2018 Volume 66 Issue 8 Pages 830-838
2018 Volume 66 Issue 8 Pages 830-838
We report the preparation of new C3- and CS-symmetrical molecules constructed on a triazine (TAZ) template. Anti-herpes simplex virus type 1 (anti-HSV-1) and cytotoxic activities against Vero cells of synthesized TAZ derivatives were evaluated. The results suggested that the presence of an electron-donating group(s) on the benzene ring in benzylamine groups on the TAZ template is an important structural factor for expressing a high level of anti-HSV-1 activity and low cytotoxicity for these C3 types of TAZ derivatives. Among the tested TAZ derivatives, compounds 4f and 7h showed the highest anti HSV-1 activities (EC50=0.98 and 1.23 µM, respectively) and low cytotoxic activities to Vero cells (50% cytotoxic concentration (CC50)=292.2 and >200 µM, respectively).
In the search for new types of antiviral active compounds, extensive efforts have been made to find promising new antiviral candidates.1) It is well known that many types of receptors or functionalized proteins on membranes in a native state often have a high order of symmetrical interfaces.2) C3- or C2-geometric symmetrical molecules have frequently been found in various synthetic biologically active compounds.3) Aiming to develop new synthetic bioactive compounds, we targeted such geometrical symmetric molecules constructed on a symmetrical template.
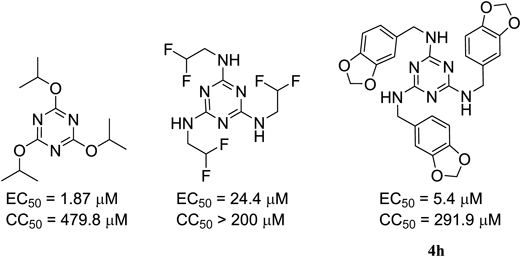
In our previous studies on antiviral compounds in the tri-substituted 1,3,5-triazine (TAZ) series, we found that many of the new C3- and CS-symmetrical TAZ derivatives synthesized previously showed a wide range of bioactivities against herpes simplex virus type 1 (HSV-1) or cytotoxicic activities to Vero cells.4) Regarding tri-substituted TAZ derivatives, we have reported that some symmetrical TAZ derivatives having alkoxy and/or alkylamino substituents show a high level of anti-HSV-1 activity5) (Fig. 1). Notably, we found that some TAZ derivatives that have benzylamine substituents on a TAZ template such as compound 4h show a high level of anti-HSV-1 activity (EC50=5.4 µM) and low cytotoxicity (50% cytotoxic concentration (CC50)=291.9 µM). In terms of a structure–activity relationship (SAR) study and finding new types of antiviral active leads, molecular modification by the introduction of benzylamine functionalities into a TAZ template seems to be interesting.
Here, we describe the synthesis of new types of symmetrical TAZ derivatives that have various benzylamine substituents on symmetrical TAZ templates and the results of their biological evaluations.
Our strategies for the synthesis of target TAZ derivatives by using 2,4,6-trichlorotriazine (TCT AZ: 1) as a starting material are summarized in Chart 1.
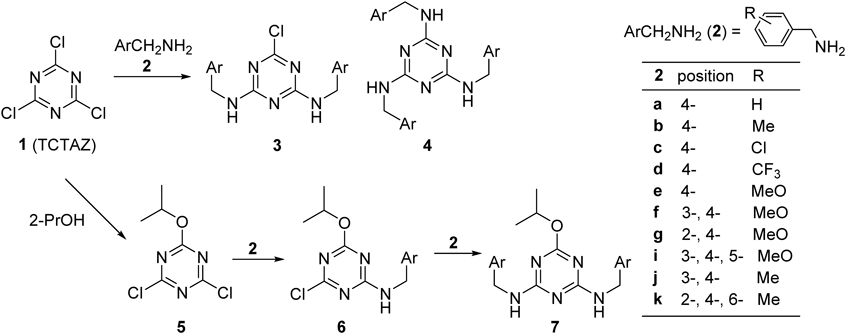
The pathway for the synthesis of C3- and CS-type symmetrical TAZ derivatives (4 and 7) together with intermediates of TAZ derivatives is shown in Chart 1. All of the substitution reactions of the starting TCT AZ (1) or monoalkoxy-dichloro-TAZ (5)5) with various benzylamines (2a–k) were performed in a one-pot reaction and the desired new TAZ derivatives (4 and 7) were obtained. All of the target C3-type TAZ derivatives (4) were obtained in moderate to good yields (42–85%) as shown in Table 1, and CS-type symmetrical TAZ derivatives (7) were also obtained in moderate yields (Table 2). For the synthesis of these new targeted C3- and CS-symmetrical tri-substituted TAZ derivatives, we used the same procedure as that described for the preparation of symmetrical TAZ derivatives (see Experimental).
| Entry | ArCH2NH2 (2) | Reaction time of MW (min)a) | Products (yield %) |
|---|---|---|---|
| 1 | 2a | 60 | 4a (85) |
| 2 | 2b | 60 | 4b (53) |
| 3 | 2b | 40b) | 3b (46)c), 4b (5)c) |
| 4 | 2c | 60 | 4c (75) |
| 5 | 2d | 60 | 4d (42) |
| 6 | 2e | 30 | 4e (66) |
| 7 | 2f | 50 | 4f (80) |
| 8 | 2g | 20 | 4g (57) |
| 9 | 2i | 20 | 4i (63) |
a) In the all reactions except for Entry 3, stirring of the reaction mixtures (ratio of 1 : 2=1 : 10) in dioxane were conducted at 0°C for 30 min, at room temperature for 1 h, and then irradiation of microwave (100 W, 100°C) for a proper shown time. b) Stirring the reaction mixture (ratio of 1 : 2b=1 : 6) in THF was conducted at room temperature for 10 min, and then irradiation of microwave (90 W, 70°C) for 20 min. c) Yields after recrystallization.
| Entry | ArCH2NH2 (2) | Ratio of 1 : 2 (: DIPEA)a) | Reaction conditionsa) | Product (yield %) |
|---|---|---|---|---|
| 1 | 2a | 1 : 1 : 1 (DIPEA) | 0°C 2 h | 6a (97) |
| 2 | 2a | 1 : 5 | MW 60 min | 7a (79) |
| 3 | 2b | 1 : 5 | MW 45 min | 7b (82)b) |
| 4 | 2e | 1 : 5 | MW 60 min | 7e (75)b) |
| 5 | 2f | 1 : 6 | MW 30 min | 7f (75)b) |
| 6 | 2g | 1 : 5 | MW 30 min | 7g (72) |
| 7 | 2h | 1 : 3 | MW 30 min | 7h (34)b) |
| 8 | 2i | 1 : 5 | MW 30 min | 7i (89) |
| 9 | 2j | 1 : 5 | MW 30 min | 7j (84)b) |
| 10 | 2k | 1 : 5 | MW 45 min | 7k (78) |
a) In the all reactions except for Entry 1, the reaction mixtures were subjected to microwave irradiation (100 W, 100°C) in dioxane. In the reaction of Entry 1, the reaction mixture with N,N-diisopropylethylamine (DIPEA) was used in THF. b) Yields after recrystallization.
All of the structures of the synthesized C3- and CS-symmetrical TAZ derivatives (4 and 7) and a few intermediates (3, 6) were confirmed by spectroscopic and analytical data. The geometries of the obtained symmetrical TAZ derivatives described above were confirmed from 13C-NMR spectroscopic data6) (see Experimental for details).
Biological Activity and DiscussionIn many synthetic receptor-type molecules mimicking the supramolecular interaction of biomolecules, it is expected that the ability to form multiple hydrogen bonds plays an important role in guest molecule recognition. Considering the hydrogen bonded interaction in a supramolecular host-guest system, it is also expected that a convergent orientation of incorporated functional groups is an effective array to produce multiple hydrogen bonding interactions between host and guest molecules.7,8) From this point of view, title C3- and CS-geometrical molecules that have donors or acceptors for hydrogen bonds on a symmetrical TAZ template are thought to offer new biologically active molecules.
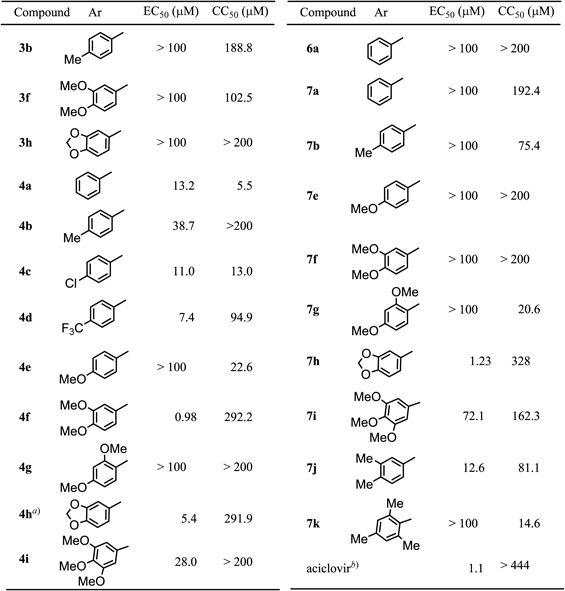
From biological evaluations of anti-HSV-1 activities (EC50) by plaque reduction assays9) and cytotoxic activities (CC50) against Vero cells of synthesized TAZ derivatives (Table 3), most of the C3-symmetrical benzylamine-type TAZ derivatives (4) presented hydrogen bond donor protons of a benzylamine (–NH–) functionality. Many of the C3-symmetrical benzylamine-type TAZ derivatives (4) showed a significantly high level of anti-HSV-1 activity, and compound 4f showed a high level of anti-HSV-1 activity (EC50=0.98 µM) and a low level of cytotoxic activity (CC50=292.2 µM) as did compound 4h5) (EC50=5.4 µM and CC50=291.9 µM) reported previously. All of the compounds that have electron-withdrawing substituents (4c and 4d) including non-substituted compound 4a showed considerably high levels of anti-HSV-1 activity (EC50=7.4–13.2 µM) and cytotoxic activity (CC50=5.5–94.9 µM). Regarding the two biological activities (anti-HSV-1 activity and cytotoxic activity), the opposite order in electron-withdrawing (attracting) effects between the two activities was observed (4a<4c<4d for anti-HSV-1 activity and 4a>4c>4d for cytotoxic activity). Lower levels of cytotoxic activity (CC50=13.0–94.9 µM) were observed for compounds 4c and 4d than for non-substituted compound 4a.
 |
a) Data were taken from ref. 5). b) Data were taken from ref. 15).
Considerably high levels of anti-HSV-1 activity (EC50=0.98–38.7 µM) were observed for C3-type compounds (4b, 4f, 4h5) and 4i) that have electron-donating substituents such as methyl or methoxy groups on the benzene rings except for compounds 4g and 4e (see Table 3), and these compounds showed cytotoxic activities over 200 µM. The obtained biological results indicated that the nature of substituents on the benzene rings prefers electron-donating substituents for expressing a high level of anti-HSV-1 activity and low level of cytotoxicity for these C3 types of TAZ derivatives. We also consider that benzylamine groups in C3-type molecules may act as hydrogen-bond donors to receptor binding sites.
The exceptional anti-HSV-1 activity for compound 4g may be due to intramolecular hydrogen bond formation between the basic benzylamine moiety and the ortho-methoxy group in the molecule 4g that weakens such intermolecular hydrogen-bonding interaction of the TAZ molecule 4g to receptor binding sites (see Fig. 2). This exceptional result for compound 4g also indicates the importance of intermolecular hydrogen bond formation ability of C3-type TAZ molecules in the supramolecular interaction with its binding site for expressing a high level of anti-HSV-1 activity.

Despite the considerably high level of cyctotoxic activity (CC50=22.6 µM) of compound 4e, the reason for exceptionally low level of anti-HSV-1 activity (EC50>100 µM) for this compound is still unclear at present. The positional effect of methoxy groups on benzene rings in these TAZ derivatives may be required for expression of anti-HSV-1 activity.
Among the CS-symmetrical benzylamine-type TAZ derivatives (7a, 7b, 7e–k) as shown in Table 3, a CS-symmetrical benzylamine-type TAZ derivative (7h) that has two benzylamine groups with electron-donating substituents and an isopropoxy group showed a considerably high level of anti-HSV-1 activity (EC50=1.23 µM); however, many structurally related CS-symmetrical derivatives (7a, 7b, 7e, 7f, 7g and 7k) showed low levels of anti-HSV-1 activity (EC50=>100 µM) compared to that of compound 7h. Compounds 7i and 7j showed moderate levels of anti-HSV-1 activity (EC50=72.1 and 12.6 µM, respectively). Interestingly, many compounds in this series (7a, 7b, 7g, 7i, 7j and 7k) showed moderate levels of cytotoxic activity (CC50=14.6–192.4 µM, see Table 3). Intermediates 3 and 6 listed in Table 3 showed almost no anti-HSV-1 or cytotoxic activity (see Table 3).
Regarding calculated log P values10) for the compounds (see Supplementary Materials), there were few distinct correlations between log P values and anti-HSV-1 activity (EC50) values shown in Table 3.
The results showing high levels of anti-HSV-1 activity for C3- or CS-symmetrical compounds (4f and 7h) strongly suggest that at least the presence of two hydrogen bond donor benzylamine protons in the target TAZ derivatives is required as an important structural factor for the expression of a high level of anti-HSV-1 activity. A similar assumption is also substantiated by our previous observation of the disappearance of anti-HSV-1 activity by N-acetylated C3-symmetrical amine-type molecules.11)
In the comparison with the anti-HSV-1 activity of acyclovir, it was found that the mode of action of acyclovir is different from the modes of action of TAZ derivatives described in this paper; however, it is noteworthy that some new symmetrical TAZ derivatives such as 4f and 7h showed similar levels of anti-HSV-1 activity with a large safety index value.
The results of calorimetric experiments12) showed sugar recognition properties of highly active compounds with good SI values (CC50/EC50) such as compound 4f (SI=298). From the point of the view of substituents as hydrogen-bond donors or acceptors, ITC experiments suggested that the reaction of compound 4f with sugars showed a thermodynamic signature different from that of the entropically driven reaction of tri-isopropoxy-substituted TAZ derivative. Thus, we consider that such an enthalpic C3-type TAZ derivative having benzylamine functionalities may act as a hydrogen-bond donor to proposed receptor binding sites. On the basis of the obtained structural information on biological activities in this series, we are further investigating chemical modifications to new TAZ framework molecules in order to find new promising leads.
Melting points were determined using a micro melting point apparatus (Yanaco MP-S3) without correction. IR spectra were measured by a JASCO FTIR-4100 IR spectrophotometer. MicromATR Vision [an apparatus of attenuated total reflectance (ATR)] was used for a neat sample operation. Low- and high-resolution mass spectra (LR-MS and HR-MS) were obtained by a JEOL JMS HX-110 double-focusing model equipped with an FAB ion source interfaced with a JEOL JMA-DA 7000 data system. lH- and 13C-NMR spectra were obtained by ECG600R. Chemical shifts were expressed in δ ppm downfield from an internal tetramethylsilane (TMS) signal for 1H-NMR and the carbon signal of the corresponding solvent [CDCl3 (77.00 ppm), dimethyl sulfoxide (DMSO)-d6 (39.50 ppm), THF-d8 (67.20 ppm)] for 13C-NMR. The signal assignments were confirmed by two-dimensional (2D)-NMR analyses: 1H–1H 2D correlation spectroscopy (COSY), 1H–13C heteronuclear multiple-quantum coherence (HMQC), and 1H–13C heteronuclear multiple-bond connectivity (HMBC). Microanalyses were performed with a Yanaco MT-6 CHN corder. Routine monitoring of reactions was carried out using precoated Kieselgel 60F254 plates (E. Merck). Microwave irradiation experiments were carried out in a CEM Discover Focused Microwave System. For the separation of crude products in small experimental scales (below 2 mmol), we applied Centrifugal chromatography separation of the reaction products on silica gel (Kanto 60N or Able-Biott) with a UV detector. Commercially available starting materials were used without further purification, and dry solvents were used in all reactions.
General Procedure for the Preparation of C3-Symmetrical Benzylamine-Type TAZ Derivatives (4) (Table 1): Example: Synthesis of N2,N4,N6-Tribenzyl-1,3,5-triazine-2,4,6-triamine (4a) (Entry 1)To a solution of TCT AZ (1, 277 mg, 1.50 mmol) in dioxane (2.5 mL), a solution of benzylamine (2a, 1.61 g, 15.0 mmol) in dioxane (1.5 mL) was added dropwise at 0°C, and the mixture was continuously stirred for 30 min at room temperature and then for another 1 h at room temperature. Then the mixture was subjected to microwave irradiation (MW) at 100°C (100 W) for 60 min with stirring. After addition of water (30 mL), the mixture was extracted with EtOAc (2×25 mL). The combined organic layer was dried over MgSO4. After evaporation of the solvent, the residual solid was purified by centrifugal chromatography (n-hexane–EtOAc=50 : 50) to give an analytically pure 4a (507 mg, 1.28 mmol, 85%) as a pale yellow solid.
4a: mp 103.0–104.5°C (from 2-PrOH). IR cm−1: 3405, 3249 (NH), 1536, 1500 (C=N), 1163 (C–N). 1H-NMR (CDCl3) δ: 4.53 (6H, br s, CH2), 5.39 (3H, br s, NH), 7.18–7.32 (15H, m, H, C2′, 3′, 4′, 5′, 6′), 13C-NMR (CDCl3) δ: 44.56 (ArCH2), 127.02 (C4′), 127.51 (C2′, 6′), 128.44 (C3′, 5′), 139.45 (C1′), 166.27 (C2, 4, 6). Positive-ion FAB-MS m/z: 397 (M+H+). HR-FAB-MS m/z: 397.2147 (Calcd for C24H25N6: 397.2147). Anal. Calcd for C24H24N6: C, 72.70; H, 6.10; N, 21.20. Found: C, 72.70; H, 6.19; N, 21.05.
N2,N4,N6-Tris(4-methylbenzyl)-1,3,5-triazine-2,4,6-triamine (4b) (Entry 2)Compound 4b was prepared from the reaction of 1 with 4-methylbenzylamine (2b) under the conditions shown in Table 1. Purification by centrifugal chromatography (CH2Cl2–EtOH=97 : 3) afforded 4b (53%) as a white solid. An analytical sample of 4b was obtained as white crystals by concentration of the filtrate.
4b: mp 116–117°C (from 2-PrOH). IR cm−1: 3440 (NH), 1575, 1504 (C=N), 1346, 1245 (C–N), 808 (1,4-disubstituted benzene). 1H-NMR (CDCl3) δ: 1.83 (1.6H, br s, NH), 2.31 (9H, s, CH3), 4.50 (6H, br s, Hα), 5.17 (0.6H, br s, NH), 5.27 (0.8H, br s, NH), 7.08 (6H, d, J=7.3 Hz, H3′, 5′), 7.16 (6H, d, J=7.3 Hz, H2′, 6′). 13C-NMR (CDCl3) δ: 21.06 (CH3), 44.40 (Cα), 127.54 (C2′, 6′), 129.13 (C3′, 5′), 136.40 (C1′), 136.62 (C4′), 166.28 (C2, 4, 6). Positive-ion FAB-MS m/z: 439 (M+H)+. HR-FAB-MS m/z: 439.2603 (Calcd for C27H31N6: 439.2610). Anal. Calcd for C27H30N6: C, 73.94; H, 6.89; N, 19.16. Found: C, 73.91; H, 6.98; N, 19.07.
6-Chloro-N2,N4-bis(4-methylbenzyl)-1,3,5-triazine-2,4-diamine (3b) (Entry 3)Compound 3b was obtained from the reaction of 1 and 2b in THF (ratio of 1 : 2b=1 : 6) under the conditions shown in Table 1 in 46% yield after recrystallization from 2-PrOH as a white solid together with 4b (5%) obtained by concentration of the filtrate.
3b: mp 235–237°C (from 2-PrOH). IR cm−1: 3248 (NH), 1541, 1511 (C=N), 1343, 1091 (C–N and/or C–O), 796, 582 (C–Cl). 1H-NMR (THF-d8) δ: 2.27, 2.28* (6H, s, CH3), 4.44–4.49 (4H, m, CH2), 4.71 (0.4H, br s, NH), 7.01–7.09 (4H, m, H3′, 5′), 7.11–7.19 (4H, m, H2′, 6′), 7.29 (0.8H, br s, NH), 7.40 (0.8H, br s, NH). 13C-NMR (THF-d8, 67.20) δ: 20.93 (CH3), 44.58, 44.75, 44.80* (CH2), 127.85, 128.14*, 128.27 (C2′, C6′) 129.47 (C3′, 5′), 136.84*, 136.90, 136.98, 137.02 (C4′), 137.13, 137.16* (C1′), 166.93, 167.08*, 167.24 (C2, 4), 169.58*, 170.10* (C6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 354 (M+H)+. HR-FAB-MS m/z: 354.1482 (Calcd for C19H21ClN5: 354.1485). Anal. Calcd for C19H20ClN5: C, 64.49; H, 5.70; N, 19.79. Found: C, 64.47; H, 5.76; N, 19.72.
N2,N4,N6-Tris(4-chlorobenzyl)-1,3,5-triazine-2,4,6-triamine (4c) [112484-31-8] (Entry 4)Compound 4c was prepared from the reaction of 1 with 4-chlorobenzylamine (2c) under the conditions shown in Table 1. Separation of the products by centrifugal chromatography (CH2Cl2–EtOH=97 : 3) gave 4c (75%) as a white solid. An analytical sample of 4c was obtained as colorless crystals by recrystallization from propionitrile (EtCN).
4c: mp 156–158°C (from EtCN). IR cm−1: 3396, 3246 (NH), 1526 (C=N), 1330, 1161 (C–N). 1H-NMR (DMSO-d6) δ: 4.31 (3.3H, br s, Hα of tautomer B), 4.40 (2.7H, d, J=5.5 Hz, Hα of tautomer A), 7.05 (0.6H, br s, NH of A), 7.15 (3.3H, br s, H2′, 6′ of B), 7.19 (1.2H, br s, NH of A and B), 7.23 (3.3H, br s, H3′, 5′ of B), 7.28 (2.7H, d, J=7.6 Hz, H2′, 6′ of A), ca. 7.32 (1.2H, br s, NH of B), 7.34 (2.7H, d, J=7.6 Hz, H3′, 5′ of A). (The integral values of the peaks at δH 7.15–7.34 were calculated from the spectral data after addition of D2O.) 13C-NMR (DMSO-d6) δ: 42.37 (Cα of A), 42.66 (Cα of B), 127.88 (C3′, C5′ of B), 127.98 (C3′, C5′ of A), 128.80 (C2′, 6′ of A), 128.96 (C2′, 6′ of B), 130.82 (C4′ of A and B), 139.86 (C1′ of A and B), 165.72 (C2, 4, 6 of A), 165.89 (C2, 4, 6 of B). Positive-ion FAB-MS m/z: 499 (M+H+). HR-FAB-MS m/z: 499.0975 (Calcd for C24H22Cl3N6: 499.0972). Anal. Calcd for C24H21Cl3N6: C, 57.67; H, 4.23; N, 16.81. Found: C, 57.63; H, 3.97; N, 16.81.
N2,N4,N6-Tris[4-(trifluoromethyl)benzyl]-1,3,5-triazine-2,4,6-triamine (4d) (Entry 5)Compound 4d was prepared from the reaction of 1 with 4-(trifluoromethyl)benzylamine (2d) under the conditions shown in Table 1. Separation of the products by centrifugal chromatography (n-hexane–EtOAc=30 : 70) gave 4d (42%) as a white solid. An analytical sample of 4d was obtained as a white solid by recrystallization from 2-PrOH.
4d: mp 128–130°C (from 2-PrOH). IR cm−1: 3462, (NH), 1530, 1502 (C=N), 1323, 1105, 1066 (C–N and/ or C–O). 1H-NMR (DMSO-d6) δ: 4.39, 4.42*, 4.51, 4.52 (6H, br s, Cα), 7.19*, 7.42 (3H, br s, NH), 7.31, 7.48* (6H, br s, C2′, 6′), 7.49, 7.66* (6H, d, J=8.2 Hz, C3′, 5′). 13C-NMR (DMSO-d6) δ: 42.74, 42.97* (Cα), 124.27* (q, J=273.1 Hz, CF3) 124.39 (q, J=271.7 Hz, CF3), 124.74*, 124.96 (C3′, 5′), 127.02*, 127.11 (q, J=31.8 Hz, C4′), 127.55, 127.67* (C2′, C6′), 145.66, 145.77* (C1′), 165.80, 166.00* (C2, 4, 6). (The observed signals of the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 601 (M+H)+. HR-FAB-MS m/z: 601.1763 (Calcd for C27H22F9N6: 601.1762). Anal. Calcd for C27H21F9N6·0.7H2O: C, 52.89; H, 3.68; N, 13.71. Found: C, 53.06; H, 3.38; N, 13.69.
N2,N4,N6-Tris(4-methoxybenzyl)-1,3,5-triazine-2,4,6-triamine (4e) (Entry 6)Compound 4e was prepared from the reaction of 1 with 4-methoxybenzylamine (2e) under the conditions shown in Table 1. Separation of the products by centrifugal chromatography (CH2Cl2–EtOH=97 : 3) gave 4e (66%) as a white solid. An analytical sample of 4e was obtained as a white solid by recrystallization from EtOH.
4e: mp 135–136°C (from EtOH). IR cm−1: 3439, 3367 (NH), 1556, 1499 (C=N), 1345, 1246, 1171, 1030 (C–N or C–O), 810 (1,4-disubstituted benzene). 1H-NMR (DMSO-d6) δ: 3.70 (9H, s, OCH3), 4.33 (6H, br s, Hα), 6.77 (3H, br s, H3′, 5′ of B), 6.81–6.87† (1H, NH), 6.83 (3H, d, J=6.9 Hz, H3′, 5′ of A), 7.01 (1.6H, br s, NH), 7.13 (3H, br s, H2′, 6′ of B), 7.18 (3H, d, J=6.9 Hz, H2′, 6′ of A), 7.16–7.23† (0.4H, NH). (The peaks with the mark† attached are assumed to be overlapped with the peaks of NH.) 13C-NMR (DMSO-d6) δ: 42.40, 42.67* (Cα) 54.93 (OCH3), 113.37 (C3′, 5′), 128.21 (C2′, 6′ of A), 128.51* (C2′, 6′ of B), 132.80*, 132.90 (C1′), 157.86 (C4′), 165.74*, 165.86 (C2, 4, 6). (Signals for only the predominant tautomer B are asterisked.) Positive-ion FAB-MS m/z: 487 (M+H)+. HR-FAB-MS m/z: 487.2460 (Calcd for C27H31N6O3: 487.2458). Anal. Calcd for C27H30N6O3: C, 66.65; H, 6.21; N, 17.27. Found: C, 66.47; H, 6.15; N, 17.19.
N2,N4,N6-Tris(3,4-dimethoxybenzyl)-1,3,5-triazine-2,4,6-triamine (4f) (Entry 7)Compound 4f was prepared from the reaction of 1 with 3,4-dimethoxybenzylamine (2f) under the conditions shown in Table 1. Separation of the products by centrifugal chromatography (CH2Cl2–EtOH=95 : 5) gave 4f (80%) as a white solid. An analytical sample of 4f was obtained as a colorless solid by recrystallization from 2-PrOH. Compound 4f was converted into hydrochloride (4f·HCl) as colorless crystals by a routine procedure.
4f: mp 47°C (from 2-PrOH). IR cm−1: 3378 (NH), 1501 (C=N), 1257, 1230, 1134, 1023 (C–N and/or C–O). 1H-NMR (CDCl3) δ: 1.80 (1.8H, br s, NH), 3.85 (9H, s, OCH3 on C3′), 3.87 (9H, s, OCH3 on C4′), 4.53 (6H, br s, ArCH2), 5.19 (0.5H, br s, NH), 5.33 (0.7H, br s, NH), 6.80 (3H, d, J=7.6 Hz, H5′), 6.85 (3H, d, J=7.6 Hz, H6′), 6.86 (3H, s, H2′). 13C-NMR (CDCl3) δ: 44.54 (ArCH2), 55.82, 55.90 (OCH3), 110.90 (C2′), 111.09 (C5′), 119.77 (C6′), 131.86 (C1′), 148.20 (C4′), 149.01 (C3′), 166.17 (C2, 4, 6). 1H-NMR (DMSO-d6) δ: 3.65, 3.70* (18H, br s, OCH3 on C3′, 4′), ca. 3.7 (1H, br s, NH), 4.34, 4.35* (6H, br s, ArCH2), 6.72–6.97 (9H, m, ArH), [7.02 (1.6H), 7.17 (0.4H)] (br s, NH). 13C-NMR (DMSO-d6) δ: 42.87, 43.13* (ArCH2), 55.35, 55.49 (OCH3 on C3′, 4′), 111.50 (C2′, 5′), 119.11, 119.49* (C6′), 133.29 (C1′), 147.48, 148.48 (C3′, 4′), 165.81 (C2, 4, 6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 577 (M+H)+. HR-FAB-MS m/z: 577.2784 (Calcd for C30H37N6O6: 577.2775). Anal. Calcd for C30H36N6O6·i-PrOH: C, 62.25; H, 6.97; N, 13.26. Found: C, 62.35; H, 6.89; N, 13.22.
4f·HCl: mp 128–131°C (from 2-PrOH–H2O). IR cm−1: 3254 (br, NH+), 1625 (NH2+), 1567, 1515 (C=N), 1263, 1234, 1137, 1023 (C–N and/or C–O). 1H-NMR (DMSO-d6) δ: 3.69*, 3.73*, 3.75 (18H, br s, OCH3 on C3′, 4′), ca. 3.7 (1H, br s, NH), 4.46*, 4.48 (6H, br s, ArCH2), 6.75–7.15 (9H, m, ArH), 8.82*, 8.88, 9.03 (3H, br s, NH). 13C-NMR (DMSO-d6) δ: 43.23*, 43.45, 43.76 (ArCH2), 55.39, 55.48*, 55.52*, 55.54 (OCH3), 111.60*, 111.71*, 111.83 (C2′, 5′), 119.81 (C6′), 130.10*, 130.48, 131.03 (C1′), 147.96, 148.03, 148.13*, 148.64* (C3′, 4′), 154.54*, 162.64 (C2, 4, 6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 577 (M+H)+. HR-FAB-MS m/z: 577.2766 (Calcd for C30H37N6O6: 577.2775). Anal. Calcd for C30H36N6O6·HCl·1.6H2O : C, 56.13; H, 6.31; N, 13.09. Found: C, 56.13; H, 6.26; N, 13.05.
N2,N4,N6-Tris(2,4-dimethoxybenzyl)-1,3,5-triazine-2,4,6-triamine (4g) (Entry 8)Compound 4g was prepared from the reaction of 1 with 2,4-dimethoxybenzylamine (2g) under the conditions shown in Table 1. Separation of the products by centrifugal chromatography (CH2Cl2–EtOH=96 : 4) gave 4g (57%) as a white solid. An analytical sample of 4g was obtained as colorless crystals by recrystallization from EtOH.
4g: mp 137–138°C (from EtOH). IR cm−1: 3444, 3421 (NH), 1556, 1499 (C=N), 1206, 1157, 1115, 1031 (C–N or C–O), 924, 810 (1,2,4-trisubstituted benzene). 1H-NMR (CDCl3) δ: 3.78 (9H, s, OCH3 on C4′ or C2′), 3.80 (9H, s, OCH3 on C2′ or C4′) 4.42, 4.52* (6H, br s, Hα), 5.05 (1H, br s, NH), 5.30 (2H, br s, NH), 6.36 (3H, br s, H5′), 6.43 (3H, br s, H3′), 7.16 (3H, d, J=8.3 Hz, H6′). 13C-NMR (CDCl3) δ: 39.72 (Cα), 55.23 (OCH3 on C2′ or C4′), 55.31 (OCH3 on C4′or C2′), 98.32 (C3′), 103.65 (C5′), 120.16 (C1′), 130.08 (C6′), 158.45 (C4′ or C2′), 160.07 (C2′or C4′), 166.15 (C2, 4, 6). Positive-ion FAB-MS m/z: 577 (M+H)+. HR-FAB-MS m/z: 577.2789 (Calcd for C30H37N6O6: 577.2775). Anal. Calcd for C30H36N6O6: C, 62.49; H, 6.29; N, 14.57. Found: C, 62.57; H, 6.29; N, 14.58.
N2,N4,N6-Tris(benzo[d][1,3]dioxol-5-ylmethyl)-1,3,5-triazine-2,4,6-triamine (4h)5)Compound 4h was prepared from the reaction of 1 with piperonylamine (2h) (see ref. 5).
N2,N4,N6-Tris(3,4,5-trimethoxybenzyl)-1,3,5-triazine-2,4,6-triamine (4i) (Entry 10)Compound 4i was prepared from the reaction of 1 with 3,4,5-trimethoxybenzylamine (2i) under the conditions shown in Table 1. Separation of the products by centrifugal chromatography (CH2Cl2–EtOH=96 : 4) gave 4i (63%) as a white solid. An analytical sample of 4i was obtained as colorless crystals by recrystallization from 2-PrOH.
4i: mp 64–65°C (from 2-PrOH). IR cm−1: 3363 (NH), 1590, 1560, 1498 (C=N), 1328, 1230, 1119 (C–N or C–O), 1003 (C–O), 811 (1,2,3,5-tetrasubstituted benzene). 1H-NMR (CDCl3) δ: 3.79 (18H, s, OCH3 on C3′, 5′), 3.82 (9H, s, OCH3 on C4′), 4.50 (6H, br s, Hα), 5.44, 5.50 (3H, br s, NH), 6.52 (6H, s, H2′, 6′). 13C-NMR (CDCl3) δ: 44.93 (Cα), 55.99 (OCH3 on C3′, 5′), 60.72 (OCH3 on C4′), 104.44 (C2′, 6′), 134.88 (C1′), 137.03 (C4′), 153.25 (C3′, 5′), 166.13 (C2, 4, 6). Positive-ion FAB-MS m/z: 667 (M+H)+. HR-FAB-MS m/z: 667.3086 (Calcd for C33H43N6O9: 667.3092). Anal. Calcd for C33H42N6O9·0.5 2-PrOH·0.5H2O: C, 58.71; H, 6.71; N, 11.91. Found: C, 58.57; H, 6.74; N, 11.78.
Preparation of Intermediate Di-benzylamino-Substituted TAZ Derivatives (3)6-Chloro-N2,N4-bis(3,4-dimethoxylbenzyl)-1,3,5-triazine-2,4-diamine (3f)This compound was prepared in a manner similar to that for the preparation of 3h described by Vidal-Mosquera et al.13) To a solution of 1 (922 mg, 5.0 mmol) in THF (100 mL), 2f (3.34 g, 20.0 mmol) was added dropwise at room temperature, and then the mixture was refluxed for 1.2 h. Then the white solids were filtered, and water (200 mL) was added. After the mixture had been stirred at 60°C for 10 min, the solid was filtered and washed with EtOH (50 mL) to give analytically pure compound 3f (1.51 g, 4.29 mmol, 86%) as a white solid.
3f: mp 193–194. IR cm−1: 3250 (NH), 1555, 1514 (C=N), 1240, 1137, 1021 (C–N and/or C–O), 799, 736 (C–Cl). 1H-NMR (DMSO-d6) δ: 3.67* (3.3H, s, OCH3 on C3′), 3.71* (3.3H, s, OCH3 on C4′), 3.72 (3.6H, br s, OCH3), 3.73 (1.8H, s, OCH3), 4.33–4.40 [4H, m, Cα: including 4.38* (d, J=6.2 Hz)], 6.72–6.96 [6H, m, ArH: including 6.73* (1.1H, dd, J=8.3, 1.4 Hz, H6′), 6.79* (ca. 1.1H, d, J=8.3 Hz, H5′), 6.89* (ca. 1.1H, d, J=1.4 Hz, H2′)], [8.05 (0.3H), 8.19 (0.3H), 8.23 (0.3H), 8.31* (1.1H)] (t, J=6.2 Hz, NH). 13C-NMR (DMSO-d6) δ: 43.19, 43.28, 43.41* (Cα), [55.29* (on C3′), 55.35, 55.42* (on 5′), 55.51] (OCH3), 111.38*, 111.51 (C2′), 111.67* (C5′), 119.22, 119.29, 119.36* (C6′), 131.43, 131.61* (C1′), 147.73*, 147.78 (C4′), 148.55,*, 148.59 (C3′), 165.23, 165.36*, 165.61 (C2, 4), 167.77*, 168.30 (C6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 446 (M+H)+. HR-FAB-MS m/z: 446.1586 (Calcd for C21H25ClN5O4: 446.1595). Anal. Calcd for C21H24ClN5O4: C, 56.57; H, 5.43; N, 15.71. Found: C, 56.35; H, 5.42; N, 15.68.
6-Chloro-N2,N4-bis(benzo[d][1,3]dioxol-5-ylmethyl)-1,3,5-triazine-2,4-diamine (3h)13)In a manner similar to that for the preparation of 3f, the reaction of 1 and 2f only by stirring for 30 min at room temperature, product 3h was obtained in 96% yield as a white solid.
3h: mp 225–226. IR cm−1: 3244 (NH), 1558 (C=N), 1280, 1038 (C–N and/or C–O), 796 (C–Cl). 1H-NMR (DMSO-d6) δ: 4.31–4.35 [4H, m, Cα: including 4.33* (d, J=6.2 Hz)], 5.96*, 5.97, 5.98 (4H, s, H2′), 6.68-6.70 [1.1H, m, ArH: including 6.69* (br d, J=8.3 Hz, H6′)], 6.72–6.78 [3H, m, ArH: including 6.77* (d, J=8.3 Hz, H7′)], 6.83–6.88 [1.9H, m, ArH: including 6.85* (br s, H4′)], [8.08 (0.5H), 8.21 (1.1H), 8.24 (1.1H), 8.33 (3.3H)] (t, J=6.2 Hz, NH). 13C-NMR (DMSO-d6) δ: 43.14, 43.23, 43.36* (Cα), 100.76 (C2′), 107.82, 107.86* (C7′), 107.90, 108.00* (C4′), 120.26, 120.37, 120.46* (C6′), 132.92, 133.07, 133.13* (C5′), 146.03 (C3a′), 146.15 (C7a′), 165.20, 165.30*, 165.56 (C2, 4), 167.81*, 168.33 (C6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 414 (M+H)+. HR-FAB-MS m/z: 414.0974 (Calcd for C19H17ClN5O4: 414.0969). Anal. Calcd for C19H16ClN5O4·0.2H2O: C, 54.67 H, 3.96; N, 16.78. Found: C, 54.63; H, 3.90; N, 16.84.
Preparation of N-Benzyl-4-chloro-6-isopropoxy-1,3,5-triazin-2-amine (6a) (Table 2, Entry 1)Compound 6a was obtained from the reaction of 5b with 2a under the conditions shown in Table 2. Thus, to a solution of intermediate 5b (2.08 g, 10.0 mmol) in THF (150 mL), benzylamine 2a (1.07 g, 8.00 mmol) and diisopropylethylamine (DIPEA, 1.29 g, 10.0 mmol) were added and the reaction mixture was stirred for 2 h at 0°C. After removal of the precipitated solids (DIPEA·HCl salt), evaporation of the solvent gave crude compound 6a (2.71 g, 9.74 mmol, 97%) as a white powder. An analytically pure sample of 6a was obtained as a white powder by recrystallization from acetonitrile (MeCN).
6a: mp 127–128°C (from MeCN). IR cm−1: 3262, 3110 (NH), 1638, 1527 (C=N), 1294, 1231, 1105 (C–N and/or C–O), 803 (C–Cl). 1H-NMR (CDCl3) δ: [1.31 (2H), 1.34* (4H)] (d, J=6.2 Hz, H2″, 3″), [4.65 (0.7H), 4.67* (1.3H)] (d, J=6.2 Hz, Hα), 5.20–5.30 (1H, m, H1″), [6.33 (0.3H), 6.78* (0.7H)] (br s, NH), 7.26–7.37 (5H, m, H2′–6′). 13C-NMR (CDCl3) δ: 21.63*, 21.67 (C2″, 3″), 44.99*, 45.07 (Cα), 71.66, 72.02* (C1″), 127.33, 127.62 (C2′, 6′) 127.66*, 127.72 (C4′), 128.70*, 128.74 (C3′, C5′), 137.45 (C1′), 167.07 (C2), 169.81, 170.33, 170.40, 171.47 (C4, 6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 279 (M+H)+. HR-FAB-MS m/z: 279.0981 (Calcd for C13H16ClN4O: 279.1013). Anal. Calcd for C13H15ClN4O: C, 56.02; H, 5.42; N, 20.10. Found: C, 55.96; H, 5.43; N, 19.93.
General Procedure for the Preparation of CS-Symmetrical Benzylamine-Type TAZ Derivatives (7) (Table 2): Example 1 (No Base by MW): Synthesis of 6-Isopropoxy-N2,N4-bis(4-methylbenzyl)-1,3,5-triazine-2,4-diamine (7b) (Entry 3)To a solution of intermediate 5b14) (416 mg, 2.0 mmol) in dioxane (4 mL) was added 2b (1.21 g, 10.0 mmol) at room temperature. Then the mixture was subjected to MW at 100°C (100 W) for 45 min with stirring. After addition of water (50 mL), the mixture was extracted with EtOAc (2×50 mL). The combined organic layer was washed with saturated aqueous NH4Cl solution and dried over MgSO4. After evaporation of the solvent, the residue was recrystallized from 2-PrOH to obtain analytically pure compound 7b as a colorless solid.
7b: mp 142–143°C (from 2-PrOH). IR cm−1: 3347, 3239 (NH), 1609, 1579 (C=N), 1158, 1104 (C–N and/or C–O). 1H-NMR (CDCl3) δ: [1.27 (br s), 1.31* (d, J=6.2 Hz)] (6H, H2″, 3″), 2.32 (6H, br s, CH3), 4.51, 4.55* (4H, br s, Hα), [5.13 (0.3H), 5.23 (0.7H)] (br s, H1″), [5.38, 5.45 (0.6H), 5.61 (1.4H)] (br s, NH), 7.11 (4H, d, J=6.9 Hz) (H3′, 5′), 7.17 (4H, d, J=6.9 Hz) (H2′, 6′). 13C-NMR (CDCl3) δ: 21.06 (CH3), 21.94 (C2″, 3″), 44.40, 44.50* (Cα), 69.02*, 69.43 (C1″), 127.38, 127.53* (C2′, 6′), 129.18 (C3′, 5′), 135.83*, 136.10 (C1′), 136.82 (C4′), 167.07, 167.35* (C2, 4), 169.93*, 170.30 (C6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 378 (M+H)+. HR-FAB-MS m/z: 378.2284 (Calcd for C22H28N5O4: 378.2294). Anal. Calcd for C22H28N5O4: C, 70.00; H, 7.21; N, 18.55. Found: C, 70.04; H, 7.22; N, 18.56.
N2,N4-Dibenzyl-6-isopropoxy-1,3,5-triazine-2,4-diamine (7a) (Entry 2)Compound 7a was prepared from the reaction of 5b with 2a under the conditions shown in Table 2 using the above method. After the workup, separation of the products by centrifugal chromatography (CH2Cl2–EtOH=98 : 2) gave 7a (79%) as a white solid. An analytically pure sample of 7a was obtained as colorless crystals by recrystallization from diisopropylether (2-Pr2O).
7a: mp 117–119°C (from 2-Pr2O). IR cm−1: 3336, 3246 (NH), 1615, 1508 (C=N), 1160, 1104 (C–N and/or C–O). 1H-NMR (CDCl3) δ: [1.24 (2H, br s), 1.28* (4H, d, J=5.5 Hz)] (H2″, 3″), 4.55, 4.59* (4H, br s, Hα), [5.12 (0.3H), 5.21* (0.7H)] (br s, H1″), [5.53 (0.5H), 5.73 (0.2H), 5.84 (0.3H), 5.97* (1H)] (br s, NH), 7.20–7.35 (10H, m, H2′–6′). 13C-NMR (CDCl3) δ: 21.89 (C2″, 3″), 44.53, 44.67* (Cα), 69.01, 69.48* (C1″), 127.11, 127.31, 127.49 (C2′, 6′, 4′), 128.46 (C3′, C5′), 138.96*, 139.21 (C1′), 167.15, 167.36* (C2, 4), 169.87, 170.21* (C6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 350 (M+H)+. HR-FAB-MS m/z: 350.1989 (Calcd for C20H24N5O: 350.1981). Anal. Calcd for C20H23N5O: C, 68.74; H, 6.63; N, 20.04. Found: C, 68.73; H, 6.74; N, 20.00.
6-Isopropoxy-N2,N4-bis(4-methoxybenzyl)-1,3,5-triazine-2,4-diamine (7e) (Entry 4)Colorless needles. mp 132–134°C (from EtOH). IR cm−1: 3392, 3261, 3101 (NH), 1583, 1530 (C=N), 1238, 1170, 1108, 1030 (C–N and/or C–O). 1H-NMR (CDCl3) δ: [1.26 (2H, br s), 1.32* (4H, d, J=5.5 Hz)] (H2″, 3″), 3.78 (6H, br s, OCH3 on C4′), 4.48, 4.53* (4H, br s, Hα), [5.13 (0.3H), 5.24* (0.7H)] (br s, H1″), [5.42 (0.3H), 5.55* (1.0H), 5.73 (0.7H)] (br s, NH), 6.83 (4H, d, J=7.6 Hz, H3′, 5′), 7.20 (4H, d, J=7.6 Hz, H2′, 6′). 13C-NMR (CDCl3) δ: 21.93 (C2″, 3″), 44.07, 44.15* (Cα), 55.22 (OCH3), 68.97*, 69.38 (C1″), 113.87 (C3′, 5′), 128.96, 128.79* (C2′, 6′), 130.93*, 131.27 (C1′), 158.79 (C4′), 166.97, 167.26* (C2, 4), 169.89*, 170.30 (C6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 410 (M+H)+. HR-FAB-MS m/z: 410.2180 (Calcd for C22H28N5O3: 410.2192). Anal. Calcd for C22H27N5O3: C, 64.53; H, 6.65; N, 17.10. Found: C, 64.51; H, 6.67; N, 17.04.
N2,N4-Bis(3,4-dimethoxybenzyl)-6-isopropoxy-1,3,5-triazine-2,4-diamine (7f) (Entry 5)Colorless crystals. mp 146–147°C (from MeCN). IR cm−1: 3397, 3248 (NH), 1553, 1516 (C=N), 1262, 1162, 1136, 1026 (C–N and/or C–O). 1H-NMR (CDCl3) δ: [1.28 (2H, br s), 1.33 (4H, d, J=4.8 Hz)] (H2″, 3″), 3.80–3.90 (12H, m, OCH3 on C3′,4′), 4.56 (4H, br s, Hα), [5.15 (0.3H), 5.26 (0.7H)] (br s, H1″), [5.42 (1.4H), 5.59 (0.6H)] (br s, NH), 6.76–6.90 (6H, m, H2′, 5′, 6′). 13C-NMR (CDCl3) δ: 21.94 (C2″, 3″), 44.62 (Cα), 55.82, 55.90* (OCH3), 69.11*, 69.59 (C1″), 110.78 (C2′), 111.12 (C5′), 119.64, 119.74* (C6′), 131.34*, 131.57 (C1′), 148.28 (C4′), 149.04 (C3′), 167.02, 167.33* (C2, 4), 170.28*, 170.31 (C6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 470 (M+H)+. HR-FAB-MS m/z: 470.2397 (Calcd for C24H32N5O5: 470.2403). Anal. Calcd for C24H31N5O5: C, 61.39; H, 6.65; N, 14.92. Found: C, 61.25; H, 6.79; N, 14.91.
N2,N4-Bis(2,4-dimethoxybenzyl)-6-isopropoxy-1,3,5-triazine-2,4-diamine (7g) (Entry 6)Colorless crystals. mp 134–136°C (from 2-PrOH). IR cm−1: 3417, 3248 (NH), 1612, 1592, 1526 (C=N), 1228, 1206, (C–N and/or C–O). 1H-NMR (CDCl3) δ: [1.22–1.29 (2H), 1.29–1.40 (4H)] (br s, H2″, 3″), [3.79 (8H), 3.82 (4H)] (s, OCH3), [4.55, 4.51 (br s), 4.56* (d, J=5.5 Hz)] (4H, Hα), 5.11*, 5.26, 5.35 (1H, br s, H1″), 5.34, 5.39, 5.50* (2H, br s, NH), 6.34–6.47 (4H, m, H3′, 5′), 7.13–7.22 (2H, m, H6′). 13C-NMR (CDCl3) δ: 21.99 (C2″, 3″), 40.03 (Cα), 55.25, 55.33 (OCH3), 68.69*, 69.06 (C1″), 98.43 (C3′), 103.67 (C5′), 119.49*, 119.70 (C1′), 129.81, 130.10*, 130.25 (C6′), 158.50 (C2′), 160.29 (C4′), 166.95, 167.20* (C2, 4), 169.88*, 170.13 (C6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 470 (M+H)+. HR-FAB-MS m/z: 470.2402 (Calcd for C24H32N5O5: 470.2403). Anal. Calcd for C24H31N5O5: C, 61.39; H, 6.65; N, 14.92. Found: C, 61.21; H, 6.72; N, 14.68.
N2,N4-Bis(benzo[d][1,3]dioxol-5-yllmethyl)-6-isopropoxy-1,3,5-triazin-2,4-diamine (7h) (Entry 7)White solid. mp 143–145°C (from EtOAc). IR cm−1: 3338, 3239, 3097 (NH), 1612, 1521 (C=N), 1246, 1232, 1102, 1038 (C–N and/or C–O). 1H-NMR (CDCl3) δ: 1.28, 1.32* (6H, d, J=6.2 Hz, H2″, 3″), 4.46, 4.50*, 4.51 (4H, br s, Hα), 5.14, 5.24* (1H, br s, H1″), 5.36, 5.42*, 5.58 (2H, br s, NH), 5.93 (4H, s, H2′), 6.70–6.85 (6H, m, H4′, 6′, 7′). 13C-NMR (CDCl3) δ: 21.96 (C2″, 3″), 44.46, 44.58* (Cα), 69.14, 69.54* (C1″), 100.98 (C2′), 108.19 (C4′, 7′), 120.64 (C6′), 132.79*, 133.10 (C5′), 146.76 (C3a′), 147.79 (C7a′), 166.99*, 167.05 (C2, 4 or C6), 167.30 (C6 or C2, 4). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 438 (M+H)+. HR-FAB-MS m/z: 438.1779 (Calcd for C22H24N5O5: 438.1777). Anal. Calcd for C22H23N5O5: C, 60.40; H, 5.30; N, 16.01. Found: C, 60.42; H, 5.27; N, 16.00.
6-Isopropoxy-N2,N4-bis(3,4,5-trimethoxybenzyl)-1,3,5-triazine-2,4-diamine (7i) (Entry 8)Colorless crystals. mp 148–150°C (from 2-PrOH). IR cm−1: 3315, 3246 (NH), 1586, 1542 (C=N), 1225, 1159, 1334, 1099 (C–N and/or C–O). 1H-NMR (CDCl3) δ: [1.29 (br s), 1.33 (d, J=4.8 Hz)] (6H, H2″, 3″), 3.82 (18H, br s, OCH3), 4.53*, 4.56 (4H, m, Hα), [5.16 (0.25H, br s), 5.26* (0.75H, br s)] (H1″), [5.45*, 5.52 (1.5H), 5.67 (0.5H)] (br s, NH), 6.54 (4H, br s, H2′, 6′). 13C-NMR (CDCl3) δ: 21.91 (C2″, 3″), 40.91, 44.93, 44.98, 45.09* (Cα), 56.07 (OCH3 on C3′, 5′), 60.79 (OCH3 on C4′), 69.24*, 69.60 (C1″), 104.39*, 104.70 (C2′, 6′), 134.48*, 134.66 (C1′), 137.16 (C3′, 5′), 153.34 (C4′), 167.11, 167.37* (C2, 4), 169.98*, 170.27 (C6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 530 (M+H)+. HR-FAB-MS m/z: 530.2621 (Calcd for C26H36N5O7: 530.2615). Anal. Calcd for C26H35N5O7: C, 58.97; H, 6.66; N, 13.22. Found: C, 58.94; H, 6.74; N, 13.20.
N2,N4-Bis(3,4-dimethylbenzyl)-6-isopropoxy-1,3,5-triazine-2,4-diamine (7j) (Entry 9)Colorless solid. mp 119–123°C (from EtOH). IR cm−1: 3397, 3247, 3105 (NH), 1614, 1577, 1523 (C=N), 1166, 1146, 1104 (C–N and/or C–O). 1H-NMR (CDCl3) δ: [1.28 (2H, br s), 1.32* (4H, d, J=5.5 Hz)] (H2″, 3″), 2.23 (12H, br s, CH3), 4.49, 4.54* (4H, br s, Hα), [5.14 (0.3H), 5.25* (0.7H)] (br s, H1″), [5.38* (1.4H), 5.52 (0.6H)] (br s, NH), 7.02–7.12 (6H, m, H2′, 5′, 6′). 13C-NMR (CDCl3) δ: 19.36 (CH3 on C4′), 19.69 (CH3 on C3′), 21.95 (C2″, 3″), 44.41, 44.52* (Cα), 68.99, 69.42* (C1″), 124.86, 125.04* (C6′), 128.74, 128.92* (C2′), 129.73 (C5′), 135.47 (C4′), 136.22*, 136.48 (C1′), 136.71 (C3′), 167.03, 167.33* (C2, 4), 169.93, 170.21* (C6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 406 (M+H)+. HR-FAB-MS m/z: 406.2594 (Calcd for C24H32N5O: 406.2607). Anal. Calcd for C24H31N5O: C, 71.08; H, 7.70; N, 17.27. Found: C, 71.03; H, 7.70; N, 17.27.
6-Isopropoxy-N2,N4-bis(2,4,6-trimethylbenzyl)-1,3,5-triazine-2,4-diamine (7k) (Entry 10)Colorless crystals. mp 146–147°C (from EtOH). IR cm−1: 3252, 3105, 2975 (NH), 1619, 1577, 1519 (C=N), 1172, 1017 (C–N and/or C–O). 1H-NMR (CDCl3) δ: [1.26 (d, J=5.5 Hz), 1.35*, 1.43 (br s)] (6H, H2″, 3″), 2.26, 2.27, 2.31* (6H, br s, CH3 on C4′), 2.36, 2.37 (12H, br s, CH3 on C2′, 6′), 4.42, 4.55, 4.58*, 4.66 (4H, br s, Hα), 4.68, 4.86* (2H, br s, NH), [5.09 (0.25H), 5.24* (0.6H), 5.43 (0.15H)] (br s, H1″), 6.86, 6.88 (4H, br s, H3′, 5′). 13C-NMR (CDCl3) δ: 19.56 (CH3 on C2′, 6′), 20.87 (CH3 on C4′), 21.96 (C2″, 3″), 39.31*, 39.43 (Cα), 68.95, 69.38*, 69.79 (C1″), 129.06 (C3′, 5′), 131.16*, 131.41 (C1′), 137.35 (C2′, 4′, 6′), 166.48, 166.76, 167.10, 167.32* (C2, 4), 169.79, 170.17*, 170.70 (C6). (The observed 1H- and 13C-signals assignable to the predominant tautomer are asterisked.) Positive-ion FAB-MS m/z: 434 (M+H)+. HR-FAB-MS m/z: 434.2905 (Calcd for C26H36N5O: 434.2920). Anal. Calcd for C26H35N5O: C, 72.02; H, 8.14; N, 16.15. Found: C, 71.81; H, 8.34; N, 16.00.
Antiviral Activity Assay and Cytotoxicity Assay of Synthesized TAZ DerivativesThe anti-HSV-1 activities (EC50) of the synthesized TAZ derivatives were measured by using a plaque reduction assay9) and their cytotoxicity against Vero cells (CC50) was also evaluated as our previous description.5) The results are summarized in Table 3 together with data for aciclovir.15) Calculated log P values10) for the compounds are also shown in Supplementary Materials (Fig. S1). There were few distinct correlations between log P values by using ChemBioDraw Ultra v.14.0 and anti-HSV-1 activity (EC50).
The authors would like to thank Ms. Kanae Yamada, Ms. Shoko Tomonaga and Ms. Junko Matsuyama for their valuable technical assistance.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.