 (d, J=11.5) | 1‴ | 131.1 | |||
| 2‴ | 131.0 | 8.10 (dd, J=1.0, 8.0) | |||
| 3‴ | 129.7 | 7.50 (t, J=8.0) | |||
| 4‴ | 134.5 | 7.61 (tt, J=1.0, 1.0, 8.0, 8.0) | |||
| 5‴ | 129.7 | 7.50 (t, J=8.0) | |||
| 6‴ | 131.0 | 8.10 (dd, J=1.0, 8.0) | |||
| 7‴ | 167.3 | ||||
a, b) Overlapped signals.
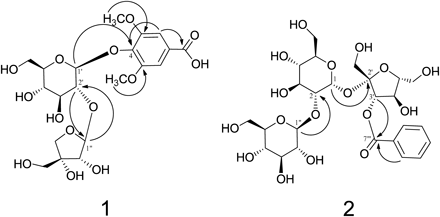
Polygalaonjiside B (2) was isolated as an off-white amorphous powder. Its molecular formula was determined to be C25H36O17 based on its HR-ESI-MS data (m/z 631.1832 [M+Na]+; Calcd for C25H36O17+Na: 631.1845) and 13C-NMR spectrum (25 13C signals). The UV absorption bands at 231 and 279sh nm suggested that it is an aromatic compound. The 1H-NMR spectrum of 2 exhibited signals characteristics of mono-substituted benzene [δ 8.10 (2H, dd, J=1.0, 8.0 Hz), δ 7.50 (2H, t, J=8.0 Hz), and δ 7.61 (1H, tt, J=1.0, 1.0, 8.0, 8.0 Hz)] and three sets of sugar proton signals. The 13C-NMR spectrum showed six aromatic carbon signals and one ester carbonyl carbon signal [δ 131.1, 131.0 (2C), 129.7 (2C), 134.5, 167.3] assignable to be a benzoyl unit, which was confirmed by acid hydrolysis followed by HPLC showing the production of benzoic acid. The sugar proton and carbon signals in the NMR spectra were assigned by 1H–1H COSY, HSQC, and HMBC experiments as shown in Table 1. These spectral data implied close similarity to those of 2-O-β-D-glucosylsucrose.16,30,31) The HMBC spectrum showed long range correlations among H-1 (δ 5.65)/C-2′ (δ 105.1), H-1″ (δ 4.41)/C-2 (δ 82.1) and H-3′ (δ 5.66)/C-7‴ (δ 167.3) (Fig. 2). Based on these spectral data, polygalaonjiside B (2) was established to be 2-O-(β-D-glucosyl)-3′-O-benzoylsucrose.
TLC-Based Evaluation of Polygala RootExamination of various TLC conditions to detect the UV-sensitive constituents of Polygala Root revealed the following conditions to be suitable to test the quality of this drug. As for the developing solvent on silica gel TLC, a mixture of ethyl acetate (EtOAc), MeOH and water (6 : 1 : 1) was found to provide well-separated spots that included three clear spots (I–III). These three spots clearly distinguished Polygala Root from a closely related crude drug, Senega (root of Polygala senega), which shows a quite different high performance (HP) TLC pattern under the same conditions as shown in Fig. 3. Furthermore, HPTLC comparison of nine samples (Table 2) of Polygala Root available on the Japanese market indicated that these three spots were characteristic of Polygala Root, and suggested to be useful as markers to estimate of their identity and quality (Fig. 4).

TLC plate was illuminated with UV 254 nm. A: Polygala Root, B: Senaga. Conditions are described in the Experimental. (Color figure can be accessed in the online version.)
| No. | Code No. | Locality | Collection date (year) |
|---|---|---|---|
| 1 | NIB-0260 | Shaanxi, China | Unknown |
| 2 | NIB-0436 | Shanxi, China | 2011 |
| 3 | NIB-0488 | Shaanxi, China | 2010 |
| 4 | NIB-0489 | Shaanxi, China | 2007 |
| 5 | NIB-0490 | Shaanxi, China | 2007 |
| 6 | NIB-0491 | Shaanxi, China | 2006 |
| 7 | NIB-0492 | Shaanxi, China | 2005 |
| 8 | NIB-0493 | Shaanxi, China | 1997 |
| 9 | NIB-0742 | Shanxi, China | Unknown |

TLC plate was illuminated with UV 254 nm. 1–9: Sample number in Table 2. Conditions are described in the Experimental. (Color figure can be accessed in the online version.)
The compounds corresponding to the three marker spots obtained from TLC analysis were identified as (spot I) tenuifoliside A (19), (spot II) tenuifoliside B (15), and (spot III) 3,6′-di-O-sinapoylsucrose (18), by comparing the respective spots of the isolated compounds (Fig. 3). Tenuifolisides A (19) and B (15) were first isolated from Polygala Root,14) whereas 3,6′-di-O-sinapoylsucrose (18) is a previously known characteristic ingredient of this drug.32) These three compounds have also been reported as marker components in the multi-component analysis of Polygala Root by ultra-performance liquid chromatography (UPLC) and LC/MS analysis,32,33) further supporting the validity that they are characteristic components of Polygala Root. These compounds have been reported to exhibit neuroprotective, cognitive improving, and cerebroprotective effects.34–37) 3,6′-Di-O-sinapoylsucrose (18) has also been reported to synergistically interact with 19.38) In addition, sinapic acid and 3,4,5-trimethoxycinnamic acid, which are aglycones of these compounds, have been reported to possess cerebroprotective and cognition-improving effects,39–41) implying these compounds to be prodrugs of the active aglycones. The single crude drug extract product of Polygala Root has been prescribed for mitigating the problem of forgetfulness after middle age, expecting a function that is related to the neuropsychological effects attributed to the three marker compounds. Therefore, these three components would be suitable markers for the pharmacological quality control of crude Polygala Root.
Further, our proposed TLC analysis was applied to five commercial single crude drug extract products of Polygala Root using generic TLC plate to obtain similar results showing the three maker spots with a Rf value of approximately 0.4–0.5 for in all products, although their relative concentrations were varied depending on the samples as shown in Fig. 5.

TLC plate was illuminated with UV 254 nm. A: Polygala Root, B: Senaga. 1–5: Commercial Polygala Root extract products. Conditions are described in the Experimental. (Color figure can be accessed in the online version.)
The confirmation test for quality control of crude drugs is required to be as simple, inexpensive, and safe as possible. The TLC method does not require expensive equipment such as HPLC or LC/MS, and the drugs can be evaluated easily and accurately. Thus, the TLC method has been adopted as the confirmation test for many crude drugs in JP17; however, no TLC method for Polygala Root has been reported thus far. The main components of the Polygala Root are known to be saponins, as described above. Detection of saponins using TLC must be carried out by heating after spraying the acid reagent, but the present method, which uses only UV irradiative detection, enables elimination of these steps. Therefore, the TLC analytical method proposed for the first time in this study is applicable for the identification and quality analysis of both the crude drug of Polygala Root and its extracts.
Optical rotations were measured using a JASCO P-1020 digital polarimeter (JASCO Corporation, Tokyo, Japan). The UV spectra were recorded using a Shimadzu UVmini-1240 (Shimadzu Corporation, Kyoto, Japan) and a JASCO V-530 (JASCO Corporation). ESI and HR-ESI-MS spectra were recorded using a micrOTOF-Q (Bruker Daltonics, Billerica, MA, U.S.A.) mass spectrometer with acetonitrile as the solvent. The NMR spectra were recorded using a Bruker AVANCE500 instrument (Bruker BioSpin, Billerica, MA, U.S.A.; 500 and 126 MHz for 1H and 13C, respectively) and chemical shifts were expressed relative to those of the solvent [MeOH-d4 (δH 3.30; δC 49.0) and dimethyl sulfoxide (DMSO)-d6 (δH 2.50; δC 39.5)] on a tetramethylsilane scale in parts per million (ppm). The standard pulse sequences programed for the instrument (AVANCE 500) were used for 2D measurement (COSY, HSQC, and HMBC). JCH was set at 10 Hz for HMBC analysis. Column chromatography was carried out over Diaion HP-20, MCI-gel CHP-20P (Mitsubishi Chemical Co., Tokyo, Japan), Chromatorex ODS (Fuji Silysia Chemical Ltd., Aichi, Japan), Sephadex LH-20 (GE Healthcare, Little Chalfont, U.K.), and YMC GEL ODS (YMC Co., Ltd., Kyoto, Japan). Preparative TLC was carried out on TLC Silica gel 60 F254 glass plates (Merck, Darmstadt, Germany). HPTLC was performed with an CAMAG HPTLC equipment (CAMAG, Muttenz, Switzerland) including a Linomat V applicator (CAMAG) and visualizer documentation system (CAMAG). The samples were spotted on HPTLC Silica gel 60 F254 glass plates (20×10 cm, Merck) or TLC Silica gel 60 F254 plates (5×7.5 cm, Merck), and the spots were detected by irradiation at 254 nm. The reversed-phase (RP) HPLC conditions used for monitoring fractions in the column chromatography were as follows. Condition 1: column, YMC-pack ODS AQ-3C2 (5 µm, 150×2.0 mm i.d., YMC Co., Ltd., Kyoto, Japan); mobile phase, solvent A 0.1% formic acid in water, and solvent B MeOH (0–30 min, 0–50% B in A, 30–50 min, 50–60% B in A, 50–70 min, 60–100% B in A); column temperature, 40°C; flow rate, 0.25 mL/min; detection wavelength, 200–400 nm. Condition 2: column, YMC-pack ODS AQ-3C2 (5 µm, 150×2.0 mm i.d., YMC Co., Ltd.); mobile phase, 10 mmol/L phosphate buffer–acetonitrile (85 : 15); column temperature, 40°C; flow-rate, 0.2 mL/min; detection wavelength, 254 nm. Condition 3: column, YMC-pack ODS AQ-3C2 (5 µm, 150×2.0 mm i.d., YMC Co., Ltd.); mobile phase, 50 mmol/L phosphate buffer–acetonitrile (75 : 25); column temperature, 35°C; flow-rate, 0.3 mL/min; detection wavelength, 250 nm.
MaterialsThe Polygala Root (Lot. no. 9A60019) used for the phytochemical investigation was purchased from Uchida Wakanyaku Ltd., Tokyo, Japan. Polygala Roots available on the Japanese market were obtained from Japan Kampo Medicines Manufactures Association, Japan Medicinal Plant Federation, and Tokyo Crude Drugs Association (model samples described by the National Institute of Biomedical Innovation, Health and Nutrition (NIBIOHN)). Senega (Lot. no. 006105001) was purchased from Tochimoto Tenkaido Co., Ltd. (Osaka, Japan). Commercial single crude drug extract products of Polygala Root were purchased from drugstores in Japan (in 2017 and 2018). All other reagents used were of special or analytical grade.
Extraction and IsolationThe Polygala Root product (100 g) was homogenized in MeOH (1 L). The homogenate was filtered and concentrated to approximately 200 mL. Next, water (200 mL) was added, and the solution was concentrated to approximately 200 mL. Extraction was performed with 900 mL n-hexane to obtain extracts from the n-hexane (944.6 mg) and H2O layers (ca. 100 mL). The H2O layer was separated using column chromatography over Diaion HP-20 with aqueous MeOH solvent (0 : 100→10 : 90→20 : 80→30 : 70→50 : 50→100 : 0) in stepwise gradient mode. First, a 10% MeOH eluate (100 mg) was separated by YMC GEL ODS column chromatography with aqueous MeOH to obtain monordicophenoide A (4, 1.5 mg) and hemsleyanumoide (6, 2.4 mg). Second, a 20% MeOH eluate (120 mg) was separated by column chromatography over YMC GEL ODS and Chromatorex ODS with aqueous MeOH to obtain polygalaonjisides A (1, 1.9 mg) and B (2, 7.5 mg) as well as sibiricose A3 (7, 6.2 mg). Third, a 50% MeOH eluate (1 g) was separated by column chromatography over YMC GEL ODS with aqueous MeOH to obtain 4-hydroxybenzoic acid (3, 0.5 mg), 4-hydroxy-3-methoxybenzoic acid (5, 1.0 mg), 3,5-dimethoxy-4-hydroxybenzoic acid (8, 0.5 mg), polygalaxanthone III (17, 20.3 mg), and 3,6′-di-O-sinapolysucrose (18, 39.8 mg). The fractions showing similar HPLC patterns (Condition 1) were combined and further purified by column chromatography over Sephadex LH-20 with ethanol and/or MCI-gel CHP-20P with aqueous MeOH to yield sibiricose A5 (9, 4.9 mg), sibiricose A6 (10, 5.6 mg), glomeratose A (13, 3.9 mg), sibiricose A1 (12, 2.1 mg), sibiricoxanthone B (14, 10.9 mg), tenuifoliside B (15, 10.0 mg), polygalaxanthone XI (16, 7.0 mg), tenuifoliside A (19, 10.5 mg), and 1′-cinnamoyl-3′-benzoyl-(2-O-β-glucosyl)-sucrose (20, 4.2 mg). Finally, the 100% MeOH eluate (2.0 g) was separated by column chromatography over YMC GEL ODS to obtain 6-(4‴-methoxybenzoyl)-3′-(3″,4″,5″-trimethoxycinnamoyl)-sucrose (21, 2.0 mg). Additionally, the 100% MeOH eluate (2.0 g) from the Polygala Root product (300 g), after similar pretreatment, was separated using preparative TLC with a 7 : 1 : 1 mixture of EtOAc, MeOH, and H2O to obtain trans-ferulic acid (11, 9.4 mg), 1,3,7-trihydroxyxanthone (22, 2.1 mg), and tenuifoliose J (23, 13.3 mg). These compounds were identified by direct comparison with authentic specimens or by comparing their spectral data with those reported in the literature. The physical and spectral data of the novel compounds 1 and 2 are as follows.
Polygalaonjiside A (1)A light brown amorphous powder. UV λmax (MeOH) nm (log ε): 208 (3.75), 252 (4.30). [α]20D −30.5° (c=2.0, MeOH). The 1H-NMR (500 MHz, MeOH-d4) and 13C-NMR (126 MHz, MeOH-d4) data are provided in Table 1. HR-ESI-MS m/z: 491.1384 ([M−H]−, Calcd for C20H28O14-H: 491.1406).
Polygalaonjiside B (2)An off-white amorphous powder. UV λmax (MeOH) nm (log ε): 231 (3.93), 279sh (3.30). [α]26D −8° (c=0.1, MeOH). The 1H-NMR (500 MHz, MeOH-d4) and 13C-NMR (126 MHz, MeOH-d4) data are provided in Table 1. HR-ESI-MS m/z: 631.1832 ([M+Na]+, Calcd for C25H36O17+Na: 631.1845).
Partial Acid HydrolysisEach solution of 1 and 2 (each 0.2 mg) was prepared in H2O (0.2 mL) and 1 mol/L HCl (0.1 mL) and heated in a boiling water bath for 8 h. After removing the solvent, the residues were separately analyzed using HPLC (Condition 2), in order to detect syringic acid in 1 and benzoic acid in 2.
Sugar AnalysisSugar configurations were determined using a previously described method. Compounds 1 and 2 (each 0.5 mg) were hydrolyzed by heating in 1 mol/L HCl (0.2 mL) and neutralized with Amberlite IRA400. After evaporation, the residues were dissolved in pyridine (0.2 mL) containing L-cysteine methyl ester hydrochloride (1.0 mg) and heated at 60°C for 1 h. o-Tolyl isothiocyanate (1.0 mg) in pyridine (0.2 mL) was then added to each mixture and heated at 60°C for 1 h. The reaction mixtures were directly analyzed using RP-HPLC (Condition 3). The peaks from 1 coincided with those of the derivatives similarly prepared from authentic D-glucose and D-apiose obtained from apiin. The peak from 2 was confirmed to be from D-glucose.
Preparation of Test Solution of Crude Polygala Root for TLCCrude Polygala Root samples were pulverized, and a 1.0 g sample was extracted with MeOH (5.0 mL) via sonication for 3 min. The extract was centrifuged, and the supernatant obtained was used as the test solution. Test solutions for commercial single crude drug Polygala Root extract products were prepared by extracting 100 mg of the product with 1.0 mL of MeOH. For TLC, aliquots (1 or 3 µL) of the test solutions were applied to the TLC or HPTLC plates, developed in a TLC chamber saturated with the mobile phase, which was a 6 : 1 : 1 (v/v/v) mixture of EtOAc, MeOH, and H2O. The spots were detected under a UV lamp at 254 nm.
A portion of this work was supported by the Japan Agency for Medical Research and Development (AMED) [under a Grant for Research on the Development of New Drugs; 17ak0101046h0002].
The authors declare no conflict of interest.
The online version of this article contains supplementary materials. Experimental details on 1H- and 13C-NMR spectra of compounds 1 and 2 are provided.