2019 Volume 67 Issue 10 Pages 1104-1115
2019 Volume 67 Issue 10 Pages 1104-1115
Licorice is one of the ancient and most frequently applied herbs for its diverse phytochemicals. At present, wild resources of licorice have rapidly declined with increasing demand and the proportion of cultivated products in the market is quickly growing. However, the different level in chemical composition between the wild and cultivated licorice may result in the discrepancy in quality and pharmacological activity. Therefore, an ultra-fast liquid chromatography coupled with triple quadrupole-time of flight tandem mass spectrometry (UFLC-Triple TOF-MS/MS) method combined with multivariate statistical analysis technology was employed to explore chemical composition differences. The result showed that total 63 components were identified from licorice samples. The wild and the cultivated licorice are obviously classified into two groups according to principal component analysis (PCA). PCA and partial least squared discrimination analysis (PLS-DA) were also introduced to rapidly find 14 candidate compounds between two ecotypes of licorice. Apart from glycyrrhizin, licorice saponin J2/G2, glyasperin D and dehydroglyasperin D also could be selected as chemical markers based on t-test and variable importance in the projection (VIP) value. Our study successfully established an effective method for exploring metabolite profiling between two ecotypes of licorice and laying the foundation for distinguishing wild and cultivated licorice.
The application of licorice was originally recorded in Shennong Materia Medica, dating back to 4000 years ago in China.1) According to traditional Chinese medical theory, licorice could enhance “qi” (kind of energy), relieve pain, treat throat disorders and act as an effective antidote.2,3) More than 60% of prescriptions contain licorice4) in that it could cooperate with other herbs in a formula to enhance the effectiveness of other ingredients.5) Therefore, licorice is described as “guolao,” like a minister charged in mediation in a dynasty.6) In addition to the prescriptions of traditional Chinese medicine requiring large amounts of licorice,7,8) baked goods, frozen dairy products, beverages, and light industrial products make good use of licorice.9) Currently, it possesses a substantial share in the global market for its traditional application and diverse phytochemicals.10) There are hundreds of compounds isolated from the genus Glycyrrhiza, including triterpene saponins, flavonoids, coumarin, and alkaloids which are responsible for hepatoprotection, neuroprotection, anti-inflammatory, detoxification and other bioactivities.11,12) Although there are different ways of using licorice between the East and the West, it is directly or indirectly related to chemical components and their bioactivities of licorice.13)
At present, Glycyrrhiza uralensis Fisch holds a large proportion of more than 90% of licorice production.14) As a result of excessive and unsustainable harvesting, the wild Glycyrrhiza uralensis Fisch has been rapidly decreasing. The cultivated-type has been widely planted in the northwest of China.15) There is hardly differentiated treatment to the wild and cultivated licorice even though their metabolites have changed a lot. Under harsh living condition, wild licorice would primarily endure drought and salt stress for the whole lifespan. On the contrary, cultivated licorice may suffer less with relatively rich fertilizer which leads to remarkably different morphological properties and metabolites.16) The different level in chemical composition between the two ecotypes of licorice may result in the discrepancy in pharmacological activity and quality.17,18) Although there are a great number of studies have been published studying the different chemical composition of the Glycyrrhiza species,19) including those from different habitats, other species and different ecological types for the discovery of some biological markers. There are also some articles comparing the accumulation of active ingredients in wild and cultivated licorice. However, they generally focused only on a limited number of compounds.20,21) Metabolomics and comprehensive chemical composition studies on wild and cultivated licorice are still limited.
The metabolomic approach offers a global analysis of low-molecular-weight metabolites in biological samples, attempting to capture global changes22) and has been applied in natural product characterization, food quality evaluation and so forth.23,24) To investigate the differential chemical composition between wild and cultivated licorice, ultra-fast liquid chromatography coupled with triple quadrupole-time of flight tandem mass spectrometry (UFLC-Triple TOF-MS/MS) combined with multivariate statistical analysis technology was employed to explore chemical composition differences. The result showed that 63 components were identified based on the accurate mass-to-charge ratio and secondary mass spectrometry fragment information from primary mass spectrometry, combined with software database search, standard comparison and related literature. Besides, the wild and the cultivated licorice are obviously classified into two groups according to principal component analysis (PCA). In addition, 14 candidate compounds were observed between two ecotypes of licorice by partial least squared discrimination analysis (PLS-DA) analysis. Most compounds, especially the content of flavonoid aglycone, were found with high content in the cultivated licorice. However, the content of confirmed bioactive compound (glycyrrhizin) was observed higher in the wild products. Apart from glycyrrhizin, licorice saponin J2/G2, glyasperin D and dehydroglyasperin D are also possibly considered as chemical markers for licorice through t-test and variable importance in the projection (VIP) value. Our investigation not only established a new method for the comprehensive chemical composition between wild and cultivated licorice, it also provided a basis for distinguishing wild and cultivated licorice.
Methanol and acetonitrile of HPLC grade were purchased from Merck (Darmstadt, Germany). Milli-Q purifying system was used to obtain ultrapure water (Millipore, Bedford, MA, U.S.A.). All other chemicals used in the experiments were of analytical reagent grade (Shanghai Yuanye-Biotechnology Co., Ltd., P. R. China). The reference substances of glycyrrhizin, isoliquiritigenin, neoliquiritin, formononetin, ononin and were obtained from Chengdu Chroma-Biotechnology Co., Ltd. (Chengdu, P. R. China); isoliquiritin and neoisoliquiritin were purchased from Chengdu Pure Chem-Standard Co., Ltd. (Chengdu); liquiritin, liquiritigenin, and glycyrrhetinic acid were offered by Liangwei Chemical Reagent Co., Ltd. (Nanjing, P. R. China); liquiritin apioside and isoliquiritin apioside were brought from Nanjing Jingzhu Bio-Technology Co., Ltd. (Nanjing, P. R. China). The purities of all chemical standards were greater than 98% determined by HPLC.
Plant MaterialsBoth cultivated and wild Glycyrrhiza uralensis Fisch of more than four-year-old were collected in Yanchi County, Ningxia Province, China (E107.41°, N37.78°). Each of them was carefully washed and grounded into powder. The botanical origins of the materials were authenticated by Professor Xunhong Liu. (Department for Authentication of Chinese Medicines, School of Pharmacy, Nanjing University of Chinese Medicine, P. R. China), Voucher specimens were deposited at Herbarium in School of Pharmacy, Nanjing University of Chinese Medicine.
Crude Sample PreparationPowders of all samples were screened through the 60-mesh sieve. Each sample of 0.5 g was accurately weighed and extracted by ultra-sonication (500 W, 40 kHz) in 25 mL 70% methanol for 1 h. After cooling down at room temperature for 15 min, methanol was added for the weight loss during extraction. The mixture was then centrifuged at 12000 rpm for 10 min. The supernatant of mixture was sucked out, diluted tenfold by solvent and filtered (0.22 µm membrane filter) prior to UFLC-Triple TOF-MS/MS analysis. Four quality control samples (QC), which were made up with a pool of all kinds of analyzed samples were also prepared so as to check signal stability and reproducibility during the instrumental analysis.
UFLC-Triple TOF-MS/MS AnalysisAll samples were analyzed by applying an UFLC system (Shimadzu, Kyoto, Japan) interfaced with Triple TOF-MS equipped with ESI source. An Agilent ZORBAX Extend-C18 column (100 × 2.1 mm, 1.8 µm) was used for all the analyses. The flow rate of the mobile phase was 0.3 mL/min and the column temperature was maintained at 35°C. The mobile phase was composed of 0.1% formic acid aqueous solution (A) and acetonitrile (B). Gradient elution was optimized and set according to the following schedule: 10–20% 35–45%B at 23–26 min; 45–50% B at 26–29 min; 50–55% B at 29–32 min; 55–65% B at 32–36 min; 65–75%B at 36–40 min; 75–95% B at 40–45 min; 2% B at 45–48 min. The flow rate was set at 0.3 mL/min. The injection volume of reference compounds and samples was 2 µL.
Triple TOF™ 5600 System-MS/MS (AB Sciex, Framingham, MA, U.S.A.), which was equipped with an electrospray ionization (ESI) source, was operated to acquire MS data. The positive ion mode was adopted with a capillary voltage of 5.0 kV. The scanning range of each sample was from m/z 50 to 1500. The optimized MS parameters were set as follow: the flow rate of curtain gas was 40 L/min; the ion source temperature was set as 550°C; the flow rate of nebulization gas was 55 L/min; the flow rate of auxiliary gas was 55 L/min; the spray voltage was −4500 V and declustering potential voltage was 100 V.
Chemometric Data AnalysisA database of chemicals isolated and identified from licorice was established by searching literature from Chinese National Knowledge Infrastructure (CNKI), Pubmed and professional chemical database like SciFinder, METLIN. Our database including compound name, formula, CAS number, molecular weights, structure types, origins and chemical structure in mol format downloaded from SciFinder.
The LC-Triple TOF-MS/MS data were collected by the Analyst TF 1.6 software (AB Sciex) and analyzed by PeakView1.2 software (AB Sciex) to identify the potential chemicals in wild and cultivated licorice. Through the analysis of the multistage MS/MS, the characteristic peaks were extracted with MS data singular point excluding, noise filtering, peak matching, peak recognition, baseline correction, peak alignment and extraction of characteristic peak. After matching according to the information in the established database, the compounds could be identified. Ions with same m/z value in different samples were set as maximum tolerance of 10 ppm and the same retention time (tR) were set as tolerance of 0.2 min. Furthermore, PCA and PLS-DA was applied to classify and distinguish wild licorice from cultivated one. t-Test was performed to show the significant difference of selected compounds between wild and cultivated licorice according to their contents.
To achieve an optimal extraction of major constituents in samples, extraction solvent (50% (v/v) methanol, 70% (v/v) methanol, 95% (v/v) methanol, 100% (v/v) methanol) and material–solvent ratio (1 : 20, 1 : 50, 1 : 100) were evaluated according to the our previous study.16) The results showed that the extraction efficiency of samples in 70% methanol solution is optimal, and suitable material–solvent ratio and ultrasonic time were 1 : 50 and 60 min, respectively.
Optimization of UFLC-Triple TOF-MS/MS ConditionsIn order to obtain the optimum chromatographic condition, several UFLC parameters including three types of columns (ThermoAcclaimTM RSLC 120 C18, ZORBAX Extend-C18, X-C18) and different kinds of mobile phases were examined. The chemical ingredients showed satisfactory sensitivity in the positive ion mode and separation when equipped with ZORBAX Extend-C18 and eluted with acetonitrile and water containing 0.1% (v/v) formic acid at 0.3 mL min−1 under 35°C.
Identification of Chemical Constituents in LicoriceIn our study, we found that ESI positive ion mode surprisingly yielded better resolution and larger LC/MS peaks than under ESI negative mode. Therefore, positive ion mode was employed. Under the chromatographic and MS condition, more than 60 compounds were identified or temporarily speculated according to accurate mass data and MS/MS fragmentation information, along with standards, database search of the software and the literature reference.
The whole chromatogram was 48 min in total and generally divided into three parts (Fig. 1). The first part includes flavonoid glycosides and triterpenoid saponins, the middle of the chromatogram is taken by triterpenoids while the last 15 min is made up with flavonoid aglycones. In addition, there are a few other ingredients such as coumarin. All the details of identified compounds were summarized in Table 1.

A, wild group; B, cultivated group.
| No. | tR (min) | Putative compound (CAS No.) | Molecular formula | ppm | Measured mass (m/z) | MS2 fragments | Structure types |
|---|---|---|---|---|---|---|---|
| 1 | 5.61 | Nicotiflorin (17650-84-9) | C27H30O15 | 3.1 | 595.1669 (+) | 463.1047[M + H − (Rha-CH2)]+, 415.0799[M + H − (Rha-CH2)-CH2O-OH]+, 375.0852[M + H − (Rha-CH2)-C6H5O-OH]+ | Flavone glycoside |
| 2 | 7.35 | Glucoliguiritin apioside (157226-47-6) | C32H40O18 | 3.3 | 713.2313 (+) | 257.0808[M + H − 2Glc-Api]+ | Flavanone glycoside |
| 3 | 7.64 | Trifolirhizin (6807-83-6) | C22H22O10 | 2.4 | 447.1295 (+) | 285.0751[M + H − Glc]+, 269.0630[M + H − Glc-O]+, 137.0233[M + H − Glc-C9H7O]+ | Coumarin |
| 4 | 7.84 | Neoliquiritin* (5088-75-5) | C21H22O9 | 4.4 | 419.1344 (+) | 257.0804[M + H − Glc]+, 239.0701[M + H − Glc − H2O]+, 137.0229[M + H − Glc-H2O-C7H3O]+, 119.0479[M + H − Glc-2H2O-C7H3O]+ | Flavone glycoside |
| 5 | 7.86 | Liquiritin apioside* (74639-14-8) | C26H30O13 | 4.3 | 551.1774 (+) | 257.0809[M + H − Glc-Ara]+, 239.0695[M + H − Glc-Ara-H2O]+, 137.0223[M + H − Glc-Ara-H2O] + | Chalcone glycoside |
| 6 | 7.88 | Liquiritin* (551-15-5) | C21H22O9 | 4.4 | 419.1344 (+) | 257.0804[M + H − Glc]+, 239.0698[M + H − Glc-H2O]+, 137.0230[M + H − Glc-H2O-C8H7]+ | Flavanone glycoside |
| 7 | 9.66 | Liquiritigenin-7,4′-diglucoside (93446-18-5) | C27H32O14 | 3.3 | 581.1878 (+) | 239.0677[M + H − 2Glc]+, 147.0561[M + H − 2Glc-Phenol]+ | Flavanone glycoside |
| 8 | 9.69 | Schaftoside/isoschaftoside (51938-32-0/52012-29-0) | C26H28O14 | 2.8 | 565.1578 (+) | 271.0590[M + H − Glc-Ara]+ | Flavone glycoside |
| 9 | 9.86 | Kaempferin (482-39-3) | C21H20O10 | 2.4 | 433.1143 (+) | 271.0592[M + H − Rha]+ | Flavone glycoside |
| 10 | 9.88 | Choerospondin (81202-36-0) | C21H22O10 | 0.6 | 435.1294 (+) | 273.0740[M + H − Glc]+, 153.0179[M + H − Glc-C8H8O]+ | Flavanone glycoside |
| 11 | 11.26 | Isoliquiritin apioside* (120926-46-7) | C26H30O13 | 4.3 | 551.1780 (+) | 257.0809[M + H − Glc-Api]+, 239.0895[M + H − Glc-Api-H2O]+, 137.0232[M + H − Glc-Api-H2O-C8H6]+ | Chalcone glycoside |
| 12 | 11.30 | Liquiritugenin-4′-apiosyl(1→2)-glucoside (N.A) | C27H30O13 | 4.2 | 563.1795 (+) | 269.0796[M + H − Glc-Api]+ | Flavone glycoside |
| 13 | 11.64 | Isoliquiritin* (5041-81-6) | C21H22O9 | 4.4 | 419.1351 (+) | 257.0804[M + H − Glc]+, 239.0698[M + H − Glc-H2O]+, 137.0230[M + H − Glc-H2O-C8H6]+ | Chalcone glycoside |
| 14 | 11.71 | Neoisoliquiritin* (59122-93-9) | C21H22O9 | 4.4 | 419.0445 (+) | 257.0818[M + H − Glc]+, 239.0709[M + H − Glc-H2O]+, 147.0442[M + H − Glc-H2O-C6H4O]+, 119.0497[M + H − Glc-H2O-C6H4O-CO]+ | Chalcone |
| 15 | 11.76 | Ononin* (486-62-4) | C22H22O9 | 4.2 | 431.1353 (+) | 269.0812[M + H − Glc]+, 237.0552[M + H − Glc-OCH3]+ | Flavone glycoside |
| 16 | 12.71 | Liquiritigenin* (578-86-9) | C15H12O4 | 2.0 | 257.0816 (+) | 165.0698[M + H − C6H5O]+, 137.0234[M + H − C6H5O-C2H3]+ | Flavonol |
| 17 | 13.01 | Licorice glycoside B (N.A) | C35H36O15 | 5.2 | 697.2166 (+) | 279.0871[M + H − liquiritin]+, 261.0744[M + H − liquiritin-H2O]+ | Chalcone glycoside |
| 18 | 15.91 | Licorice glycoside D2 (202657-65-6) | C35H36O15 | 5.2 | 697.2167 (+) | 257.0787[M + H − C20H25O11]+, 215.0696[M + H − C20H25O11-C2HO]+, 137.0230[M + H − C20H25O11-C2HO-C6H6]+ | Flavone glycoside |
| 19 | 16.59 | Licorice saponin A3 (1262326-47-5) | C48H72O21 | 7.4 | 1001.4662 (+) | 825.4235[M + H − Glucuronic acid]+, 649.3418[M + H − 2Glucuronic acid]+, 487.3407[M + H − 2Glucuronic acid-Glc]+, 469.3304[M + H − 2Glucuronic acid-Glc-H2O]+, 451.3107[M + H − 2Glucuronic acid-Glc-2H2O]+ | Triterpenoid saponin |
| 20 | 17.16 | Uralsaponin F (1208004-79-8) | C44H64O19 | 6.1 | 897.4169 (+) | 545.3469[M + H − 2Glucuronic acid]+, 527.3351[M + H − 2Glucuronic acid-H2O]+, 497.3223[M + H − 2Glucuronic acid-H2O-CH2O]+ | Triterpenoid saponin |
| 21 | 17.44 | Uralsaponin T (1616062-85-1) | C48H74O19 | 6.3 | 955.4974 (+) | 603.4213[M + H − 2Glucuronic acid]+, 585.4149[M + H − 2Glucuronic acid-H2O]+, 439.3575[M + H − 2Glucuronic acid-H2O-Rha]+ | Triterpenoid saponin |
| 22 | 18.37 | Uralsaponin D (1262489-44-0) | C42H58O18 | 6.3 | 851.3756 (+) | 499.3067[M + H − 2Glucuronic acid]+, 481.2931[M + H − 2Glucuronic acid-H2O]+, 463.2877[M + H − 2Glucuronic acid-2H2O]+ | Triterpenoid saponin |
| 23 | 19.54 | 24-Hydroxyl-licorice-saponin E2/ uralsaponin E (934987-86-7/1262489-45-1) | C42H60O17 | 4.8 | 837.3942 (+) | 485.3262[M + H − 2Glucuronic acid]+, 467.3156[M + H − 2Glucuronic acid-H2O]+, 449.3050[M + H − Glucuronic acid-2H2O]+, 437.3050[M + H − Glucuronic acid-2H2O-C]+ | Triterpenoid saponin |
| 24 | 20.14 | Uralsaponin R (1616062-83-9) | C48H74O20 | 6.9 | 971.4842 (+) | 439.3568[M + H − Glc-Rha-Glucuronic acid-COOH]+ | Triterpenoid saponin |
| 25 | 20.23 | Isoliquiritigenin* (961-29-5) | C15H12O4 | 2.0 | 257.1100 (+) | 137.0228[M + H − C8H8O]+ | Chalcone |
| 26 | 20.73 | Uralsaponin Q (1616062-82-8) | C47H72O19 | 7.2 | 941.4817 (+) | 647.3736[M + H − Rha-Xyl]+, 471.3449[M + H − Rha-Xyl-Glucuronic acid]+, 453.3351[M + H − Rha-Xyl-Glucuronic acid-H2O]+ | Triterpenoid saponin |
| 27 | 20.74 | Formononetin* (485-72-3) | C16H12O4 | 3.4 | 269.0811 (+) | 253.0503[M + H − CH3]+, 237.0555[M + H − CH3-O]+, 213.0914[M + H − CH3-O-C2H]+, 137.0236[M + H − CH3-O-C2H-C6H4]+ | Isoflavone |
| 28 | 21.33 | Glabrolide (10401-33-9) | C30H44O4 | 4.3 | 469.3314 (+) | 451.3189[M + H − H2O]+, 439.3204[M + H − H2O-C]+, 395.2634[M + H − H2O-C3H8]+ | Triterpenoid |
| 29 | 21.36 | Licorice saponin E2 (119417-96-8) | C42H60O16 | 4.9 | 821.4006 (+) | 469.3330[M + H − 2Glucuronic acid]+, 451.3207[M + H − 2Glucuronic acid-H2O]+ | Triterpenoid saponin |
| 30 | 21.39 | Glabric acid (22327-86-2) | C30H46O5 | 3.7 | 487.3451 (+) | 469.3352[M + H − H2O]+, 451.3213[M + H − 2H2O]+, 317.2475[M + H − COOH-OH-C10H14]+ | Triterpenoid |
| 31 | 21.99 | Licorice saponin G2 (118441-84-2) | C42H62O17 | 6.9 | 839.4117 (+) | 487.3408[M + H − 2Glucuronic acid)]+, 469.3308[M + H − 2Glucuronic acid-H2O]+, 451.3198[M + H − 2Glucuronic acid-2H2O]+, 439.3202[M + H − 2Glucuronic acid-C]+ | Triterpenoid saponin |
| 32 | 23.03 | 3-O-β-D-Glucuronopyranosyl glycyrrhetinic acid (34096-83-8) | C36H54O10 | 4.7 | 647.3756 (+) | 453.3352[M + H − Glucuronic acid-H2O]+, 435.3242[M + H − Glucuronic acid-2H2O]+, 407.3291[M + H − Glucuronic acid-H2O-COOH]+ | Triterpenoid saponin |
| 33 | 23.09 | Glycyrrhizin* (1405-86-3) | C42H62O16 | 3.6 | 823.4146 (+) | 471.3448[M + H − 2Glucuronic acid]+, 453.3347[M + H − 2Glucuronic acid-H2O]+, 407.3286[M + H − 2Glucuronic acid-COOH]+ | Triterpenoid saponin |
| 34 | 23.92 | Glycyuralin E (1879910-26-5) | C21H22O6 | 4.1 | 371.1501 (+) | 297.0750[M + H − C4H8-H2O]+, 165.0535[M + H − C12H13O2-H2O]+, 135.0427[M + H − C13H13O2-2H2O]+ | Other |
| 35 | 24.77 | Neochalcoside (81793-82-0) | C21H22O8 | 3.6 | 403.1418 (+) | 373.0917[M + H − CH3OH]+, 357.0569[M + H − CH3OH-CH4]+, 327.0863[M + H − CH3OH-CH4-CH2O]+ | Chalcone glycoside |
| 36 | 24.86 | Licorice saponin B2 (118536-86-0) | C42H64O15 | 6.2 | 809.4383 (+) | 457.3655[M + H − 2Glucuronic acid-]+, 439.3570[M + H − 2Glucuronic acid-H2O]+, 421.3474[M + H − 2Glucuronic acid-2H2O]+ | Triterpenoid saponin |
| 37 | 26.14 | Licorice saponin J2 (938042-18-3) | C42H64O16 | 5.4 | 825.4308 (+) | 455.3505[M + H − 2Glucuronic acid]+, 437.3399[M + H − 2Glucuronic acid-H2O]+, 315.2314[M + H − 2Glucuronic acid-H2O-C9H14]+, 301.2158[M + H − 2Glucuronic acid-H2O-C9H14-CH2]+, 287.1995[M + H − 2Glucuronic acid-H2O-C9H14-2CH2]+ | Triterpenoid saponin |
| 38 | 26.77 | Tetrahydroxy-methoxychalcone (197227-39-7) | C16H14O6 | 2.8 | 303.0869 (+) | 165.0578[M + H − C6H5O2-CO]+, 135.0417[M + H − C6H5O2-CO-OCH3]+, 107.0505[M + H − C6H5O2-CO-OCH3-C2H4]+ | Chalcone |
| 39 | 26.80 | Glicophenone (303175-66-8) | C20H22O6 | 0 | 359.1507 (+) | 257.0851[M + H − C5H10-OCH3]+ | Isoflavone |
| 40 | 27.02 | Glycybridin H (2138843-61-3) | C21H24O5 | 2.2 | 357.1341 (+) | 301.0685[M + H − C4H8]+, 283.0772[M + H − C4H8-H2O]+, 179.0343[M + H − C4H8-H2O-C7H4O]+, 165.5554[M + H − C4H8-H2O-C8H2O]+, 151.0387[M + H − C4H8-H2O-C9H4O]+, 139.0398[M + H − C4H8-H2O-C10H4O]+, 125.0596[M + H − C4H8-H2O-C10H4O-CH3]+, 107.0477[M + H − C4H8-H2O-C10H4O-OCH3]+ | Isoflavan |
| 41 | 27.09 | Glyasperin C (142474-53-1) | C21H24O5 | 2.2 | 357.1713 (+) | 283.0676[M + H − C4H8-H2O]+, 165.0555[M + H − C4H8-H2O-C8H6O]+, 137.0532[M + H − C4H8-H2O-C8H6O-CO]+, 123.0421[M + H − C4H8-H2O-C8H6O-CO-CH2]+ | Isoflavan |
| 42 | 27.22 | (2R,3R)-3,4′,7-Trihydroxy-3′-prenylflavane (1217305-78-6) | C20H20O5 | 0.7 | 341.1393 (+) | 137.0239[M + H − C13H16O2]+ | Isoflavanone |
| 43 | 27.95 | 8-Methylretusin (37816-20-9) | C17H14O5 | 0.3 | 299.0926 (+) | 267.0652[M + H − OCH3]+, 248.9875[M + H − OCH3-H2O]+, 159.0507[M + H − OCH3-H2O-C6HO]+ | Isoflavone |
| 44 | 28.76 | Corylifol B (775351-90-1) | C20H20O5 | 0.7 | 341.1393 (+) | 269.0452[M + H − C4H8-H2O]+, 229.0827[M + H − C4H8-H2O-C3H2]+, 165.0692[M + H − C12H11-O-OH]+ | Chalcone |
| 45 | 28.98 | Licoagrocarpin (202815-29-0) | C21H22O4 | 2.5 | 339.1243 (+) | 283.0597[M + H − C4H8]+, 255.0648[M + H − C4H8-C-CH3]+ | Pterocarpan |
| 46 | 29.17 | Licochalcone C (144506-14-9) | C21H22O4 | 2.5 | 339.1245 (+) | 283.0611[M + H − C4H8]+, 271.0602[M + H − C4H8-C]+, 255.0648[M + H − C4H8-C-CH3]+ | Chalcone |
| 47 | 29.66 | Hirtellanine I (1369378-20-0) | C21H18O6 | 3.6 | 367.1964 (+) | 337.1090[M + H − OCH3]+, 321.1122[M + H − OCH3-CH3]+ | Isoflavone |
| 48 | 30.32 | Morachalcone A (76472-88-3) | C20H20O5 | 0.7 | 341.1392 (+) | 255.0932[M + H − C5H8-H2O]+, 165.0672[M + H − C5H8-H2O-Phenol]+, 137.0200[M + H − C5H8-H2O-Phenol-CO]+ | Chalcone |
| 49 | 30.50 | Glycyuralin A (1879910-22-1) | C26H34O6 | 4.8 | 443.2452 (+) | 369.1699[M + H − C4H9O]+, 247.1355[M + H − C4H9O-C7H6O2]+, 233.1162[M + H − C4H9O-C7H6O2-CH3]+ | Isoflavan |
| 50 | 30.57 | Luteone/6-C-prenylorobol/gancaonin L/2,3-dehydrokievitone(41743-56-0/66777-71-7/129145-50-2/74161-25-4) | C20H18O6 | 2.7 | 355.1192 (+) | 299.0553[M + H − C4H8]+, 243.0655[M + H − C4H8-C3H4O]+ /299.0553[M + H − C4H8]+, 165.0160[M + H − C4H8-C7H2O3]+ | Isoflavone/isoflavan/isoflavanone |
| 51 | 30.74 | Glycyuralin F (1879910-27-6) | C20H20O7 | 2.1 | 373.1658 (+) | 296.0423[M + H − C5H10O-H2O]+, 179.0693[M + H − C5H10O-H2O-C6H2O]+,149.0587[M + H − C5H10O-H2O-C6H2O-CO]+ | Isoflavone |
| 52 | 27.95 | Isotrifoliol (329319-08-6) | C16H10O6 | 3.4 | 299.0926 (+) | 281.0466[M + H − H2O]+, 248.9875[M + H − H2O-OCH3]+, 159.0507[M + H − H2O-OCH3-C6H2O]+ | Coumarin |
| 53 | 31.77 | Kanzonol K (156281-30-0) | C26H28O6 | 5.3 | 437.1619 (+) | 381.1345[M + H − C4H8]+, 369.1331[M + H − C4H8-C]+, 237.0572[M + H − C4H8-C-C8H4O2]+ | Isoflavone |
| 54 | 32.96 | Glyasperin D (142561-10-2) | C22H26O5 | 3.4 | 371.1874 (+) | 250.9766[M + H − Phenol-H2O]+, 219.0994[M + H − Phenol-H2O-CH3-O]+, 205.1093[M + H − Phenol-H2O-CH3-O-CH2]+ | Isoflavan |
| 55 | 33.42 | Glyasperin B (142488-54-8) | C21H22O6 | 4.1 | 371.1875 (+) | 153.0532[M + H − C13H14O3]+, 137.0596[M + H − C13H14O3-O]+, 123.0811[M + H − C14H16O3-O]+ | Isoflavanone |
| 56 | 33.86 | Dehydroglyasperin D (517885-72-2) | C22H24O5 | 0.6 | 369.0243 (+) | 299.0912[M + H − C5H9]+, 285.0758[M + H − C5H9-CH3]+, 163.0390[M + H − C5H9-CH3-C7H6O2]+ | Isoflavan |
| 57 | 34.12 | Licoricidin (30508-27-1) | C26H32O5 | 5.9 | 425.0516 (+) | 221.1167[M + H − C13H6O2]+, 189.0904[M + H − C13H6O2-CH2O]+, 177.0527[M + H − C13H6O2-CH2O-C]+, 135.0438[M + H − C13H6O2-CH2O-C-C2HO]+ | Isoflavan |
| 58 | 34.33 | Isoglycyrol (23013-86-7) | C21H18O6 | 3.6 | 367.1551 (+) | 335.1259[M + H − OCH3]+, 321.1076[M + H − OCH3-CH3]+, 203.0679[M + H -OCH3-CH3-C8H6O]+ | Coumarin |
| 59 | 35.15 | Angustone A (90686-13-8) | C25H26O6 | 7.6 | 423.1841 (+) | 367.1175[M + H − C4H8]+, 311.0556[M + H − 2C4H8]+, 299.0557[M + H − 2C4H8-C]+ | Isoflavone |
| 60 | 35.81 | Glycyrrhetinic acid* (471-53-4) | C30H46O4 | 3.2 | 471.3471 (+) | 453.3368[M + H − H2O]+, 435.3267[M + H − 2H2O]+, 407.3325[M + H − 2H2O-CO]+ | Triterpenoid |
| 61 | 36.03 | Gancaonin G (126716-34-5) | C21H20O5 | 4.3 | 353.1398 (+) | 297.0749[M + H − C4H8]+, 267.0654[M + H − C4H8-H2O-C]+, 165.0678[M + H − C4H8-H2O-C-OCH3-(Ph-H5)]+ | Isoflavone |
| 62 | 37.16 | 5,3′,4′-Trihydroxy-8,6′-di-(3-methyl-2-butenyl)-flavone (134958-52-4) | C25H26O5 | 6.3 | 407.1887 (+) | 295.0608[M + H − 2C4H8]+, 117.0175[M + H − 2C4H8-C8H6O]+ | Flavone |
| 63 | 38.93 | Licorisoflavan A (129314-37-0) | C27H34O5 | 4.8 | 439.0877 (+) | 327.1224[M + H − 2C4H8]+, 193.0859[M + H − 2C4H8-C7H4O2-CH3]+ | Isoflavan |
Note: “*” means comparing with reference compound; N.A means “CAS No” is not available.
By comparing with those of the reference compounds in respect of retention times and characteristic fragment ions, 12 components were unambiguously identified. All the proposed fragmentations of tentatively identified compounds were shown in Table 1.
The results showed that the identified triterpenoids are divided into two categories according to their structure (Fig. 2). In Structure type 1 (Table 2), the difference between these compounds lies in the C-3, C-11, C-21, C-22, C-23 and C-30 positions in the substituents to which they are attached. Most of them have in common that the R2 substituent sites are linked to glucuronic acid. The diagnostic fragmentation of these triterpenoids is the loss of glucuronic acids, Rha-Xyl and/or water to produce [M + H − 176]+, [M + H − 294]+ or [M + H − 18]+. Taking compound 26 as an example, its fragmentation law has been elaborated as follows. This compound exhibited an [M + H]+ ion at m/z 941.4817, corresponding to a molecular formula of C47H72O19. In the MS/MS spectrum [M + H]+, fragment ions at m/z 647.3736, 471.3449 and 453.3351 correspond to [M + H − Rha-Xyl]+, [M + H − Rha-Xyl-Glucuronic acid]+, [M + H − Rha-Xyl-Glucuronic acid − H2O]+, respectively. Thus compound 26 could be temporarily deduced as uralsaponin Q.25)

 | |||||||
|---|---|---|---|---|---|---|---|
| Peak No. | Compounds | R1 | R2 | R3 | R4 | R5 | R6 |
| 33 | Glycyrrhizin | –CH3 | –2 Glucuronic acid | =O | –COOH | –H | –H |
| 32 | 3-O-β-D-Glucuronopyranosyl glycyrrhetinic acid | –CH3 | –Glucuronic acid | =O | –COOH | –H | –H |
| 37 | Licorice saponin J2 | –CH2OH | –2 Glucuronic acid | –H | –COOH | –H | –H |
| 31 | Licorice saponin G2 | –CH2OH | –2 Glucuronic acid | =O | –COOH | –H | –H |
| 36 | Licorice saponin B2 | –CH3 | –2 Glucuronic acid | –H | –COOH | –H | –H |
| 19 | Licorice saponin A3 | –CH2OH | –2 Glucuronic acid | =O | –Glucuronic acid | –H | –H |
| 20 | Uralsaponin F | –CH2OH | –2 Glucuronic acid | =O | –COOH | –H | –OOCCH3 |
| 21 | Uralsaponin T | –CH3 | –2 Glucuronic acid | =O | –CH3 | –H | Rha |
| 26 | Uralsaponin Q | –CH3 | –Glucuronic acid-Rha-Xyl | =O | –COOH | –OH | –H |
| 24 | Uralsaponin R | –CH3 | –Glucuronic acid-Glc-Rha | =O | –COOH | –OH | –H |
| 30 | Glabric acid | –CH3 | –OH | =O | –COOH | –OH | –H |
| 60 | Glycyrrhetinic acid | –CH3 | –OH | =O | –COOH | –H | –H |
Among the other triterpenoids in our rearch (Table 3), glabrolide is not linked to glucuronic acid while the other four compounds are all cleaved from glucuronic acid, followed by H2O to produce [M + H − 2 Glucuronic acid]+, [M + H − 2 Glucuronic acid − H2O]+ and obtain the corresponding molecular weight. Glabrolide produced an [M + H]+ ion at m/z 469.3314 with an elemental composition of C30H44O4. MS2 gave rise to the product ions at m/z 451.3189, 439.3204, and 395.2634, respectively.26)
 | ||||
|---|---|---|---|---|
| Peak No. | Compounds | R1 | R2 | R3 |
| 23 | 24-Hydroxyl-licorice-saponin E2 | –CH3 | –CH2OH | –2 Glucuronic acid |
| 23 | Uralsaponin E | –CH2OH | –CH3 | –2 Glucuronic acid |
| 28 | Glabrolide | –CH3 | –CH3 | –OH |
| 29 | Licorice saponin E2 | –CH3 | –CH3 | –2 Glucuronic acid |
| 22 | Uralsaponin D | –COOH | –CH3 | –2 Glucuronic acid |
In addition, flavonoids and their corresponding glycoside took the largest proportion of all chemical compounds in licorice.27) The identified compounds included kinds of structures such as flavonoid, isoflavone, isoflavanone, flavanone, isoflavane, chalcone and dihydrochalcone (Tables 4–9). Several sugar fragments consisting of Glc [M + H − 162]+, Ara [M + H − 132]+, Api [M + H − 132]+ and Rha [M + H − 146]+ are most common substituents linked to flavonoids. In addition, the presence of group X1 (− C4H8) showed high frequency and its cleavages were commonly observed.
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak No. | Compounds | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 |
| 1 | Nicotiflorin | –H | –OH | –H | –OH | –Glc–Rha | –H | –H | –OH | –H | –H |
| 12 | Liquiritugenin-4′-apiosyl(1→2)-glucoside | –H | –OH | –H | –H | –OH | –H | –H | –Glc–Api | –H | –H |
| 8 | Schaftoside | –Ara | –OH | –Glc | –OH | –H | –H | –H | –OH | –H | –H |
| 8 | Isoschaftoside | –Glc | –OH | –Ara | –OH | –H | –H | –H | –OH | –H | –H |
| 9 | Kaempferin | –H | –OH | –H | –OH | –Rha | –H | –H | –OH | –H | –H |
| 62 | 5,3′,4′-Trihydroxy-8,6′-di-(3-methyl-2-butenyl)-flavone | –H | –OH | –X1 | –OH | –H | –H | –X1 | –OH | –H | –H |
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak No. | Compounds | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 |
| 27 | Formononetin | –H | –OH | –H | –H | –H | –H | –OCH3 | –H | –H |
| 50 | Luteone | –H | –OH | –X1 | –OH | –OH | –H | –OH | –H | –H |
| 50 | 6-C-Prenylorobol | –H | –OH | –X1 | –OH | –H | –H | –OH | –OH | –H |
| 50 | Gancaonin L | –X1 | –OH | –H | –OH | –H | –H | –OH | –OH | –H |
| 50 | 2,3-Dehydrokievitone | –X1 | –OH | –H | –OH | –OH | –H | –OH | –H | –H |
| 47 | Hirtellanine I | –H | –OH | –H | –H | –OCH3 | –X3 | –X3 | –H | –OH |
| 43 | 8-Methylretusin | –OCH3 | –OH | –H | –H | –H | –H | –OCH3 | –H | –H |
| 61 | Gancaonin G | –H | –OCH3 | –X1 | –OH | –H | –H | –OH | –H | –H |
| 51 | Glycyuralin F | –H | –OH | –X4 | –OH | –H | –H | –OH | –OH | –H |
| 59 | Angustone A | –H | –OH | –X1 | –OH | –OH | –X1 | –OH | –H | –H |
| 53 | Kanzonol K | –H | –OCH3 | –X1 | –OH | –OH | –X1 | –OH | –H | –H |
| 15 | Ononin | –H | –Glc | –H | –H | –H | –H | –OCH3 | –H | –H |
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak No. | Compounds | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 |
| 2 | Glucoliguiritin apioside | –H | –Glc | –H | –H | –H | –H | –H | –Glc–Api | –H | –H |
| 10 | Choerospondin | –H | –OH | –H | –OH | –H | –H | –H | –Glc | –H | –H |
| 4 | Neoliquiritin | –H | –Glc | –H | –H | –H | –H | –H | –OH | –H | –H |
| 7 | Liquiritigenin-7,4′-diglucoside | –H | –Glc | –H | –H | –H | –H | –H | –Glc | –H | –H |
| 16 | Liquiritigenin | –H | –OH | –H | –H | –H | –H | –H | –OH | –H | –H |
| 42 | (2R,3R)-3,4′,7-Trihydroxy-3′-prenylflavane | –H | –OH | –H | –H | –OH | –H | –X1 | –OH | –H | –H |
| 5 | Liquiritin apioside | –H | –Api | –H | –H | –H | –H | –H | –Glc | –H | –H |
| 6 | Liquiritin | –H | –OH | –H | –H | –H | –H | –H | –Glc | –H | –H |
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak No. | Compounds | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 |
| 55 | Glyasperin B | –H | –OCH3 | –X1 | –OH | –H | –OH | –H | –OH | –H | –H |
 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak No. | Compounds | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | R11 |
| 49 | Glycyuralin A | –H | –X3 | –X3 | –OCH3 | –H | –H | –OH | –X4 | –OH | –H | –H |
| 63 | Licorisoflavan A | –H | –OCH3 | –X1 | –OCH3 | –H | –H | –OH | –X1 | –OH | –H | –H |
| 54 | Glyasperin D | –H | –OCH3 | –X1 | –OCH3 | –H | –H | –OH | –H | –OH | –H | –H |
| 40 | Glycybridin H | –X1 | –OH | –H | –H | –H | –H | –OH | –OH | –OCH3 | –H | –H |
| 57 | Licoricidin | –H | –OH | –X1 | –OCH3 | –H | –H | –OH | –X1 | –OH | –H | –H |
| 41 | Glyasperin C | –H | –OH | –X1 | –OCH3 | –H | –H | –OH | –H | –OH | –H | –H |
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak No. | Compounds | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 |
| 11 | Isoliquiritin apioside | –H | –OH | –H | –H | –OH | –H | –H | –Glc–Api | –H | –H |
| 14 | Neoisoliquiritin | –H | –Glc | –H | –OH | –H | –H | –H | –OH | –H | –H |
| 35 | Neochalcoside | –H | –H | –H | –OH | –H | –H | –H | –Glc | –H | –H |
| 13 | Isoliquiritin | –H | –OH | –H | –OH | –H | –H | –H | –Glc | –H | –H |
| 25 | Isoliquiritigenin | –H | –OH | –H | –OH | –H | –H | –H | –H | –OH | –H |
| 44 | Corylifol B | –H | –OH | –X1 | –OH | –H | –H | –OH | –OH | –H | –H |
| 46 | Licochalcone C | –H | –OH | –H | –H | –H | –OCH3 | –X1 | –OH | –H | –H |
| 48 | Morachalcone A | –H | –OH | –X1 | –OH | –H | –H | –H | –OH | –H | –OH |
| 38 | Tetrahydroxy-methoxychalcone | –H | –OH | –OH | –H | –H | –OCH3 | –OH | –OH | –H | –H |
First of all, the cleavage law of flavonoids basically starts from the attached sugar chain. For example, liquiritugenin-4′-apiosyl(1→2)-glucoside, detected at 11.30 min, showed [M + H]+ion at m/z 563.1795, and the molecular formula of C27H30O13 is proposed. In the ESI+MS/MS experiment, the characteristic fragment ions at m/z 294 [M + H − Glc-Api]+ was found. Schaftoside and isoschaftoside are isomers, showing the same [M + H]+ ion at m/z 565.1578.28) The characteristic loss of 294 Da [M + H − Glc-Ara]+ could be detected but they cannot be completely distinguished from each other in this study. In addition, without any sugar chain, 5,3′,4′-trihydroxy-8,6′-di-(3-methyl-2-butenyl)-flavone suggested an [M + H]+ ion at m/z 407.1887 (C25H26O5) and displayed the losses of the group X1 [M + H − 2C4H8]+.
Compounds such as isoflavone, flavanone and chalcone are partially linked with sugar chains as well, suggesting the structure diversity of flavonoids in licorice. Compound 4 yielded intensive [M + H]+ molecular ion peaks at m/z 419.1344, corresponding to the molecular formula of C21H22O9. The characteristic fragment ion m/z 257.0804, 239.0698 and 137.0230 were observed, originated from [M + H − Glc]+, [M + H − Glc-H2O]+ and [M + H − Glc-H2O-C8H7]+ respectively. Similarly, compounds 5, 6, 7, 9, 10, 11, 14, and 15 with sugar chains were tentatively identified and some of them were unambiguously identified by comparing their retention times and MS data with the reference compound.
Apart from glycosyl, methoxy (–OCH3) and X1 (–C4H8) group were often attached to different positions of the licorice flavonoids. Thus production of [M + H − OCH3]+ and [M + H − C4H8]+ could also be found in the MS/MS spectrum. Ononin, for instance, was detected at 11.76 min, showed an [M + H]+ ion at m/z 431.1353, suggesting a molecular formula of C22H22O9. In the MS2 spectrum, the fragment ion at m/z 269.0812 and 237.0552 were yielded by the loss of Glc and OCH3. Compound 47 (hirtellanine I) produced [M + H]+ ions at m/z 367.1964. In MS/MS mode, characteristic fragment ions at m/z 337.1090[M + H-OCH3]+ and 321.1122[M + H-OCH3–CH3]+ were obtained. Furthermore, the presence of 56 Da (− C4H8) commonly was observed in luteone/6-C-prenylorobol/gancaonin L/2,3-dehydrokievitone, gancaonin C, gancaonin G, licorisoflavan A, glycybridin H and glyasperin C in the MS2 experiment.29,30)
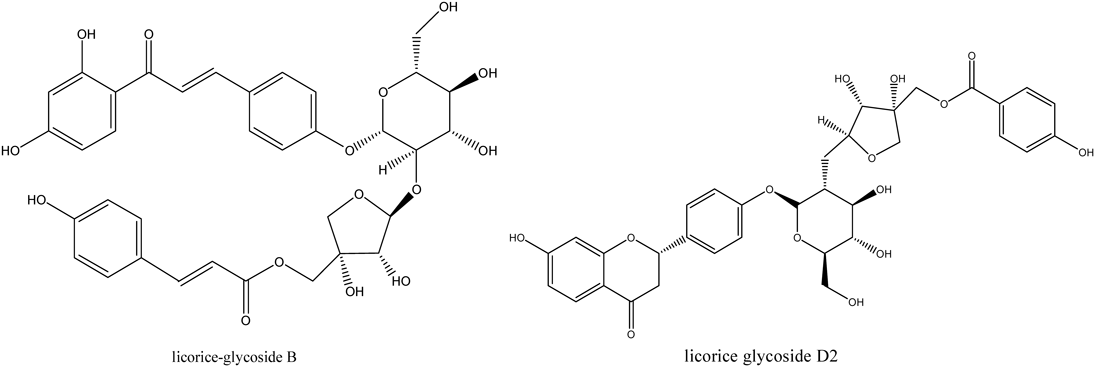
Although coumarin is not the typical chemical in licorice, several coumarins and its derivatives were also detected in this experiment. The characteristic fragmentation was derived from the cleavage of the OCH3 (isoglycyrol), H2O (isotrifoliol) and Glc (trifolirhizin) groups31) (Table 10). Finally, two dimeric structures of flavonoids were characterized in this research. Their basic structure possesses one Api-group, one Glc-group and flavonoid skeleton. The fragmentation mechanism of them starts from glycosidic bonds, followed by the fragmentation of H2O (licorice glycoside B) and cleavage of C-ring (licorice glycoside D2).
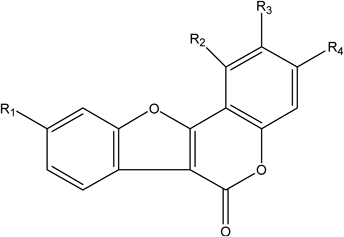 | |||||
|---|---|---|---|---|---|
| Peak No. | Compounds | R1 | R2 | R3 | R4 |
| 52 | Isotrifoliol | –OH | –OCH3 | –H | –OH |
| 58 | Isoglycyrol | –OH | –OCH3 | –X3 | –X2 |
 | |||||
| Peak No. | Compounds | R1 | R2 | R3 | R4 |
| 3 | Trifolirhizin | –X5 | –X5 | –Glc | –H |
| 45 | Licoagrocarpin | –OCH3 | –H | –OH | –X1 |
The unsupervised principal component analysis PCA was performed to obtain the cluster of wild and cultivated licorice by the chemical constituents. After Pareto scaling with mean-centering, the data were displayed as scores in a coordinate system of latent variables, which were based on the two groups of licorice sample. The first, second and third principal components contribute to 31.2, 24.4 and 14.5% of the total variance, respectively. The ellipse was set as 95% confidence limits for the data. As is shown in Fig. 3(A), quality control samples gathered together in the middle of the axis. Besides, wild licorice samples were mostly located in the negative axis while cultivated ones were mostly displayed in positive axis. The results proved that licorice from different growing environment displayed significant differences in chemical composition. However, PCA could not highlight specific metabolites with statistically significant differences among those samples.

QC, quality control; C, cultivated group; W, wild group.
To find potential chemical markers that contributed most to the differences between cultivated and wild licorice, UFLC–QTOF-MS/MS data were further processed by PLS-DA. The clustering results of PLS-DA have been shown in Fig. 3(B). The reliable confidence level was 95%, R2Y = 0.916, Q2 = 0.819, which proved that the model was effective. To identify significant chemical markers between wild and cultivated licorice, the variable importance in the projection (VIP) value and loading plot that indicate the relative contribution of each variable was performed in PLS-DA model. The important variables were also picked out as potential markers when VIP value was considered to be large than 1.0. Besides, the distance of each point detected in the loading plot represents the contribution to classification. The size of the point was set according to the VIP value. Furthermore, t-test was also introduced to verify the significance of those chosen chemical constituents based on peak intensity. Finally, VIP (Figs. 4A, B) and t-test analysis (Fig. 4C) both demonstrated that there are indeed chemical composition changes between wild and cultivated licorice. As a result, 14 candidate compounds were selected as potential chemical markers.

In details, although licoricidin has the greatest contribution and its VIP value is greater than 3, but the content of licoricidin in both wild and cultivated licorice was unstable, which means it does not have the universal meaning as a marker. The VIP value greater than 2 is 3-O-β-D-glucuronopyranosyl glycyrrhetinic acid, licorice saponin G2, licorice-saponin J2, dehydroglyasperin D and glyasperin D, three saponins and two isoflavanones. The content of 3-O-β-D-glucuronopyranosyl glycyrrhetinic acid showed slight differences between two types of licorice while licorice-saponin J2 in wild licorice was much higher than that of cultivated products. On the contrary, the content of licorice saponin G2, dehydroglyasperin D and glyasperin D showed opposite results, indicating that saponins and flavonoids are still the key components for licorice markers. Those chosen compounds should be analyzed in combination with the content of the chemical components. The VIP value among remaining compounds was between 1–2, including quality control components of glycyrrhizin (VIP greater than 1.5) and isoliquiritin, neoisoliquiritin, hirtellanine I, glycyuralin E (Table 11), liquiritigenin, glycybridin H, ononin, formononetin, glabrolide, licorice saponin B2 and isoglycyrol in licorice. Overall, apart from several common licorice compounds (glycyrrhizin, for instance), licorice saponin J2/G2, glyasperin D and dehydroglyasperin D (VIP greater than 2) are also possibly considered to be as a chemical markers for licorice based on our study.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Peak No. | Compounds | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
| 34 | Glycyuralin E | –OH | –H | –H | –OH | –X2 | –X2 | –OCH3 |
Since the wild and cultivated licorice come from the same genus, the composition of the chemical components is basically consistent. In this experiment, the contents of most ingredients were higher in the cultivated products (Fig. 4C). However, the contents of confirmed bioactive compound (glycyrrhizin) performed better in the wild licorice. Besides, a great number of investigations proved that difference of chemical contents could be found between wild and cultivated licorice.15) Combined with literature evidence, our results suggested that quality of traditional Chinese medicine should not only be evaluated from the content of a few compounds and the accumulative rules between bioactive compounds along with other chemicals should be explored further. It is worth noting that these different compounds also cover the major components of licorice, such as triterpenoids, kinds of flavonoids along with their glycosides. The selection of chemical marker should take into account the representative components of each category based on the comprehensive study. The chemical composition of licorice is complex and varied, and the relation among various types of compounds could also be explored for setting the chemical markers in licorice from overall quality aspect.
In this research, a UFLC-Q-TOF-MS method was successfully established to provide the possibility for observing the chemical profiling between wild and cultivated licorice. Ultimately, 63 components with various structures including triterpenoids, triterpenoid saponins, coumarin, flavone, flavanone, chalcone, flavanone, isoflavone, isoflavan, isoflavanone and their glycosides were identified or deduced on the basis of MS or MS/MS information and data from literatures. Glycyrrhizin, licorice saponin J2/G2, glyasperin D and dehydroglyasperin D could be considered as chemical markers for wild and cultivated licorice. Our investigation could not only provide a powerful platform for metabolites profiling and lay the way for distinguishing the two ecotypes of licorice.
This study was financially supported by National Key R&D Program of China (2018YFC1706500).
The authors declare no conflict of interest.