2019 年 67 巻 10 号 p. 1042-1045
2019 年 67 巻 10 号 p. 1042-1045
Biaryls are important compounds with widespread applications in many fields. Tetramethylammonium fluoride tetrahydrate was found to promote the biaryl coupling of aryl iodides bearing electron-withdrawing substituents with unactivated arenes. The reaction takes place at temperatures between 100 and 150°C and can be applied to a wide range of aromatic and heteroaromatic rings, affording the products in moderate to high yields. The reaction does not require strong bases or expensive additives that are employed in the existing methods and can be conducted in air and moisture without any precautions.
Biaryls, compounds containing the aryl–aryl bond, are used in various fields such as pharmaceuticals, agrochemicals, and materials science.1,2) Hence, much effort has been devoted to the development of new synthetic methods for these compounds. To date, transition metal-catalyzed cross-coupling reactions have been the most commonly employed method to prepare biaryls via C–C bond formation.3–5) However, these methods require organometallic reagents, which are usually unstable and need to be synthesized first. For instance, the direct C–H arylation of arenes with aryl halides has attracted considerable attention, and the synthesis has been extensively reported using several transition metals (Pd, Rh, Ru, Ir, and Cu).6–16) However, the development of cheap and environment-friendly methods is in high demand.
As an alternative method, a transition metal-free coupling of haloarenes with unactivated arenes for the construction of biaryls has been achieved using radical sources such as Bu3SnH and (Me3Si)3SiH.17–25) The reaction proceeds via a homolytic aromatic substitution (HAS) mechanism, wherein the aryl radicals generated by dehalogenation of haloarenes add to the unactivated arenes to afford cyclohexadienyl-type radicals. These radicals almost always undergo rearomatization (Chart 1). Furthermore, base-mediated biaryl coupling reactions that proceed via benzyne26–28) or radical reaction mechanism have been developed recently. As a pioneering work of biaryl synthesis via radical pathway, in 2008, Itami and colleagues presented a t-BuOK-mediated biaryl coupling of aryl halides and electron-deficient heterocycles involving base-promoted homolytic aromatic substitution (BHAS), in which strong bases acted as a single electron donor to haloarenes, resulting in the generation of aryl radicals.29) The subsequent works30–51) reported that some additives, such as 1,10-phenanthroline,30,32,40,50) 1,2-dimethylethylenediamine,31) proline,37) alcohols,42,49)p-tolylsulfonyl hydrazide,44) and urea51)etc. were effective in the coupling reaction in the presence of an inorganic base (t-BuOK or t-BuONa). Other base-mediated coupling such as CsOH-52) and lithium bis(trimethylsilyl)amide (LiHMDS)-53)mediated coupling reactions have also been reported. Although these methods are efficient for the construction of biaryls, most of these reactions require to be conducted under an inert atmosphere because of the strong bases. In 2014, Ryu and colleagues reported that the Bu4NBH3CN-mediated biaryl synthesis under photoirradiation in air proceeded via a BHAS-based mechanism.54) It is considered that the reaction is promoted by oxygen. In the same year, Guo and colleagues developed the photoinduced coupling reaction in air using a catalytic amount of N,N,N′,N′-tetramethylethylenediamine (TMEDA).55) Though the reactions can be easily carried out in air, such reactions have been rarely reported (Chart 2).
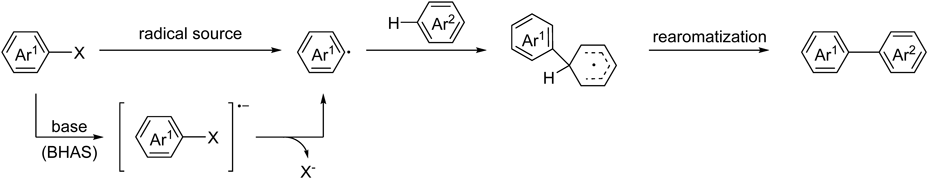

Our group has developed transition metal-free reactions involving C–C bond formation by amide bases generated in situ from fluoride and aminosilane.56–61) Very recently, we reported the amide base-mediated biaryl coupling of aryl iodides and arenes.62) Tetramethylammonium fluoride (TMAF) and hexamethyldisilazane (HMDS) were used to generate the amide base in situ. During the investigation, it was found that the coupling of 2-nitroiodobenzene with benzene was driven by TMAF in the absence of HMDS. It has already been reported that the dehalogenation of 2-nitrohalobenzenes proceeds with CsF in N,N-dimethylformamide (DMF) via a single electron transfer to the substrate63); however, a coupling reaction with arenes in the presence of fluoride has not yet been developed. In this study, we further investigated the coupling of aryl iodides with unactivated arenes using a fluoride, wherein the unactivated arenes acted as the solvents. The reaction proceeded smoothly in air in the presence of tetraalkylammonium fluoride hydrate. This method did not require strong bases or expensive additives and could be conducted in air and moisture without any precautions.
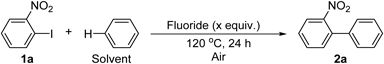
We first studied the coupling of the substrate 2-nitroiodobenzene, 1a, with benzene as solvent (Table 1). When the coupling reaction was conducted in Ar atmosphere in the presence of TMAF, which was used as a fluoride source in our previous work,62) biaryl product 2a was obtained in low yield (entry 1). The use of TMAF·4H2O, which is air stable, gave the same yield (entry 2). Subsequently, the reaction was carried out in air using TMAF·4H2O, and this improved the yield of 2a (entry 3). The yield of 2a was higher in O2 atmosphere as compared with that in Ar atmosphere, suggesting that O2 promoted the desired reaction (entry 4).24,54) The reaction could also be carried out effectively in air in the presence of tetrabutylammonium fluoride trihydrate (TBAF·3H2O) (entry 5). However, the reaction did not proceed at all in the presence of alkali metal fluorides (entries 6 and 7). A drop in the yield was observed on reducing the amount of TMAF·4H2O (entries 8 and 9). In addition, lowering of the reaction temperature to 100°C decreased the yield (entry 10).
 | ||
|---|---|---|
| Entry | Fluoride (equiv.) | Yield (%)[a] |
| 1[b] | TMAF (3.0) | 30 |
| 2[b] | TMAF⋅4H2O (3.0) | 30 |
| 3 | TMAF⋅4H2O (3.0) | 82(74)[c] |
| 4[d] | TMAF⋅4H2O (3.0) | 76 |
| 5 | TBAF⋅3H2O (3.0) | 50 |
| 6 | KF (3.0) | 0 |
| 7 | CsF (3.0) | 0 |
| 8 | TMAF⋅4H2O (1.0) | 38 |
| 9 | TMAF⋅4H2O (2.0) | 45 |
| 10[e] | TMAF⋅4H2O (3.0) | 23 |
[a] Determined by 1H-NMR using 1,1,2-trichloroethane as an internal standard. [b] Carried out under Ar atmosphere. [c] Isolated yield is given in parentheses. [d] Carried out under O2 atmosphere. [e] Reaction temperature = 100°C.
Next, we investigated the substrate scope of the reaction using the optimal reaction conditions (Table 2). The coupling of 3-iodonitrobenzene 1b with benzene required higher temperatures (entry 2). The coupling of 4-iodonitrobenzene 1c with benzene proceeded by the aromatic nucleophilic substitution of the substrate with the hydroxide ion (OH−) and resulted in the formation of 4-nitrophenol in 70% yield. The addition of 3,5-di-tert-butyl-4-hydroxytoluene (BHT) and K2CO3 could prevent this undesirable reaction, and the yield of the biaryl product 2c was increased (entry 3).
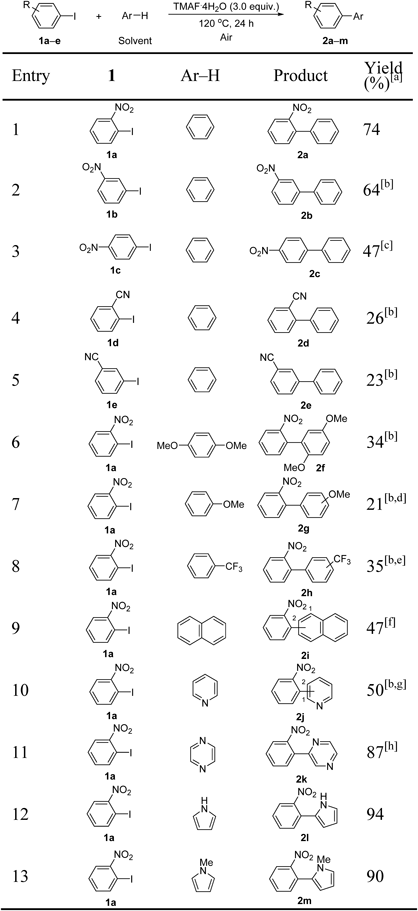 |
[a] Reaction conditions: 1 (0.20 mmol), 2 (1.0 mL), TMAF·4H2O (0.60 mmol), 120°C, 24 h, air. [b] Reaction temperature = 150°C. [c] With BHT (3.0 eq) and K2CO3 (1.2 eq) at 110°C. [d] o:m:p = 1.0 : 0.37 : 0.41. [e] o:m:p = 1.0 : 1.4 : 1.0. [f] C1 : C2 = 1.0 : 0.34. [g] C1:C2 = 1.0 : 1.5. [h] Reaction temperature = 100°C.
When iodobenzonitriles were employed, the reactions proceeded at 150°C in low yields (entries 4 and 5). We further investigated a variety of substituted arenes as solvents. Benzene derivatives lowered the yields regardless of the electron-donating or electron-withdrawing nature of the substituents, even at 150°C (entries 6–8). Monosubstituted benzenes afforded a mixture of regioisomers, indicating the involvement of aryl radicals (entries 7 and 8). Reactions with N-containing heteroarenes proceeded efficiently (entries 10–13). The coupling reaction with pyrrole (entry 12) or 1-methylpyrrole (entry 13) gave only the 2-substituted coupling product.64–66)
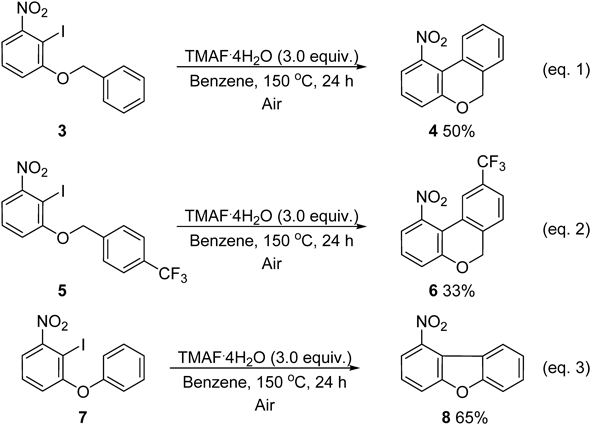
We then applied these reaction conditions for carrying out the intramolecular cyclization of aryl ethers using 1-(benzyloxy)-2-iodo-3-nitrobenzenes 3 and 5, and 2-iodo-1-nitro-3-phenoxybenzene 7 as the substrates (Chart 3). The reaction produced fused three-ring compounds at 150°C in moderate to good yields. While 3 or 5 generated 4 or 6 having a six-atom ring at the center (eqs. 1 and 2), 7 generated 8 (eq. 3) having a five-atom ring at the center. Thus, these reaction conditions could be used for the further development of transition metal-free syntheses of fused ring compounds.
To investigate the reaction mechanism, we performed the reaction by adding the radical scavenger, TEMPO or galvinoxyl free radical. None of the desired products were detected, indicating that the reaction might proceed via a radical process (Chart 4). Further mechanistic studies are currently underway.

In summary, we developed a new transition metal-free method to prepare biaryl compounds using TMAF·4H2O. The cross-coupling between aryl iodides having electron-withdrawing groups and unactivated arenes proceeds in the range 100–150°C and affords the products in moderate to high yields. This reaction can be carried out in air, unlike the ones involving strong inorganic bases, thereby enabling the easy handling of reagents for the reaction. Additionally, intramolecular cyclization of aryl ethers proceeds under these reaction conditions to afford fused ring compounds in good yields. Investigations to unveil the detailed reaction mechanism and broaden the substrate scope is currently in progress.
This work was financially supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant No. 17K15418. This work was also supported by Grand for Basic Science Research Projects from The Sumitomo Foundation, the Platform Project for Supporting Drug Discovery and Life Science Research funded by Japan Agency for Medical Research and Development (AMED), Tohoku University Center for Gender Equality Promotion (TUMUG), and Morinomiyako Project for Empowering Women in Research.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.